
Research Article
Austin J Surg. 2015; 2(6): 1075.
The Differential Expression of Cancer Stem Cell Markers CD44, CD24 and ALDH1 in Breast Cancer Histological Types
Lee JS¹, Kim YM²* and Kim WK²*
¹Department of Surgery, Haeundae Paik Hospital, Inje University, South Korea
²Department of Pathology, Haeundae Paik Hospital, Inje University, South Korea
*Corresponding author: Kim WK and Kim YM, Department of Pathology, Haeundae Paik Hospital, Inje University College of Medicine, 875 Haeun-daero, Haeundae-gu, Busan 612-030, South Korea
Received: July 29, 2015; Accepted: November 06, 2015; Published: November 17, 2015
Abstract
Aldehyde Dehydrogenase 1(ALDH-1) and CD44+CD24- are the most consistently used biomarkers to identify and characterize breast Cancer Stem Cells (CSCs). However, expression of CSCs in Specific Histologic Types (SHTs) of Invasive Ductal Carcinomas (IDCs) remains unclear. We aimed to determine the distribution of CD44, CD24 and ALDH-1 breast CSC markers in SHTs of IDC compared with that in IDC-Not Otherwise Specified (NOS). From October 2013 to February 2014, we analyzed 168 IDC cases for double immunohistochemical staining of CD44, CD24 and single expression of ALDH- 1. The distribution of these CSC markers, CD44+CD24-, ALDH-1 and CSC phenotypes (CD44+CD24-/ ALDH-1) were retrospectively evaluated among the distinct SHTs and intrinsic subtypes compared with IDC-NOS. Medullary, metaplastic and apocrine carcinomas enriched in ALDH-1 population (100%, 100%, and 66.7%, respectively, vs. 44.1% in IDC-NOS, P=0.04). Medullary, papillary, and metaplastic carcinomas displayed significant increases in CSC phenotype frequency (66.7%, 28.6% and 100%, respectively, vs. 20% in IDCNOS, P=0.03). In IDC-SHT, mucinous carcinoma was correlated with luminal A subtype and medullary, papillary, metaplastic, apocrine, and micropapillary carcinomas were correlated with triple negative breast cancer (P=0.01). CK5/6(+), EGFR(+), ER(-), PR(-), and high HG were associated with ALDH-1(+) or CSC phenotype, but HER2(-) was associated with CD44+CD24-(+). Within distinct SHTs, medullary and metaplastic carcinomas are highly associated with the basal-like subtype and the CSC phenotype. In conclusion, we demonstrated the described CD44+/CD24-, ALDH-1, and CSC phenotypes may identify CSCs with distinct levels of differentiation and several SHTs are distinguished entities from IDC-NOS with regard to CSC marker expression.
Keywords: Breast cancer; Cancer stem cell; Basal-like; Intrinsic subtype
Abbreviations
ALDH-1: Aldehyde Dehydrogenase-1; CSC: Cancer Stem Cell; SHT: Specific Histologic Type; IDC: Invasive Ductal Carcinoma; NOS: Not Otherwise Specified; CK5/6(+): Cytokeratin 5/6; EGFR: Epidermal Growth Factor Receptor; ER: Estrogen Receptor; PR: Progesterone Receptor; HG: Histologic Grade; HER2: her-2/neu over expressed; TNBC: Triple Negative Breast Cancer
Introduction
Breast cancer is a heterogeneous disease, comprising various histological types, with distinct clinical presentations and underlying molecular signatures. Despite an increased knowledge about breast cancers in clinicians, development of metastasis cannot be always avoided in patients. One explanation for treatment failure is the Cancer Stem Cell (CSC) theory, which hypothesizes that cancer, may originate from and be sustained by a small population of cells that display the ability to maintain tumor growth by self-renewal and differentiation, as well as resistance to chemotherapy and radiotherapy [1-3]. In the case of breast tumors, Al-Hajj et al. [4] were the first to isolate a highly tumorigenic subpopulation of tumor cells with a CD44+CD24- lineage phenotype. Subsequently, Ginestier et al. [5] presented evidence that Aldehyde Dehydrogenase-1 (ALDH- 1) is a marker of stem/progenitor cells of the normal and malignant human breast. Based on this current knowledge, there is evidence to support the idea that the use of CD44 and CD24 cell surface markers in combination with ALDH-1 activity is the most accurate method to identify and isolate CSC-like cells within breast cancer populations. However, none of these CSC markers is expressed exclusively by stem cells, and a considerable number of cells that express these markers are not stem cells, resulting in phenotype heterogeneity within putative CSC populations [6,7]. Moreover, the overlap between high CD44+CD24-(+) and ALDH-1 expressions in primary tumors is quite small (approximately 1%) [5], thus it is imperative to improve CSC identification in routine formalin-fixed and paraffin-embedded tissue samples. Furthermore, regarding Special Histological Types (SHTs) that comprise up to 25% of invasive breast cancers [8], only a few studies have been conducted to explore the role of CD44 and CD24 in Micro papillary Carcinomas of the breast (IMPC) [9]. In a recent study by Park et al. [10], several stem cell-related markers were tested, but only Invasive Ductal Carcinomas (IDC)-Not Otherwise Specified (NOS) cases were studied. Others used cohorts mainly composed of IDC-NOS samples with only few cases of SHT [11,12]. Therefore, the frequency of the CSC phenotype in SHT breast carcinomas and whether each SHT has its own CSC remains largely unknown. In present study, we analyzed the expression of the main established breast CSC markers, CD44, CD24, and ALDH-1, in a large series of IDCs to evaluate their distribution among the different intrinsic subtypes and SHTs.
Methods and Materials
Patient selection
We prospectively collected 188 primary breast cancer specimens between October 2013 and February 2014 at our institute through our breast cancer center. One hundred sixty eight breast cancer patients were included for this study after exclusions. The following exclusion criteria for breast cancer patients or healthy subjects were applied in this study: (a) neoadjuvant chemotherapy (n=2); (b) recurrent breast cancer (n=1); (c) bilateral breast cancer (n=5); (d) ductal carcinoma in situ (n=7); (e) invasive lobular carcinoma (n=4); and (f) lobular carcinoma in situ (n=1). All patients provided written informed consents, and use of biological specimens, as well as clinical data for research purposes, were approved by the Institutional Review Boards of Haeundae-Paik Hospital, Inje University (Haeundae-paik 2013- 60).
Tissue specimens
Tissue Microarrays (TMA) were constructed from representative tissue columns (2.0mm in diameter) of formalin-fixed paraffin embedded tissue. For each surgical case, two cores from every individual tumor were made into TMA. All patients were female, with a median age of 52.3±10.2 years. Clinicopathological information was obtained by reviewing medical records, pathological reports and hematoxylin and eosin stained slides, and included the followings: tumor size, ipsilateral axillary lymph node status, histologic subtype, Bloom–Richardson histologic grade, lymphovascular tumor emboli, Estrogen Receptor (ER) status, Progesterone Receptor (PR) status, Human Epidermal Growth Factor Receptor 2 (HER2) status, and Ki67 index, as well as expressions of basal cell markers, Epidermal Growth Factor Receptor (EGFR) and cytokeratin 5/6.
Immunohistochemical staining of CD44 and CD24
Sections (4μm thick) of the TMA blocks were mounted on Superfast Plus Slides (Thermo Scientific). Double-immunostaining was performed using a detection kit (Ventanaultraview universal kit) by a Ventana Benchmark XT Autostainer (Ventana medical system). CD44 staining (1:200 dilution; Clone 156-3C11, Neomarkers, Fremont, CA, USA) was visualized with AP Red (Ventanaultraview universal AP red kit), whereas CD24 (1:50 dilution; polyclonal CD24 antibody, Biorbyt, Cambridge, UK) was visualized using Diaminobenzidine (DAB) (Ventanaultraview universal DAB kit).
Immunohistochemical staining of ALDH-1
For ALDH-1 immunostaining, ALDH-1 antibody (1:100 dilution; clone 44/ALDH, BD Biosciences, San Jose, CA, USA) was used. Staining was performed using the Ventanaultraview universal DAB kit according to the manufacturer’s protocols. Paraffin sections of normal liver tissue were used as a positive control. The cytoplasmic staining of cancer cells was considered ALDH-1 positive. Positive control for the specificity of ALDH-1 staining was carried out in normal hepatic tissue showing diffuse strong cytoplasmic expression.
Immunohistochemical scoring
All cases were independently reviewed and scored by two pathologists (W.G. Kim and Y.M. Kim) who were blinded to clinical diagnosis and there was inter-pathologist discussion for all unclear cases.
CD44 showed predominant membranous staining patterns and the scoring was carried out as follows: 0, 0% positive tumor cells; 1, 1–10% positive cells; 2, 11–50% positive cells; 3, 51–75% positive cells; and 4, 76–100% positive cells. Conversely, CD24 staining was detected mainly in the cytoplasm with some membranous staining pattern. The same scoring system described for CD44 was applied for CD24.
The proportion of CD44+CD24-(+) tumor cells was determined as the percentage of cells positive for AP Red staining but negative for DAB staining (Figure 1A-1D). For subsequent analyses, tumors with score of 2 to 4 (>10% staining) were considered positive for CD44+CD24- phenotype. The frequencies of CD44-CD24+ cells, CD44+CD24+ cells and CD44-CD24- tumor cells were determined in a similar fashion.
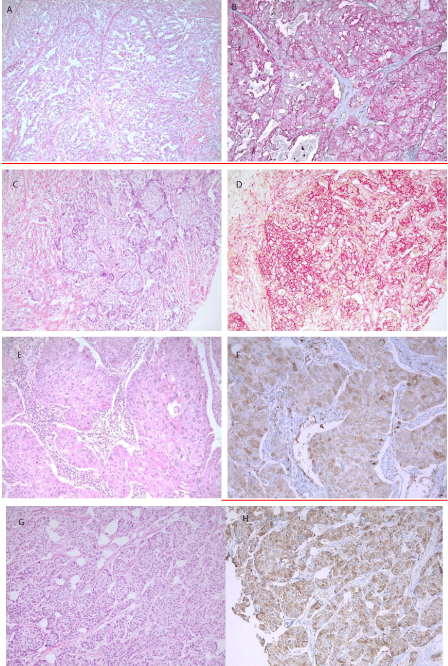
Figure 1: Hematoxylin & Eosin (HE) stains and immunohistochemical expression of CD44+CD24- and ALDH-1 in IDC-SHT and IDC-NOS. Double immunostaining
of CD44 and CD24 shows CD44 is stained with AP red and CD24 with DAB. Invasive ductal carcinoma, papillary type (A, HEx20) shows predominant membranous
staining in CD44 in AP red in all the tumor cells without CD24 expressions (B, CD44+CD24-x20). IDC, NOS (C, HEx20), showing predominant CD44+CD24-
phenotype (D, CD44+CD24-x20). Metaplastic carcinoma with squamoid differentiation (E, HEx20) shows diffuse strong positive ALDH-1 expressions in most of the
tumor cells (F, ALDH-1x20). An example of IDC, NOS (G, HEx20) with diffuse cytoplasmic ALDH-1 expressions (H, ALDH-1x20) is demonstrated.
ALDH-1 showed cytoplasmic staining in epithelial tumor cells. Immunohistochemical staining of ALDH-1 was classified as 4+ (>75% to 100% positive tumor cells), 3+ (>50% and =75%), 2+ (>10% to =50%), 1+ (1 to =10% positive tumor cells) and 0 for no expression of ALDH-1 in tumor cells. For subsequent analyses, tumors with 1+ to 4+ staining were considered ALDH-1 positive (Figure 1E-1H).
Cancer Stem Cell (CSC) phenotype was defined as the tumors with both CD44+CD24-(+) and ALDH-1(+) expressions [ALDH- 1(+)/CD44+CD24-(+)].
For Estrogen Receptor (ER) and Progesterone Receptor (PR), tumors with any positive staining for each receptor were considered positive. HER2 was scored using the ASCO guidelines, as follows: positive for HER2, IHC 3 (uniform intense membrane staining of >10%); equivocal for HER2, IHC 2 (weak and complete membranous staining of >10% of the tumor cells); negative for HER2, IHC 0–1 (<10% of the tumor cells or faint or barely any staining for partial membranous staining in >10% of the tumor cells).All equivocal HER2 results were confirmed by Silver In Situ Hybridization (SISH) for definite HER2 gene status [13]. The expression of Ki67 was considered positive in all tumor cells with nuclear expression of any intensity. CK5/6 and EGFR were positive for any membranous expression for the tumor cells.
Histology
Formalin-fixed paraffin-embedded tissues of 168 invasive ductal cancer samples, included 141 IDC-NOS and 27 IDC-SHT samples, which were comprised of five mucinous, seven medullary, seven papillary, two metaplastic, three apocrine, and three micropapillary carcinomas. Although intrinsic subtypes of breast cancer were originally defined by gene expression profiling using DNA microarrays, most archival formalin-fixed paraffin-embedded samples are not amenable to cDNA microarray and subsequent studies revealed that subtypes can be accurately determined using immunohistochemistry as a surrogate for molecular classifications. Subtype definitions in this study were used for two different classifications, as follows: luminal A (ER+ and/or PR+, HER2-), luminal B (ER+ and/or PR+, HER2+), HER2+ (ER-, PR-, HER2+), basal-like (ER-, PR-, HER2-, CK5/6+ and/or EGFR+) and unclassified (negative for all markers) [13,14]. We also classified breast cancer subtypes by the St. Gallen intrinsic breast cancer subtypes: luminal A, luminal B (HER2-), luminal B (HER2+), HER2+, and TNBC [15].
Statistical analysis
Statistical analyses were carried out to assess the relationship between ALDH-1(+) and/or CD44+CD24-(+) in primary tumors and SHTs with clinical variables. Associations between the different parameters were assessed using the ?2 test or Fisher’s exact test when appropriate.
All analyses were performed using SAS version 8.2 (SAS Institute Inc., Cary, NC, USA). All tests were two-sided and Fisher’s exact test was used whereby P-values of less than 0.05 were considered as significant.
Results
Clinicopathological parameters
Mean age of the patients was 52.3 (±10.2) years and mean tumor size was 2.26 (±1.33) cm. Ninety patients (53.6%) were stage I, 71 patients (42.3%) were stage II, and seven patients (4.2%) were stage III. A total of 141 patients (83.9%) were IDC-NOS and 27 patients (16.1%) were IDC-SHT. Other comparisons of clinicopathological parameters between IDC-NOS and IDC-SHT are shown in Table 1.
IDC-NOS
IDC-SHT
P-value
Age (mean) (years)
52.18
52.81
0.79
Tumor size (mean) (cm)
2.25
2.33
0.73
Number of metastatic lymph node
1.94
2.07
0.89
Number of patients (%)
Total number=141
Number of patients (%)
Total number=27
OP BCT
101(71.6)
18(66.7)
0.60
MRM
40(28.4)
9(33.3)
Stage I
79(56.0)
11(40.7)
0.30
II
56(39.7)
15(55.6)
III
6(4.3)
1(3.7)
ER(+)
69(51.1)
12(36.4)
0.67
PR(+)
45(33.3)
8(24.2)
0.81
HER2(3+)
31(23.0)
2(6.1)
0.11
LVI(+)
48(35.5)
9(6.7)
0.94
HG(3)
71(52.6)
15(11.1)
0.82
Ki-67(+)
71(52.6)
12(36.4)
0.67
Intrinsic subtypesa
0.02
Luminal A
29(21.5)
6(18.2)
Luminal B(HER2-)
20(14.8)
2(6.0)
Luminal B(HER2+)
26(19.2)
4(12.1)
HER2
33(24.4)
1(3.0)
TNBC
33(24.4)
14(42.4)
aSt. Gallen’s intrinsic subtypes classification
IDC: Invasive Ductal Carcinoma; NOS: Not Otherwise Specified; SHT: Special Histologic Types; OP: Operation; BCT: Breast Conserving Therapy; MRM: Modified Radical Mastectomy; ER: Estrogen Receptor; PR= Progesterone Receptor; HER2: her-2/neu over expressed; LVI: Lymphovascular Invasion; HG: Histologic Grade; TNBC: Triple Negative Breast Cancer
Table 1: Comparison of pathological variables between IDC-NOS and IDC-SHT.
Association between CD44+CD24-(+) expression, ALDH- 1(+) expression and CSC phenotypes within IDC-NOS and IDC-SHT and associations with clinicopathological parameters
CD44+CD24-(+) was observed in 46.4% of 168 patients, with 46.1% (65/141) of IDC-NOS, and 48.1% (13/27) of IDC-SHT (P=0.84). ALDH-1(+) was observed in 45.2% of 168 patients, with 44% (62/141) of IDC-NOS and 51.9% (14/27) of IDC-SHT (P=0.45). CSC phenotypes were observed in 22.0% of 168 patients, with 21.7% (30/135) of IDC-NOS and 25.9% (7/33) of IDC-SHT (P=0.59) (Table 2). ALDH-1(+) expression and CSC phenotype expressions were associated with a few breast cancer special types. ALDH-1(+) was associated with medullary, metaplastic, and apocrine carcinoma, while ALDH-1(-) was associated with micropapillary, papillary, and mucinous carcinoma (P=0.04) (Table 3). Positive CSC phenotype [ALDH-1(+)/CD44+CD24-(+)] expression was also associated with medullary, papillary and metaplastic carcinoma, but negative CSC phenotypes [ALDH-1(-)/CD44+CD24-(-)] was associated with mucinous, apocrine, and micropapillary carcinoma (P=0.03) (Table 3).
IDC-NOS
IDC-SHT
P-value
ALDH-1+(-)
79(56%)
13(48.1%)
0.45
ALDH-1+(+)
62(44%)
14(51.9%)
CD44+CD24-(-)
76(53.9%)
14(51.9%)
0.84
CD44+CD24-(+)
65(46.1%)
13(48.1%)
ALDH-1+/CD44+CD24-(-)
111(78.7%)
20(74.1%)
0.59
ALDH-1+/CD44+CD24-(+)
30(21.7%)
7(25.9%)
IDC: Invasive Ductal Carcinoma; NOS: Not Otherwise Specified; SHT: Special Histologic Types; ALDH-1: Aldehyde Dehydrogenase-1; CD: Cluster of Differentiation
Table 2: Comparison of stem cell markers between IDC-NOS and IDC-SHT.
Histologic types
IDC,NOS
Mucinous
Medullary
Papillary
Metaplastic
Apocrine
Micropapillary
P-value
ALDH-1(-)
79(56.0%)
4(80.0%)
0
4(57.1%)
0
1(33.3%)
4(100%)
0.04
ALDH-1(+)
62(44.0%)
1(20%)
6(100%)
3(42.9%)
2(100%)
2(66.7%)
0
CD44+CD24-(-)
78(55.4%)
3(60%)
2(33.3%)
3(42.9%)
0
2(66.7%)
2(66.7%)
0.88
CD44+CD24-(+)
63(44.6%)
2(40.0%)
4(66.7%)
4(57.1%)
3(100%)
1(33.3%)
1(33.3%)
ALDH-1+/CD44+CD24-(-)
112(79.4%)
5(100%)
2(33.3%)
5(71.4%)
0
4(100%)
3(100%)
0.03
ALDH-1+/CD44+CD24-(+)
29(20.6%)
0
4(66.7%)
2(28.6%)
2(100%)
0
0
IDC: Invasive Ductal Carcinoma; NOS: Not Otherwise Specified; ALDH-1: Aldehyde Dehydrogenase-1; CD: Cluster of Differentiation
Table 3: Associations between CSC markers and histological types.
Association between histologic types and intrinsic subtypes
In IDC-SHT, mucinous carcinomas were correlated with luminal A subtype, whereas medullary, papillary, metaplastic, apocrine, and micropapillary carcinomas were correlated with TNBC (P=0.01) (Table 4).
Histologic types
IDC,NOS
Mucinous
Medullary
Papillary
Metaplastic
Apocrine
Micropapillary
P-value
Luminal A
29(20.6%)
3(60.0%)
0
2(28.6%)
0
0
1(33.3%)
0.01
Luminal B (HER2-)
21(14.9%)
0
0
0
0
0
1(33.3%)
Luminal B (HER2+)
27(19.2%)
1(20%)
0
2(28.6%)
0
0
0
HER2
33(23.4%)
1(20%)
0
0
0
0
0
TNBC
31(21.9%)
0
7(100%)
3(42.9%)
2(100%)
3(100%)
1(33.3%)
141
5
7
7
2
3
3
IDC: Invasive Ductal Carcinoma; NOS: Not Otherwise Specified; HER2: her-2/neu over expressed; TNBC: Triple-Negative Breast Cancer
Table 4: Breast cancer intrinsic subtypes according to histological type in invasive ductal carcinomas.
Association between CD44+CD24-(+), ALDH-1(+), CSC phenotypes(+) and prognostic factors, and intrinsic molecular subtypes
CSC phenotypes (+) and ALDH-1(+) demonstrated a few associations with the classic prognostic factors, as well as with other studied biomarkers. ALDH-1 expression was significantly associated with CK5/6 (P=0.03), EGFR (P=0.02), ER negativity (P<0.001), PR negativity (P=0.02), high HG (P=0.002), high Ki-67 (P<0.001), and TNBC or basal-like subtype (P<0.001); no association was found with HER2 expression (P=0.86) and lymph node metastasis (P=0.75). CSC phenotypes were associated with these classic prognostic factors, and intrinsic breast cancer subtypes like ALDH-1 expression. But CD44+CD24- was not associated with classic prognostic factors, biomarkers, or intrinsic breast cancer subtypes except negative HER2 expression (P=0.02). This result was similar between the different classifications of intrinsic subtypes (Table 5).
ALDH-1
CD44+CD24-
ALDH1+/CD44+CD24-
(-)
(+)
P-value
(-)
(+)
P-value
(-)
(+)
P-value
LN(-)
57
45
0.75
55
47
1.0
78
24
0.70
LN(+)
35
31
35
31
53
13
CK5/6(-)
80
55
0.03
76
59
0.23
111
24
0.03
CK5/6(+)
12
20
14
18
20
12
EGFR(-)
79
52
0.02
74
57
0.09
108
23
0.01
EGFR(+)
13
22
14
21
21
14
ER(-)
35
52
<0.001
42
45
0.15
60
27
0.003
ER(+)
57
24
48
33
71
10
PR(-)
56
59
0.02
58
57
0.24
84
31
0.02
PR(+)
36
17
32
21
47
6
HG(I)
13
4
0.002
6
11
0.22
15
2
0.06
HG(II)
43
21
34
30
55
9
HG(III)
36
50
50
36
61
25
HER2(-)
66
56
0.86
58
64
0.02
92
30
0.21
HER2(+)
26
20
32
14
39
7
Ki-67(low)
61
24
<0.001
47
38
0.75
77
8
<0.001
Ki-67(high)
31
52
43
40
54
29
Intrinsic subtypea
Luminal A
25
10
<0.001
19
16
0.34
32
3
0.003
Luminal B
(HER2-)
12
10
12
10
16
6
Luminal B
(HER2+)
24
6
20
10
28
2
HER2
16
18
20
14
26
8
Basal
-like
11
22
13
20
20
13
Unclassified
4
10
6
8
9
5
Intrinsic subtypesb
Luminal A
25
10
<0.001
19
16
0.22
32
3
<0.003
Luminal B
(HER2-)
12
10
12
10
16
6
Luminal B
(HER2+)
24
6
20
10
28
2
HER2
16
18
20
14
26
8
TNBC
15
32
19
28
29
18
aSt. Gallen’s intrinsic subtype’s classification
bCarey and Nielsen’s intrinsic subtypes classification
ALDH-1: Aldehyde Dehydrogenase-1; CD: Cluster of Differentiation; CSC: Cancer Stem Cell; LN: Lymph Node; CK: Cytokeratin; EGFR: Epidermal Growth Factor Receptor; ER: Estrogen Receptor; PR: Progesterone Receptor; HG: Histologic Grade; HER2: her-2/neu overexpressed; TNBC: Triple Negative Breast Cancer
Table 5: Association between CD44+CD24-, ALDH-1+, CSC phenotypes (ALDH1+/CD44+CD24-) and clinicopathological parameters including molecular subtypes.
Discussion
The existence of stem/progenitor cells in the breast has long been postulated in view of the organ’s morphological changes throughout life, particularly during and after pregnancy [16]. In fact, adult mammary stem/progenitor cells are thought to be responsible for the massive expansion and differentiation of epithelial tissue during pregnancy and tissue renewal in interpregnancy periods [17]. It has been suggested that early full-term pregnancy may be associated with lower lifetime breast cancer risk because transformation and terminal differentiation of the stem/progenitor cells preferentially occur in pregnancy, decreasing the remaining stem/progenitor cells in number for malignant transformation [18,19].
When investigating ALDH-1 in SHTs, only apocrine, metaplastic and medullary carcinomas demonstrated increased prevalence of expression of this marker over IDC-NOS. ALDH-1 expression was also associated with high grade, triple-negative breast cancers and the expression of basal-like markers, similar to that previously described by other investigators [11,20]. However, another study showed increased prevalence of ALDH-1 expression only in papillary and medullary carcinomas over IDC-NOS [21]. When the CSC immunophenotypes were assessed, we found a different prevalence of CD44+CD24- tumor cells in the SHT series in comparison with that observed in the IDC-NOS series [12,22,23]. Though not statistically significant, medullary, papillary, and metaplastic carcinomas were associated with a higher prevalence of the CD44+CD24-. More interestingly, medullary, papillary and metaplastic carcinomas demonstrated this CD44+CD24-(+)/ALDH-1(+), CSC phenotype, with a significant increase in its prevalence over IDC-NOS. Therefore, the use of this CSC phenotype specifically identified these two breast cancer SHTs; medullary and metaplastic, consistently associated with triple-negative breast cancer, consistent with previous studies [11,12,20].
The CD44+CD24-(+)/ALDH-1(+)was associated with a worse patient prognosis in a subgroup of ER-negative tumors [11], and was also associated with ER and PR negativity, triple-negative tumors, and with the presence of basal markers, namely EGFR and CK5/6 [21]. Previous studies have already demonstrated an association between basal-like carcinomas and the CD44+CD24-(+) phenotype [24,25]. These results highlight the biological heterogeneity of breast cancer and an enrichment of putative tumor-initiating cells in the aggressive basal-like tumor subtypes. Furthermore, it seems to reflect the fact that whenever CSC markers are present in tumors, they probably identify the tumor cell origin more than cells harboring a higher selective advantage for tumor progression, because highly aggressive HER-2 overexpressing tumors did not show an increased expression of these markers, as observed in the present study.
Previous works also detected small percentages of ALDH-1(+) cases in invasive breast cancer, ranging from 4% to 19% [26,27]. In our study, we found 45.2% of tumors with ALDH-1 expression, higher than previous results. Remarkably, the majority of cases showing a predominant ALDH-1(+) population were significantly associated with basal-like tumors. Zhong et al. [28] reported that the proportion of ALDH-1 expression increased significantly in the breast after recurrence, but the proportion of CD44+CD24- tumor cells did not. However, other studies disputed that ALDH-1expressing tumors did not significantly correlate with poor clinical outcomes [12,29].
The major limitation of this study was the small number of patients included in the IDC-SHTs group and further investigation with a larger group of participants is required to confirm our study results. In summary, the described CD44+CD24-(+), ALDH-1(+), and CSC phenotypes seem to identify CSCs with distinct levels of differentiation, with the CD44+CD24- phenotype being more related to HER-2(+) invasive breast cancer putatively originating from luminal committed progenitors, whereas ALDH-1(+) and CSC phenotypes are markers of basal-like triple negative breast cancer putatively originating from the most primitive mammary stem cells. Also, we demonstrated that the prevalence of CSC markers is heterogeneous among breast cancer SHTs, thus providing further evidence that several SHTs are distinguished entities from IDCNOS with regard to CSC marker expression. Moreover, we provide evidence using the CSC phenotypes that we can identify SHTs characteristically associated with basal-like breast cancer.
Contributions
JSL: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing, final approval of manuscript; WGK and YMK: data analysis and interpretation.
Funding
The present research was funded by the Korean Breast Cancer Foundation.
Compliance with Ethical Standards
The institutional review board of our institute approved this study (Haeundae-paik 2013-60). The biospecimens and data used in this study were provided by the Biobank of InJe University Paik Hospital (InJe Biobank), a member of the Korea Biobank network ( IJB-13-00)
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009; 15: 4234-4241.
- Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008; 26: 2813-2820.
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008; 100: 672-679.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003; 100: 3983-3988.
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007; 1: 555-567.
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells –perspective on current status and future directions: AACR Workshop on cancer stem cells. Cancer res. 2006; 66: 9339-9344.
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008; 8: 755-768.
- Reis-Filho JS, Lakhani SR. Breast cancer special types: why bother? J Pathol. 2008; 216: 394-398.
- Gong Y, Sun X, Huo L, Wiley EL, Rao MS. Expression of cell adhesion molecules, CD44s and E-cadherin, and microvessel density in invasive micropapillary carcinoma of the breast. Histopathology. 2005; 46: 24-30.
- Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res. 2010; 16: 876-887.
- Ali HR, Dawson SJ, Blows FM, Provenzano E, Pharoah PD, Caldas C. Cancer stem cell markers in breast cancer: pathological, clinical and prognostic significance. Breast Cancer Res. 2011; 13: R118.
- Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008; 10: R53.
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007; 13: 2329-2334.
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004; 10: 5367-5374.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24: 2206-2223.
- Song LL, Miele L. Cancer stem cells--an old idea that's new again: implications for the diagnosis and treatment of breast cancer. Expert Opin Biol Ther. 2007; 7: 431-438.
- Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003; 3: 832-844.
- Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005; 7: 131-142.
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975; 255: 197-200.
- Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ADLH-1: expression distribution with intrinsic molecular subtypes. J ClinPathol. 2011; 64: 937-938.
- de Beça FF, Caetano P, Gerhard R, Alvarenga CA, Gomes M, Paredes J, et al. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol. 2013; 66: 187-191.
- Li W, Liu F, Lei T, Xu X, Liu B, Cui L, et al. The clinicopathological significance of CD44+/CD24-/low and CD24+ tumor cells in invasive micropapillary carcinoma of the breast. Pathol Res Pract. 2010; 206: 828-834.
- Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, et al. The clinicopathologic and prognostic significance of CD44+/CD24(-/low) and CD44-/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008; 39: 1096-1102.
- Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008; 10: R25.
- Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006; 8: R59.
- Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010; 123: 97-108.
- Fogel M, Friederichs J, Zeller Y, Husar M, Smirnov A, Roitman L, et al. CD24 is a marker for human breast carcinoma. Cancer Lett. 1999; 143: 87-94.
- Zhong Y, Shen S, Zhou Y, Mao F, Guan J, Lin Y, et al. ALDH1 is a better clinical indicator for relapse of invasive ductal breast cancer than the CD44+/CD24- phenotype. Med Oncol. 2014; 31: 864.
- Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010; 5: e10277.