
Research Article
J Surg. 2016; 3(1): 1082.
The Impact of Tumor Size on the Prognosis of Gastric Cancer: Experiences from a European Study Group
Dittmar Y*, Ardelt M, Scheuerlein H, Rauchfuss F, Dondorf F and Settmacher U
Department of General, University Hospital of Jena, Germany
*Corresponding author: Dittmar Y, Department of General, University Hospital of Jena, Helios-Klinikum Meiningen GmbH, Visceral and Vascular Surgery, Bergstrasse 3, 98617 Meiningen, Germany
Received: February 10, 2016; Accepted: April 07, 2016; Published: April 12, 2016
Abstract
Background: To investigate the impact of tumor size as a prognostic marker in the clinical course of resected gastric cancer on a German study group.
Methods: Based on a prospectively maintained data base, we included 573 cases of resected gastric cancer. Tumor size was measured postoperatively on the pathological specimen by the pathologist. The optimal cut-off point for tumor size was estimated by using the Cox regression model. We performed a tumorsize stratified analysis for several clinical and pathohistological factors in terms of their frequency and size-related impact on survival. The influence of tumor size on the pattern of tumor recurrence was analyzed.
Results: We found longer overall 5-year survival for patients with smaller tumors (69.5% and 36.8%, respectively, p<0.0001). Tumor size was an independent prognostic factor (p=0.042). Tumor size was a significant prognostic factor in curatively resected patients (R0), in both diffuse and intestinal type gastric cancer according to the Lauren classification, in the T2 category, in both well/moderate and poor/un- differentiated tumors, in both node-positive and node-negative categories, in cases with and without lymphangiosis, venous infiltration, cardia tumor location as well as tubular pathohistological growth pattern. Tumor recurrence was less frequent (28 vs. 39%, respectively, p=0.021) and at a later interval in smaller tumors (19 vs. 13 months, respectively, p<0.0001). Lung metastases were observed significantly more frequently in the subgroup of larger tumors.
Conclusion: Tumor size is a strong prognostic factor. In the development of a more individually designed cancer treatment tumor size might be a useful marker.
Keywords: Gastric cancer; Survival; Outcome; Tumor size; Prognostic factors
Introduction
Gastric cancer is known to be the second most frequent reason for tumor-related death worldwide [1]. With a global incidence of 952.000 new cases annually, gastric cancer is now the 5th most frequent malignancy [2].
The late onset of symptoms usually in an advanced tumor stage as well as the lack of eligible screening procedures for gastric cancer cases without symptoms both lead to the sustained poor prognosis [3]. The biology of gastric cancer implicates a strong association between tumor stage and overall survival [4]. The only hope for cure from gastric cancer is the curative resection which currently can be performed in approximately 50% of newly diagnosed cases. For those curatively resected patients, the evaluation of prognostic factors is essential to predict survival and the incidence of tumor recurrence [5]. The nodal stage is the factor with the highest predictive value but there is a wide variety of further markers which influence the further clinical course [6]. TNM-associated criteria as well as other pathohistological factors like the classification according to Lauren are well accepted prognostic markers [5]. Moreover, there is a rapidly growing number of molecular-based markers both influencing individual therapy and predicting individual prognosis. In this context, the value of the tumor size is not fully understood. Several authors judged tumor size to be a significant prognostic marker for gastric cancer [7-10]. In other solid tumor entities, tumor size is included in various staging systems. Tumor size is a well-established predictive factor in parenchymal organ malignancies, such as thyroid gland or the liver. More recently, tumor size has been shown to be a predictive factor for survival and recurrence in tumors derived from non-parenchymal organs, too. Tumor size can be measured preoperatively by via endoscopy whereas the accurate estimation of the depth of tumor invasion can be performed only after the tumor resection. Thus, tumor size is a preoperatively available parameter.
With our study, we intended to analyze the prognostic value of tumor size as related to the main tumor stages for resected gastric cancer.
Patients and Methods
Patient cohort, inclusion and exclusion criteria
From 1995 to 2012 we treated 1074 patients with gastric cancer in our Department of General, Visceral and Vascular Surgery. 752 of these patients underwent gastric resection. In 573 cases the tumor size was clearly identified. We used a prospectively maintained gastric cancer data base to collect detailed demographical, clinical and pathohistological data from these patients. All patients with adenocarcinomas of the stomach and information on its size who underwent resection were considered for our study. Patients with permanent immunosuppression as well as those who had a neuroendocrine tumor differentiation were excluded from the study. In addition, we did not enrol emergency resections as well as procedures which had been performed for cases which were refractory to interventional treatment of severe tumor-associated complications (palliatively intended resections).
Definitions
To stratify the cases of our study group, we used the TNM classification from 1997 because the majority of gastric cancers included in this study were diagnosed before 2010. Those cases which were included from 2010 onward we converted to the original classification system.
Tumor measurement and definition of cut off point
Tumor size was measured after surgical resection by the pathologist and was defined as the largest diameter of macroscopically detectable tumor mass.
To identify the optimal cut off point, we stratified all measured tumor sizes in centimetre groups (1 to 10 millimetres as 1 centimetre, 11 to 20 millimetres as 2 centimetres and so on). As a second step, we analyzed these categorical data by using the Cox regression model. The position with the highest chi square value was chosen as the optimal cut off point.
Statistical methods and literature search
Data collection and statistical analysis were performed using SPSS 19.0. For the univariate analysis, categorical data were evaluated by cross-linked tables and the exact Fisher-test. The results were regarded as statistically significant if the p-value was lower than 0.05. The Kaplan-Meyer method was used for survival analyses.
For comparison of subgroups, statistical significance was measured by the log rank test. For the multivariate analysis, we used the Cox regression model and included all criteria with a p-value below 0.1 in the univariate analysis.
Medline was searched for literature using the following search strategy: (“gastric cancer” or “gastric adenocarcinoma”) and (“tumor size”) and (“survival” or “outcome” or “clinicopathological factors” or “prognostic factors” or “predictive factors”).
Results
General description of the study group
The study group consisted of 223 women (38.8%) and 350 men (61.3%). Patient age ranged from 23 to 94 years with a median age of 66 years. All patients underwent gastric resection.
Tumor size and definition of the cut off point
The tumor size ranged from 2 to 200 mm and had a median value of 45 mm. The Cox regression showed the optimal cut off point to be 4 cm with a maximum chi square value of 40.725 (p=0.000). Details of the analysis are shown in Table 1. Figure 1 shows that the most frequent tumor size was 3 cm followed by 4 cm.
a
Chi square value
P value
Hazard ratio
1
14.374
0.000
6.639 (1,649-26.731)
2
32.084
0.000
3.926 (2.192-7.033)
3
32.823
0.000
2.444 (1.757-3.398)
4
40.725
0.000
2.453 (1.844-3.264)
5
31.460
0.000
2.167 (1.656-2.836)
6
29.023
0.000
2.172 (1.653-2.853)
7
22.260
0.000
2.142 (1.590-2.885)
8
11.499
0.001
1.905 (1.346-2.697)
9
9.153
0.002
2.023 (1.332-3.072)
10
3.853
0.050
1.896 (1.058-3.398)
11
1.780
0.182
1.682 (0.829-3.413)
12
3.411
0.065
2.632 ( 1.079-6.417)
13
4.702
0.030
3.211 (1.317-7.829)
15
4.648
0.031
3.756 (1.388-10.166)
17
4.648
0.031
3.756 (1.388-10.166)
18
2.811
0.094
3.193 (1.016-10.033)
Table 1: Evaluation of optimal cut-off point.
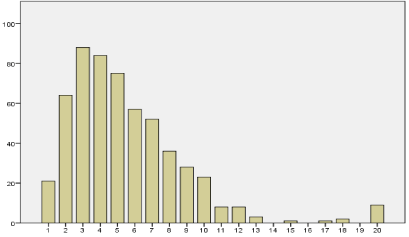
Figure 1: Distribution of frequency of tumor size as categorized in 1 cm steps
(vertical axis: number of cases, horizontal axis: tumor diameter in cm).
Tumor size and distribution of demographic and pathohistological factors
Low T-stage (T1), node-negative tumors, well and moderate grade differentiation, UICC stage Ia and Ib, tubular histological growth pattern as well as the presence of intestinal metaplasia within the gastric mucosa were observed significantly more frequently in smaller tumors. On the other hand, more advanced T-stages, nodepositive tumors with more than six positive nodes, tumors with distant metastases, lymphangiosis, venous tumor infiltration and perineural sheath infiltration, poorly differentiated tumors, UICC stage IIIa or higher and heterogeneous histological growth pattern were found more frequently in large tumors. Details of tumor-size dependent incidence of all analyzed factors are shown in Table 2.
Criterion (number of cases in brackets)
Incidence in tumors up to 40mm (in %)
Incidence in tumors from 41mm (in %)
P value
Demographical data
Gender
Male (350)
Female (223)
48.1
42.4
51.9
57.6
0.193
Age
Up to 45 years (61)
46 years and above (512)
44.8
45.9
55.2
54.1
0.890
Criteria according to the TNM classification
T stage
T1 (84)
T2 (294)
T3 (135)
T4 (46)
84.1
48.8
21.8
20.5
15.9
51.2
78.2
79.5
0.000
0.102
0.000
0.000
N stage
N0 (214)
N1 (1-6 positive nodes) (194)
N2 (7-15 positive nodes) (99)
N3 (>15 positive nodes) (65)
67.8
40.3
26.5
20.3
32.2
59.7
73.5
79.7
0.000
0.059
0.000
0.000
M1 (86)
29.3
70.7
0.001
Lymphangiosis (265)
32.7
67.3
0.000
Venous tumor invasion (108)
31.4
68.6
0.001
Perineural sheath invasion (87)
30.9
69.1
0.003
Tumor differentiation
G1/G2 (170)
G3/G4 (399)
62.9
39.3
37.1
60.7
0.000
0.000
Radicality of surgery
R0 (curative) (488)
R1 (31)
R2 (non-curative) (54)
49.2
38.7
25.9
50.8
61.3
74.1
n.s.
0.003
0.003
UICC stage
Ia (82)
Ib (104)
II (143)
IIIa (80)
IIIb (22)
IV (142)
88
68.3
42
32.5
14.3
23
12
31.7
58
67.5
85.7
77
0.000
0.000
0.331
0.011
0.003
0.000
Further pathohistological criteria
Macroscopical growth pattern
Exophytic (109)
Ulcerous (168)
Patelliformous ulcerated (96)
Flat (194)
43.1
51.2
44.8
44.6
56.9
48.8
55.2
55.4
0.456
0.167
0.737
0.535
Histological growth pattern
Tubular (233)
Cribiform/ trabecular (36)
Solide (34)
Diffuse (185(
Heterogeneous (81)
56.9
36.1
35.3
42.7
36.3
43.1
63.9
64.7
57.3
63.8
0.000
0.229
0.216
0.244
0.049
Mucin production
Extracellular (EZ) (131)
Intracellular (IZ) (195)
Signet ring cells (SRC) (173)
EZ a/o IZ a/o SRC (268)
39.7
43.6
44.5
41.8
60.3
56.4
55.5
58.2
0.044
0.332
0.584
0.036
Tumor location
Cardia (203)
45.8
54.2
0.930
Lauren classification
Intestinal (232)
Diffuse (320)
50.9
40.5
49.1
59.5
n.s.
0.054
Peritumoral desmoplasia (123)
43.9
56.1
0.506
Gastric mucosa features
Intestinal metaplasia (114)
Chronic gastritis (303)
57.0
47.5
43.0
52.5
0.010
0.615
Postoperative morbidity
All complications (169)
45.0
55.0
0.646
Anastomotic leakage (47)
40.4
59.6
0.446
Table 2: Tumor-size dependent incidence of all analyzed factors.
Tumor size and survival analysis
Our data showed that the median overall survival was more favourable in smaller tumors (183 and 31 months, respectively, log rank p<0.0001). The associated overall 5-year-survival was 69.5% and 36.8%, respectively, as shown in Figure 2.
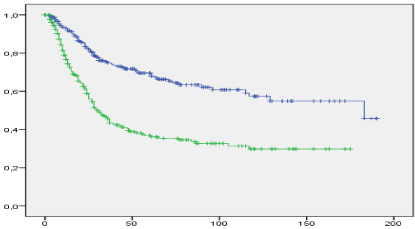
Figure 2: Overall survival as related to tumor size (blue line: tumors up to
4cm in diameter, green line: tumors more than 4cm in diameter; vertical axis:
cumulative survival, horizontal axis: survival time in months, n=573).
Similar results were seen for median tumor free survival (not reached and 21 months, Log Rank p<0.0001). The classification in several tumor size categories showed different prognostic subgroups which might be regarded as similar to T- or N-categories: The survival analysis showed a continuous decrease of survival time with increasing tumor size. Figure 3 shows the associated Kaplan-Meyer- Curve.
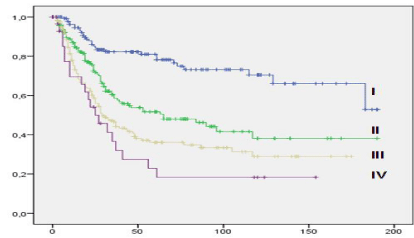
Figure 3: Overall survival related to stratified tumor size (I: smaller than 3
cm, II: 3 – 5 cm, III: 5 – 10 cm, IV: larger than 10 cm; vertical axis: cumulative
survival, horizontal axis: survival time in months, n=573).
The multivariate analysis revealed tumor size to be an independent prognostic factor (p=0.041).
Survival and tumor-size associated subgroups
In the next step, we analysed whether tumor size is a prognostic factor in different subgroups. Table 3 demonstrates these results in detail. Tumor size was a significant prognostic factor in both diffuse and intestinal type according to the Lauren classification, in the T2 category, in both well/moderate and poor/un- differentiated tumors and in both node-positive and node negative categories. Furthermore, tumor size was relevant for the prognosis in tumors with and without lymphangiosis, venous infiltration, cardia tumor location as well as tubular pathohistological growth pattern. There was no significant difference between small and large tumors in terms of overall median survival time when stratifying the study group in individual UICC stages by using the 4 cm cut-off point. By using an alternative cutoff- point of 6cm, there was a statistically significant survival benefit for smaller tumours in the UICC stages II and IIIb (data not shown).
Median overall survival (in months)
p-value
(Log Rank)
Factor
Small tumours
Large tumours
Classification acc. to Lauren
Diffuse
Intestinal
115
Not reached
25
37
<0.0001
<0.0001
T-category
T1
T2
T3
T4
Not reached
117
26
19
Not reached
37
27
24
0.322
<0.0001
0.822
0.779
Degree of differentiation
G1/2
G3/4
Not reached
115
65
28
<0.0001
<0.0001
Lymph node stage
N-positive
N-negative
46
Not reached
25
Not reached
0.001
0.031
UICC stage
Ia
Ib
II
IIIa
IIIb
IV
Not reached
Not reached
73
29
9
23
Not reached
Not reached
52
29
27
17
0.507
0.261
0.386
0.442
0.787
0.316
Tumor location
Cardia
Non-cardia
96
183
25
35
0.002
<0.0001
Lymphangiosis
Yes
No
39
Not reached
25
117
0.002
<0.0001
Venous infiltration
Yes
No
53
183
19
37
0.012
<0.0001
Pathohistological growth pattern
Tubular
Non-tubular
Not reached
77
37
28
<0.0001
<0.0001
Table 3: Impact of tumor size on median overall survival stratified to different factors.
We observed a statistically significant longer survival for curatively resected patients (R0) with smaller tumors as compared with those of larger tumors (overall 5-year-survival 77% and 44.6%, n=482, log rank: p<0.0001), whereas there were no statistically significant differences in the subgroups of R1- and R2-resected patients (data not shown).
Tumor size and patterns of tumor recurrence
27.6% of patients with smaller tumors developed tumor recurrence whereas in patients with larger tumors the percentage was 38.5% (exact Fisher Test p=0.021). Metachronous lung metastases (n=14) developed significantly more frequently in patients with tumors larger than 4cm (n=12). There was no tumor-size-dependent difference for the incidence of liver metastases, local recurrence, metachronous lymph node metastases and for peritoneal carcinosis (data not shown). The number of tumor sites at the time of diagnosis of tumour recurrence as well was not tumor-size-dependent (data not shown). Interestingly, we found a statistically significant tumor-sizedependent difference for the tumor free interval which was longer in patients with smaller primary tumors (19 and 13 months, Log Rank p<0.0001).
The role of chemotherapy in our study population
In 102 cases (21%) of curatively resected patients (n=482) a chemotherapy was part of the primary treatment (56 cases of neoadjuvant chemotherapy and 46 cases of adjuvant chemotherapy). In the subgroup of R1-resections 17 out of 29 cases underwent chemotherapy, for non-curatively resected patients (R2) it was 35 out of 52 cases.
We observed a survival advantage for patients with smaller tumors (all resections included) as compared to those with larger tumors both in the subgroup of resection only (80.1% and 41.3%, respectively, log rank: p<0.0001) and resection combined with chemotherapy (40.3% and 26.8%, respectively, log rank: p=0.014). The poorer survival in patients who underwent chemotherapy as compared to those who underwent resection only can be explained by more advanced tumor stages in the chemotherapy subgroup.
Discussion
Gastric cancer is well known for its unfortunate combination of high incidence with poor prognosis. Whereas in the first decades of gastric surgery the perioperative mortality rate was the dominating problem, the management of tumor recurrence became the leading challenge in the modern era [11]. Ever since, the evaluation of potential prognostic markers became a focus of clinical research in the field of gastric cancer. Achieving complete eradication of tumor tissue by a curatively intended resection was one of the first predictive factors identified for survival. It was followed by the lymph node stage. Both factors decisively influenced the evolution the surgical technique. From its introduction in 1950 by the UICC and by Pierre Denoix, several pathohistological factors are used to categorize gastric cancer with intention to optimize its treatment and follow-up-care [12]. For the characterization of the primary tumor the involvement of the most distant tissue layer is the basis for the specification of tumor extent. It has been shown in numerous studies that this “vertical extent” of the primary tumor is strongly associated with overall survival [13,14]. For other tumor entities, alternative parameters were established to stratify the extent of primary tumors, for instance the number of involved layers as well as thickness of tumors in case of malignant melanoma. Tumor size – the “horizontal extent” of the primary tumor in mucosa-derived cancers – is another way to define primary tumor extent and is used for various entities, such as liver cancer, breast cancer and lung cancer. The potential impact of tumor size in gastric cancer has been described by various authors before [7-11,15]. It has been shown that tumor size is a strong and independent prognostic factor in terms of overall survival and the incidence of tumor recurrence [10]. Furthermore, it has been demonstrated, that tumor size influence overall outcome of surgery in gastric cancer patients in Asian study groups. However, only few data derived from non-Asian study groups are available.
In agreement with other studies, we found tumor size to be a statistically significant and independent predictive marker for survival. However, the comparability of our results against other publications which focussed on the impact of tumor size is limited because the time of measurement, the used technique as well as the choice of the cut-off point varied remarkably in the other reports. The majority of authors defined the largest diameter of the primary tumor as the “tumor size”.
There are two established methods to measure tumor size: the estimation preoperatively by upper endoscopy or postoperatively as part of the pathohistological examination of the resected specimen. The preoperative method can be used.
Regardless of neoadjuvant therapy protocols whereas the postoperative method probably does not reflect the original tumor extent depending on the individual treatment response. The following findings led us to use the pathohistological method:
-Tumor size was measured more precisely (estimation in millimetres)
-The endoscopic and pathohistological measurement results differed in a remarkable number of cases
-Tumor size frequently was not specified directly as a numerical value
-Only few patients received neoadjuvant treatment, the majority of our patients did not undergo chemotherapy prior to resection.
There have been different suggestions for the optimal cut-off point ranging from 3 to 10cm [10,16]. Furthermore, several authors converted the numeric data of tumor size into categorical data with different intervals [17].
Adachi and co-workers divided the study group (n=479) in three tumor-size-dependent subgroups and found a significant influence of tumor size on 10-year-survival (92% for less than 4cm, 66% for 4-10cm and 33% for more than 10cm) [17]. Based on a large study group of 2379 patients, Guo and co-workers found a significant survival benefit for patients with tumors not larger than 4cm as compared to those with a tumor size of more than 4cm (5-year-survival 69% and 40%, respectively) [10]. Similar results were presented by Xu and co-workers in 2009 by using a cut-off point of 3cm (5-year-survival 84% and 67%, respectively) [16]. I’m and co-workers defined a cutoff point of 6cm for advanced gastric cancer and also found a better 5-year-survival for those patients whose presented with smaller tumors (71% and 48%, respectively) [15].
Our cut-off point was statistically calculated by estimation of the highest chi square value by using the Cox regression model. Whereas the highest statistical significance was observed at the level of 4cm, the most frequent tumour diameter was 3cm. With an overall 5-year survival of 70% and 37%, respectively, for smaller and larger tumors we observed similar results as compared to the abovementioned studies.
The analysis of the tumor-size-dependent constellation of TNMassociated criteria showed that there is a strong correlation between tumor stage and tumor size. In addition, further pathohistological criteria, – such as intestinal metaplasia, tubular Growth pattern and higher degree of tumor differentiation (G1 and G2) - were found to have a higher incidence in smaller tumours. These findings suggest that tumor size is a marker which may indicate both the stage of the tumor disease and its biological behaviour. Guo and co-workers found a similar tumor-size-dependent constellation of clinical and pathohistological factors: intestinal type tumors, advanced patient age and earlier TNM-associated stages were found to be statistically more frequently in the subgroup of patients with smaller tumors [10]. Zu and co-workers, too, found a strong correlation between tumor size and tumor stage as well as biological behavior [9]. Interestingly, both authors observed younger age and a higher incidence for tumor location in the distal stomach in the subgroup of patients with smaller tumors.
For the whole study group, we found tumor size to be a strong predictive factor for survival. This finding was published by several authors before. As a first approximation this relationship may be caused by an accumulation of cases with more advanced tumor stages in the subgroup of large gastric cancer. Therefore, we performed a tumor-size-dependent comparison of various pathohistological criteria. Indeed, regarding the T-category, we found a relationship between tumor size and the T-stages 1, 3 and 4 but not for stage 2. This is partly in contrast to findings of other working groups: Hongliang and co-workers found a statistically significant difference of overall survival for the T-stages 2, 3 and 4 [9]. On the other hand, we found numerous pathohistological factors where overall survival was different between large and small tumors, among them nodal stage, classification according to Lauren, degree of tumor differentiation as well as lymphangiosis and venous infiltration. The impact of tumor size on survival has been described for node-negative and nodepositive gastric cancer by several authors [9,18-20].
The analysis of tumor-size-dependent recurrence pattern showed a higher incidence of recurrence for larger tumors. Furthermore, we found that metachronous lung metastases developed more frequently from larger primary tumors, whereas for all other recurrence locations there was no tumor-size-dependent correlation with the frequency of tumor recurrence. To our knowledge this has not been published by any other authors before.
Conclusion
Tumor size seems to be a strong prognostic factor that can even be measured prior to systemic treatment. Thus, in the development towards a more individually designed cancer treatment, tumor size might be a useful marker. In the future, the development of a scorichromeg system involving tumor size could serve as an additional tool in the management of gastric cancer. This hypothesis should be tested by a clinical study in the future.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007; 57: 43-66.
- Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014; 40: 250-260.
- Schmidt N, Peitz U, Lippert H, Malfertheiner P. Missing gastric cancer in dyspepsia. Aliment Pharmacol Ther. 2005; 21: 813-820.
- Sarela AI, Miner TJ, Karpeh MS, Coit DG, Jaques DP, Brennan MF. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg. 2006; 243: 189-195.
- Buzzoni R, Bajetta E, Di Bartolomeo M, Miceli R, Beretta E, Ferrario E, et al. Pathological features as predictors of recurrence after radical resection of gastric cancer. Br J Surg. 2006; 93: 205-209.
- Dittmar Y, Rauchfuss F, Goetz M, Jandt K, Scheuerlein H, Heise M, et al. Non-curative gastric resection for patients with stage 4 gastric cancer--a single center experience and current review of literature. Langenbecks Arch Surg. 2012; 397: 745-753.
- Quan J, Zhang R, Liang H, Li F, Liu H, Zhang H, et al. The impact of tumor size on survival of patients with pT4aN0M0 gastric cancer. Am Surg. 2013; 79: 328-331.
- Xu M, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, et al. Does tumor size improve the accuracy of prognostic predictions in node-negative gastric cancer (pT1-4aN0M0 stage)? PLoS One. 2014; 9: e101061.
- Zu H, Wang F, Ma Y, Xue Y. Stage-stratified analysis of prognostic significance of tumor size in patients with gastric cancer. PLoS One. 2013; 8: e54502.
- Guo P, Li Y, Zhu Z, Sun Z, Lu C, Wang Z, et al. Prognostic value of tumor size in gastric cancer: an analysis of 2,379 patients. Tumour Biol. 2013; 34: 1027-1035.
- Li JH, Zhang SW, Liu J, Shao MZ, Chen L. Review of clinical investigation on recurrence of gastric cancer following curative resection. Chin Med J (Engl). 2012; 125: 1479-1495.
- Denoix PF, Schwartz D. [General rules for classification of cancers and presentation of the therapeutic results]. Regles generals de classification des cancers et de presentation des resultants therapeutiques. Mem Acad Chir (Paris). 1959; 85: 415-424.
- Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998; 228: 449-461.
- Meyer L, Steinert R, Nowak L, Gellert K, Ludwig K, Saeger D, et al. [Prospective multicenter trial of gastric cancer surgery--a contribution to clinical research on quality control]. Prospektive Multizenterstudie zur Chirurgie des Magenkarzinoms--Ein Beitrag zur klinischen Versorgungsforschung. Zentralbl Chir. 2005; 130: 97-105.
- Im WJ, Kim MG, Ha TK, Kwon SJ. Tumor size as a prognostic factor in gastric cancer patient. J Gastric Cancer. 2012; 12: 164-172.
- Xu CY, Shen JG, Shen JY, Chen WJ, Wang LB. Ulcer size as a novel indicator marker is correlated with prognosis of ulcerative gastric cancer. Dig Surg. 2009; 26: 312-316.
- Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumor size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997; 4: 137-140.
- Kim DY, Joo JK, Park YK, Ryu SY, Kim YJ, Kim SK. Predictors of long-term survival in node-positive gastric carcinoma patients with curative resection. Langenbecks Arch Surg. 2007; 392: 131-134.
- Maehara Y, Tomoda M, Tomisaki S, Ohmori M, Baba H, Akazawa K, et al. Surgical treatment and outcome for node-negative gastric cancer. Surgery. 1997; 121: 633-639.
- Saito H, Kuroda H, Matsunaga T, Fukuda K, Tatebe S, Tsujitani S, et al. Prognostic indicators in node-negative advanced gastric cancer patients. J Surg Oncol. 2010; 101: 622-625.