
Case Report
Austin J Surg. 2025; 12(3): 1355.
Mature Hepatic Teratoma in an Adult: A Case Report and Literature Review
El Waled Moustapha EL GHOTH
Department of Surgery, Rabat, Morocco
*Corresponding author: El Waled Moustapha EL GHOTH, Department of Surgery, Rabat, Morocco Email: elwaledmoustapha@gmail.com
Received: August 13, 2025 Accepted: September 09, 2025 Published: September 11, 2025
Abstract
Hepatic teratoma is a rare germ cell tumor in adults, most often seen in young children. We report the case of a 52-year-old woman who presented with an incidentally discovered right hepatic mass. Surgical excision confirmed the diagnosis of mature hepatic teratoma. Through this observation, we discuss the diagnostic, therapeutic, and prognostic aspects of this exceptional entity.
Introduction
Teratomas are complex germ cell tumors arising from totipotent primordial germ cells, capable of differentiating into tissues derived from the three embryonic germ layers: ectoderm, mesoderm, and endoderm [1]. They occur mainly in the gonads (ovaries and testes) but can also arise in extragonadal locations, such as the anterior mediastinum, sacrococcygeal region, retroperitoneum, and more rarely, the liver [2].
Hepatic teratoma is an exceptional entity in the adult population, with an estimated incidence of less than 1% of all extragonadal teratomas [2]. To date, most documented cases involve children, particularly females under three years of age, suggesting a developmental or embryonic origin [3,4].
In adults, the occurrence of a mature hepatic teratoma is extremely rare and poorly characterized, making diagnosis challenging. Clinical presentation is often nonspecific, and the diagnosis is usually made incidentally during imaging performed for other reasons. Imaging plays a key role in the diagnostic approach, but only histopathological analysis can provide a definitive diagnosis, particularly to exclude immature or potentially malignant forms [5,6].
Given the rarity of this entity, each clinical observation makes a valuable contribution to understanding its pathophysiology, clinical features, management, and prognosis. The objective of this work is to report a new case of mature hepatic teratoma in an adult woman and to discuss, in light of literature data, the main clinical, radiological, histological, and therapeutic aspects of this rare pathology.
Case Presentation
The patient was a 52-year-old woman, with no medical history, no history of hydatid exposure, and no toxic habits. However, she had undergone surgery in 2010 for ductal carcinoma in situ of the right breast. She presented with right hypochondrial pain.
Physical examination was unremarkable.
Ultrasound of the right liver (anterior sector) revealed a heterogeneous cystic image with a thin wall, suggestive of a type IV hydatid cyst. Abdominal CT confirmed a multiloculated cystic lesion containing fatty, calcified, and fluid components, suggestive of a hepatic teratoma. An MRI scan further supported the diagnosis, showing a grossly oval lesion in the hepatic dome measuring 57 × 48 mm. The lesion contained a fluid level with a supernatant displaying T1 hypersignal, T2 hyposignal, and a dependent portion in T1 and T2 isosignal, without enhancement after gadolinium injection — findings suggestive of a modified hydatid cyst.
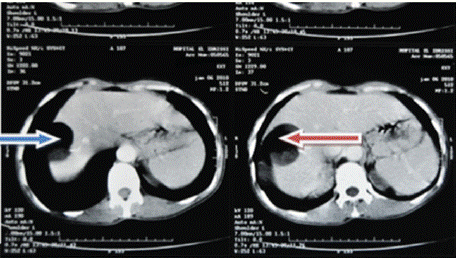
Figure 1: Axial abdominal MRI C+.
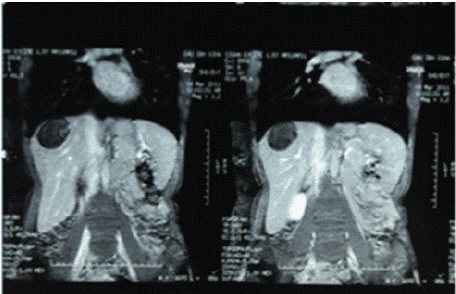
Figure 2: Abdominal MRI C-, frontal section showing a rounded formation
with clear boundaries in the right liver with heterogeneous content.
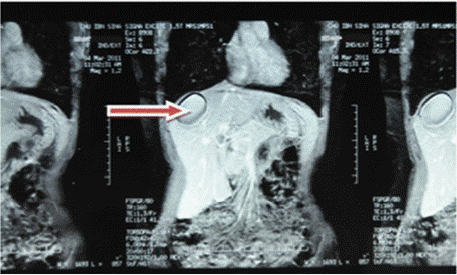
Figure 3: Red arrow: C+ abdominal MRI frontal section showing a rounded
lesion in the right liver with a watery appearance and no contrast uptake at
the periphery.
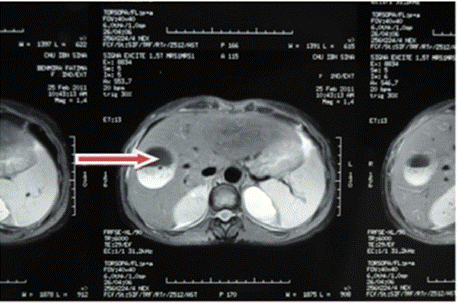
Figure 4: Red arrow: well-defined rounded lesion in segment VIII with
probable cystic NHA image.
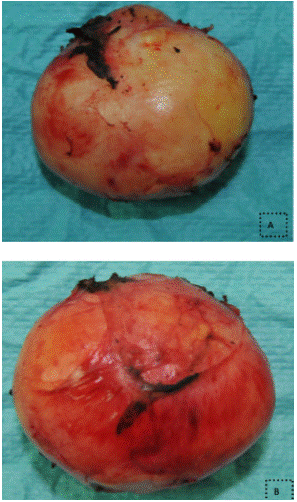
Figure 5: (images A and B) Surgical specimen after resection of an 80g
mass measuring 5x3x5x0.5 cm.
The patient underwent surgery via a right subcostal incision.
Exploration
The peritoneal cavity appeared normal. The liver was of normal size.
Segment VIII contained an oval lesion measuring 70 × 60 mm on intraoperative ultrasound, with a protruding whitish dome. The lesion’s contents appeared thick.
At this stage, the diagnosis favored was that of a modified type IV hydatid cyst. The cyst wall appeared thick.
A closed cyst resection was decided upon.
The resection was performed under vascular clamping of the hepatic pedicle for 25 minutes (Pringle maneuver).
Histopathological examination concluded a mature teratoma, with no immature or malignant component.
The postoperative course was uneventful.
An 18-month follow-up showed no recurrence.
Discussion
Germ cell tumors are rare entities originating from totipotent primordial germ cells. When they occur in the liver, they present a real diagnostic and therapeutic challenge due to their extreme rarity and varied clinical and radiological presentation [1].
Hepatic teratomas represent only a small fraction of extragonadal teratomas, with an incidence estimated at less than 1% of primary liver tumors [2]. Most reported cases involve female children under 3 years old, with a predilection for the right lobe [3,4]. This distribution suggests a developmental origin due to aberrant migration of primordial germ cells during embryogenesis.
Histologically, teratomas are composed of tissues from all three embryonic layers: ectoderm (skin, neural structures), mesoderm (muscle, adipose tissue), and endoderm (digestive tract, respiratory tissue) [5]. Mature teratomas, such as in our patient, are made up of well-differentiated tissues, unlike immature teratomas, which are potentially malignant and rich in undifferentiated neuroectodermal elements [6].
Two main theories explain their pathogenesis: the germ cell theory, based on ectopic migration of germ cells during embryonic life, and the embryonic theory, suggesting derivation from pluripotent cells trapped in embryonic fusion lines [7,8]. The germ cell hypothesis is most widely accepted for deep locations such as the liver.
Diagnosis is often incidental, as in our case, due to nonspecific symptoms. Imaging may reveal a solid-cystic hepatic mass with calcifications, fatty tissue, or dental-like structures [9]. Ultrasound provides an initial assessment, but CT and MRI are superior for identifying different tissue components and guiding surgery [10].
Definitive diagnosis requires histopathology to confirm maturity and exclude malignancy. The treatment of choice is complete surgical resection, which offers excellent long-term prognosis when no malignant transformation is present [11]. Follow-up is clinical and radiological, with very low but not zero recurrence risk [12].
Conclusion
Hepatic teratoma is an extremely rare pathological entity in adults. Its diagnosis is based on imaging and histological confirmation after resection. Surgical treatment is curative for mature forms. The rarity of this location justifies the publication of each case to enrich knowledge of this pathology.
Informed Consent
The patient's informed consent was obtained for the publication of this clinical case.
References
- Rescorla FJ. Teratomas and other germ cell tumors. Curr Opin Pediatr. 1997; 9: 344–353.
- Tapper D, Lack EE. Teratomas in infancy and childhood. A 54-year experience at the Children’s Hospital Medical Center. Ann Surg. 1983; 198: 398–410.
- Billmire DF, Grosfeld JL. Teratomas in children: analysis of 142 cases. J Pediatr Surg. 1986; 21: 548–551.
- Mignogna C, Barni T, Paglierani M, Gheri CF, Vannucchi S, Taddei A, et al. Hepatic teratoma: report of a case and review of the literature. Pathol Res Pract. 2001; 197: 567–570.
- Göbel U, Schneider DT, Calaminus G, Haas RJ, Schmidt P, Harms D. Germcell tumors in childhood and adolescence. Ann Oncol. 2000; 11: 263–271.
- Watanabe M, Watanabe K, Shirai Y, Hatakeyama K. A mature cystic teratoma of the liver in an adult: report of a case. Surg Today. 2000; 30: 947–950.
- Teilum G. Classification of endodermal sinus tumor (mesoblastoma vitellinum) and so-called “embryonal carcinoma” of the ovary. Acta Pathol Microbiol Scand. 1965; 64: 407–429.
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumors in a broader perspective. Nat Rev Cancer. 2005; 5: 210–222.
- Lee WK, Mossman D, Little AF. CT appearances of cystic teratomas in the abdomen and pelvis. Australas Radiol. 2006; 50: 193–198.
- Kocaoglu M, Frush DP, Donnelly LF. Teratomas in children: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2006; 186: 859–871.
- Khan Z, Pandey A, Joshi A, Khanna R, Khanna AK. Mature cystic teratoma of the liver: a rare entity. J Cancer Res Ther. 2015; 11: 669.
- Hwang SK, Lee HJ, Park SH, Cho KJ, Kim SH. Mature cystic teratoma of the liver with malignant transformation: a case report. Korean J Radiol. 2007; 8: 443–446.