Special Article - Pancreatic cancer Surgery
Austin J Surg. 2018; 5(1): 1119.
TGF-β Pathway Activity in Pancreatic Versus Biliary Tract Cancers
Aghamaliyev U1#, Rech A1*#, Hablawetz P1, Kiesslich T3, Gaiser T4, Thomas M5, Breitkopf- Heinlein K6 and Rückert F1
¹Department of Surgery, University Medicine Mannheim and Heidelberg, Germany
²Department of Internal Medicine I, Paracelsus Medical University, Austria
³Laboratory for Tumor Biology and Experimental Therapies (TREAT), Institute of Physiology and Pathophysiology, Paracelsus Medical University, Austria
4Department of Pathology, University Medicine Mannheim and Heidelberg, Germany
5Dr. Margarete Fischer-Bosch Institute of Clinical Pharmacology and University of Tuebingen, Germany
6Department of Internal Medicin II, University Medicine Mannheim and Heidelberg, Germany #These authors have contributed equally to this study
*Corresponding author: Andrea Rech, Department of Surgery, Medical Faculty Mannheim, University of Heidelberg, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, University of Heidelberg, Germany
Received: November 20, 2017; Accepted: January 03, 2018; Published: January 10, 2018
Abstract
Background and Aims: It has been observed that pancreato-biliary cancers go along with a prominent desmoplastic reaction, which seems to play a crucial role in migration and metastasis. Transforming Growth Factor β (TGF-β) is a key-player in tumor progression and metastasis contributes to this desmoplastic reaction. The histological characteristics of the desmoplastic reaction in Cholangio Cellular Carcinoma (CCC) seem to be comparable to those in Pancreatic Ductal Adeno Carcinoma (PDAC). The aim of the present study therefore was to analyze differences in TGF-β signaling between PDAC and CCC.
Material and Methods: Established cell lines of CCC and PDAC were analyzed for expression of each of the 27 TGF-β pathway signaling genes using the Fluidigm’s Biomark high-throughput qPCR chip platform. Eighteen PDAC and ten CCC cell lines served as samples. Furthermore, to validate the findings we either performed Western Blotting or immune histochemistry of Activin Receptor-Like Kinase 1 (Alk1) and Thrombospondin 1 (TSP-1).
Results: The Fluidigm microfluidics dynamic array showed a significantly lower expression of Alk1 (0.00-7.39 95% C.I.; p=0.004) in CCC cell lines when compared to PDAC cell lines. In contrast, the expression of TSP-1 (5,8 E+152; 3,307 - 4,76 E+170 95% C.I.; p=0.004) and Cyclin D1 (3.008; 0.394-26.330 95% CI; p=0.004) was significantly up regulated in CCC cell lines. However, these findings could neither be confirmed by Western Blotting nor by immune histochemistry.
Discussion: The majority of the identified members of the canonical TGF-β pathway seemed to be expressed homogenously in CCC and PDAC cell lines. Alk1 and TSP-1 had a distinct differential expression in CCC cell lines in The Fluidigm microfluidics dynamic array. A reason for a lack of confirmation by other methods could be the complexicity of mRNA and protein expression of the TGF-β pathway and the influence of cell-cell interactions in the in vivo situation, e.g., via inflammatory stroma reaction.
Keywords: Pancreato-biliary cancers; Pancreatic cancer; Cholangiocellular cancer; TGF-beta pathway; Thrombospondin-1; Desmoplastic reaction
Introduction
Pancreato-biliary cancers are aggressive gastrointestinal malignancies, characterized by a poor prognosis [1]. These cancer types have been observed to have a prominent desmoplastic reaction [2,3]. Profound evidence suggests, that the desmoplastic reaction leads to a poorly perfused and poorly vascularized tissue. As an effect, delivery and efficacy of chemotherapeutics is limited [2]. It has been further reported that interaction between cancer cells and their surrounding microenvironment might play a crucial role in migration and metastasis formation in these cancers [4]. The desmoplastic reaction therefore seems to play a role in important clinical characteristics of the tumors.
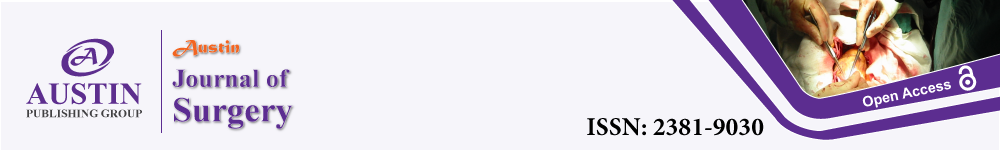
Histopathologic analyses of the desmoplastic reaction in pancreato-biliary cancers is characterized by an abundant extracellular matrix, fibroblasts, stellate cells, immune cells, nerve cells, growth factors and cytokines. The desmoplastic reaction is associated with an abnormal vasculature with numerous circuitous small leaky blood vessels and capillaries [4,5] (Figure 1). The extracellular matrix itself is composed of a variety of fibrous proteins, glycoproteins, proteinases and glycosaminoglycans as well as modulators of the cell-matrix interaction such as periostin, tenascin C, SPARC and Thrombospondin 1 (TSP-1) [6-9].
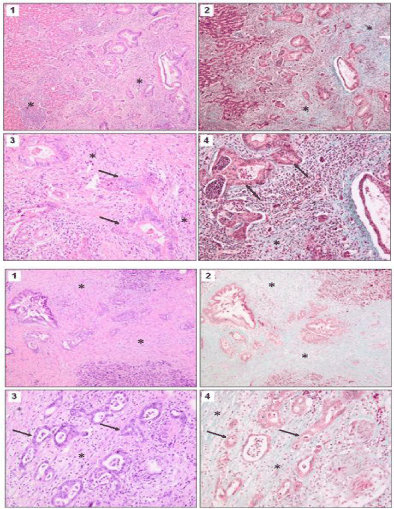
Figure 1: Desmoplastic reaction in cholangiocellular cancer (A) and
pancreatic cancer (B) with * marking desmoplastic reaction. Staining with H/E
(1) and Masson-Goldner (2) (x 40) and details of H/E (3) and Masson-Goldner
(4) (x 100). Arrows indicate tumor cells.
This abundant connective tissue deposition is due to growth factor production by the tumor cells and the cells of the tumor microenvironment. It has been shown that Transforming Growth Factor β (TGF-β), a pleiotropic cytokine, contributes not only to this desmoplastic reaction but also to pancreatic and biliary tract cancer progression and metastasis [10-12]. The role of TGF-β was extensively analyzed in pancreatic cancer (PDAC). Its importance in PDAC pathophysiology is mirrored in the fact that more than 50% of human PDAC show mutations in the TGFβ canonical pathway [13]. The histological composition of the desmoplastic reaction in Cholangio-Carcinoma (CCC) seems to be comparable to the desmoplastic reaction in PDAC. However, there is only limited data on the molecular background of the desmoplastic reaction in CCC. Therefore the aim of the present study was to compare TGF-β signaling activity in PDAC with CCC.
Cancer cells as well as cells of the stromal compartment take part in the development of the desmoplastic reaction and the signaling processes within the tumor show a high layer of complexity [14]. We limited the in vitro analyses to cancer cells, but validated the data in whole tumor tissue samples.
Material and Methods
Identification of TGF-β pathway members
The TGF-β pathway related genes were assembled from electronic databases, such as the Kyoto Encyclopedia of Genes and Genomes (www.genome.ad.jp/kegg), Gene Data Base of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) and with Wiki Pathways (www.wikipathways.org). Keywords for the search were ‘‘TGF-β’’, ‘‘TGF-β pathway’’, ‘‘TGF-β receptor”.
Cell culture
Cells were cultured under standard cell culture conditions at 37°C and 5% CO2. Medium was changed every 2-3 days. Supplementary information 1 shows the cell lines used for the in vitro investigations. Cholangio cellular carcinoma cell lines TFK-1, CCSW-1, BDC, EGI- 1, Sk-Ch-A1, gallbladder cancer cell lines Mz-ChA1, Mz-Ch-A2 and immortalized cholangiocyte cell line MMNK1 were cultured as described previously [15]. Primary pancreatic cell lines (PaCaDD cell lines) and the cell line CCC-5 were established previously by our lab and maintained as described previously [16-18].
Fluidigm microfluidics dynamic arrays
Parallel quantification of expression of 96 genes was performed using Fluidigm’s BioMark HD high-throughput quantitative chip platform (Fluidigm, San Francisco, CA), following the manufacturer’s instructions [19]. The IDs of all corresponding TaqMan assays are available upon request. The mRNA expression levels were normalized to the beta actin (ACTB) and glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) mRNA expression. Relative gene expression changes were normalized and calculated using the deltaCt (ΔΔ Ct) method [20].
Western blot analyses
Total cell protein was harvested on ice with RIPA lysis buffer (1x Tris-buffer, 1% Nonidet P40, 0.5% sodium deoxycholate, and 0.1% sodium dodecylsulfate) in the presence of freshly added protease and phosphatase inhibitors (Roche, Mannheim, Germany). Protein concentration was measured with a Bio-Rad protein assay (Biorad, Munich, Germany). Proteins were separated by 4-12% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (NuPAGE, Invitrogen) and transferred to nitrocellulose membranes (Pierce, Rockford, IL). To detect TSP-1 or ALK1, respectively, rabbit polyclonal antibodies from Abcam (ab85762) and Thermo Fisher Scientific (PA514921) were used. For house-keeping gene detection, monoclonal mouse β-actin antibodies from Sigma (clone AC-74) and polyclonal rabbit GAPDH antibodies from Santa Cruz (sc-25778) were used. Incubation with the primary antibodies occurred at 4°C overnight. Horseradish peroxidase-linked secondary antibodies were from Santa Cruz. The membranes were developed with Supersignal Ultra (Pierce, Hamburg, Germany) and chemiluminescence was detected digitally with a Fujifilm LAS 1000 image detection system.
Immunohistochemistry
Immunostaining was carried out on paraffin-embedded tissue sections using the same antibodies against TSP-1 or ALK1 as for Western blotting (see above). One investigator (TG) blinded to both clinical and pathological data assessed the slides. Protein expression in PDACs and CCCs was quantified using a visual grading system with a range between 0= no immune expression or weak staining in any proportion of the cells, or moderate expression in </=5% of the cells; 1=moderate staining in 5-50% of the cells; 2=moderate staining in >50% of the cells or strong staining in any proportion of the tissue. According to staining intensity, tissues were classified as expressing TSP-1 low, medium or high [21]. The local ethics committee approved the analysis of surgical samples for the purpose of this study.
Statistics
Two-sided, unpaired student’s t-test was used to identify significant differences of continuous variables between groups. Categorial variables were tested using ?2 (Pearson) for the cross tables. A p-value < 0.05 was considered significant. Statistical analysis of RT-q PCR was performed using Excel (Microsoft Corporation, Redmond, USA) (Table 1).
Carcinoma cells
Stromal tumor tissue
PDAC patient 1
2
-
PDAC patient 2
2
2
PDAC patient 3
2
2
PDAC patient 4
2
2
PDAC patient 5
2
-
PDAC patient 6
2
2
PDAC patient 7
0
2
PDAC patient 8
2
1
PDAC patient 9
2
1
PDAC patient 10
2
1
CCC patient 1
2
CCC patient 2
0
1
CCC patient 3
2
1
CCC patient 4
2
1
CCC patient 5
2
2
CCC patient 6
2
1
CCC patient 7
2
1
CCC patient 8
1
1
CCC patient 9
2
0
CCC patient 10
2
1
CCC patient 11
2
1
CCC patient 12
1
1
Positive control
2
0
Table 1: Expression of TSP-1 in pancreatic cancer and cholangiocellular cancer samples. Statistical analysis (chi-square Test) did not show a difference in expression of this protein between the two tumor entities (0= no immunoexpression; 1=moderate staining; 2= strong staining).
Results
Literature search and construction of a pathway map for the TGF-β pathway, we selected 24 genes encoding proteins that belong to the well-described core of the mammalian TGF-β pathway including their downstream targets often described in the literature. The candidate genes are enlisted in (Figure 2). Due to the massive inter linkage of the TGF-β pathway with other pathways we cannot claim perfection. The information derived from the electronic data banks was used to construct a pathway map of the TGF-β pathway (Figure 3).
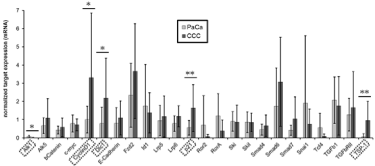
Figure 2: Expression of 24 genes associated with the TGF-β signal transduction pathway was measured by qRT-PCR in 9 CCC (dark grey bars) and 15 PDAC
(light grey bars) cell lines. The average +/- SD of the two groups of cell lines is shown. * indicates p>0.05 and ** indicates p>0.01 as calculated by student’s t-test.
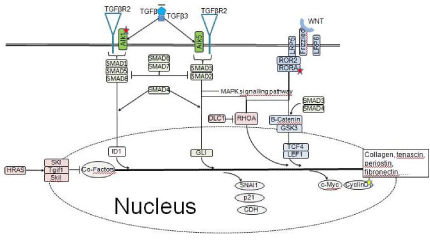
Figure 3: Map of the TGF-β signaling pathway.
Gene expression analysis of TGF-β pathway-associated gene groups can distinguish between pancreatic cancer and cholangiocellular cancer cell lines
We measured gene expression levels for each of the 24 signaling genes of the canonical TGF-β pathway using Fluidigm’s Biomark high-throughput qPCR chip platform in fifteen PDAC and nine CCC cell lines. Two reference genes were used in order to obtain more accurate and reliable normalization data that is [19].
Average expression levels of five genes were found to be differently expressed in CCC versus PDAC cell lines (Figure 2) with statistical significance. Expression of Alk1 was significantly down regulated in CCC cell lines. On the contrary, we observed that expressions of TSP- 1, CyclinD1, Dlc1 and p21 were higher in CCC cell lines compared to PDAC cell lines (Figure 2). Because TSP-1 is involved in activation of latent TGF-β [22] and Alk-1 is a TGF-β type I receptor, it can be expected that differential expression of both genes may directly affect TGF-β signal transduction. Therefore these two genes were chosen for further validation.
Validation of candidate genes by Western Blotting and immune histochemistry
To validate findings from the Fluidigm analysis we performed Western Blots for Alk1 and TSP-1 in protein lysates of CCC and PDAC cell lines. Surprisingly, higher expression of Alk1 in PDAC cell lines could not be confirmed on the protein level (Figure 4A). Concerning expression of TSP-1 we found stronger expression in the CCC cell lines than in PDAC cell lines (Figure 4B). However, this did not reach statistical significance. Because this tendency was in accordance to our findings on the RNA level we performed Immune Histo Chemistry (IHC) in tissue samples from CCC and PDAC patients. IHC showed a strong cytoplasmatic staining for TSP-1 in CCC cancer cells (Figure 5A), but also in cells of the tumor stroma (Figure 5B). However, the same holds true for PDAC, which also showed a staining in cancer cells and cells of the tumor stroma (Figure 5C and D). A breast cancer sample served as positive control (not shown). Semi-quantitative analysis of protein expression was performed by a pathologist. A statistical analysis by Chi-square test could not proof that expression of TSP-1 was higher in CCC than in PDAC.
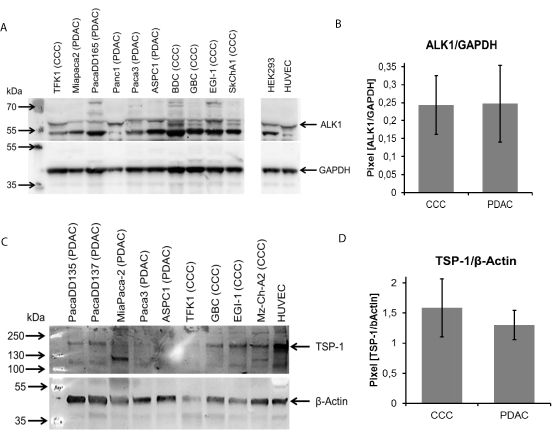
Figure 4: Western Blot of protein lysates from diverse CCC and PDAC cell lines. (A) Detection of Alk1 and (B) TSP-1. The bar diagramms show the densitometric
analyses of the blots as average +/- SD of the two groups of cell lines.
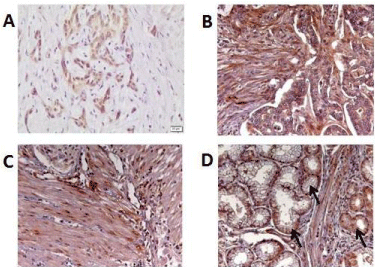
Figure 5: Immunohistochemistal staining of PDAC and CCC patient samples
for TSP-1. Representative samples of CCC (A) and (B) and PDAC (C) and
(D) are shown (Magnification: 100x). Within the sample shown in (D) there
are also normal acinar cells (arrow).
Discussion
Recently, it has been recognized, that the desmoplastic reaction plays an important role for clinical characteristics of CCC and PDAC [2,4,23]. TGF-β is a major profibrotic cytokine and the signaling pathway takes part in the development of the desmoplastic reaction in CCC as well as PDAC [3,13]. However, data on the molecular background of the formation of the desmoplastic reaction in CCC is limited. To evaluate similarities and discrepancies in the activation of the canonical TGF-β signaling pathway in CCC and PDAC we compared the expression of pathway members in cell lines of both tumor entities. The TGF-β pathway is one of the best investigated intracellular pathways. However, it comprises a multitude of signaling molecules and displays complex interactions. Twenty-seven TGF-β core signaling associated genes were identified by database search. To better understand the information from gene expression analysis and to allow judgments about disturbances in the information flow within the TGF-β pathway we constructed a pathway map [24]. To assess interactions between the identified genes we evaluated databases manually. By setting TGF-β receptors as starting point for the signaling we constructed a model of the pathway to visualize the interactions. Although we could not experimentally proof those interactions, there is good experimental evidence due to the great number of references.
The analysis of gene expression by high throughput q-PCR showed differential expression of five genes in CCC. We found down-regulation of Alk1 and observed that expression of TSP-1, Dlc1, p21 and CyclinD1 was up regulated in CCC. Most interestingly, the major part of the identified pathway members seemed to be expressed homogenously in CCC and PDAC cell lines. As it was previously reported that the TGF-β pathway is highly dysregulated in PDAC the high homogeneity of expression was not anticipated [13]. However, a high number of cell lines were tested and the results seem to be coherent. Alk1 and TSP-1 are both prominent members of the pathway and had a distinct differential expression in CCC cell lines. Therefore these two genes were chosen for further validation. Differential expression of Alk1 could not be confirmed on the protein level by means of western blotting. However, TSP-1 protein levels seemed to be up regulated in the tested CCC cell lines. To test if this was also the case under in vivo conditions we analyzed the expression in surgical samples from patients with CCC and PDAC. Although immunohistochemistry showed a strong cytoplasmatic staining for TSP-1 in CCC cancer cells, this was also true for cells of the tumor stroma. PDAC samples also showed a staining in cancer cells and cells of the tumor stroma. Literature data have suggested the homeotrimeric extracellular matrix protein TSP-1 as a major activator of TGF-β1 and evidence suggests a central role of TSP1- in mediating tissue fibrosis through interaction with latent TGF-β [25,26]. Therefore it would be reasonable that up regulation of TSP-1, as seen in high throughput qRT-PCR, might be responsible for formation of desmoplasia in CCC. The reason for increased levels in stroma cells might be that TSP-1 expression is regulated by various cytokines such as plateletderived growth factor, fibroblast growth factor-2, or TGF-β being frequently expressed de novo at sites of inflammation [26,27]. Most cancers have inflammatory microenvironments which might explain the up regulation of TSP-1 in stroma cells in vivo. In conclusion, although we found five significantly differently expressed members of the TGF-β core signaling pathway in CCC versus PDAC cell lines we could not reproduce these differences in vivo. This discrepancy might be due to the very high complexity of TGF-β signaling and the in vivo interaction between the cells of the different tumor compartments.
Acknowledgment
This work was supported by grants from VAND e.V. (Vereinigung aserbaidschanischer Mediziner in Deutschland) and the Vetter- Stiftung. We are thankful to Emine Tüzen and Katarina Abramovic for technical assistance.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017; 67: 7-30.
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009; 324: 1457-1461.
- Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013; 58: 1992-2000.
- Heits N, Heinze T, Bernsmeier A, Kerber J, Hauser C, Becker T, et al. Influence of mTOR-inhibitors and mycophenolic acid on human cholangiocellular carcinoma and cancer associated fibroblasts. BMC Cancer. 2016; 16: 322.
- Apte MV, Xu Z, Pothula S, Goldstein D, Pirola RC, Wilson JS, et al. Pancreatic cancer: The microenvironment needs attention too. Pancreatology. 2015; 15: S32-38.
- Park EH, Kim S, Jo JY, Kim SJ, Hwang Y, Kim JM, et al. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis. 2013; 34: 694-702.
- Paron I, Berchtold S, Voros J, Shamarla M, Erkan M, Höfler H, et al. Tenascin-C enhances pancreatic cancer cell growth and motility and affects cell adhesion through activation of the integrin pathway. PLoS One. 2011; 6: e21684.
- Aghamaliyev U, Hajiyeva Y, Rückert F. Desmoplastic reaction in pancreatic ductal adenocarcinoma. Pancreas Open J. 2016; 1: 22-29.
- Nie S, Lo A, Wu J, Zhu J, Tan Z, Simeone DM, et al. Glycoprotein biomarker panel for pancreatic cancer discovered by quantitative proteomics analysis. J Proteome Res. 2014; 13: 1873-1884.
- Huang CK, Aihara A, Iwagami Y, Yu T, Carlson R, Koga H, et al. Expression of transforming growth factor beta1 promotes cholangiocarcinoma development and progression. Cancer Lett. 2016; 380: 153-162.
- Duangkumpha K, Techasen A, Loilome W, Namwat N, Thanan R, Khuntikeo N, et al. BMP-7 blocks the effects of TGF-beta-induced EMT in cholangiocarcinoma. Tumour Biol. 2014; 35: 9667-9676.
- Neuzillet C, de Gramont A, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, et al. Perspectives of TGF-beta inhibition in pancreatic and hepatocellular carcinomas. Oncotarget. 2014; 5: 78-94.
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008; 321: 1801-1806.
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007; 6: 1186-1197.
- Mayr C, Wagner A, Neureiter D, Pichler M, Jakab M, Illig R, et al. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement Altern Med. 2015; 15: 194.
- Zach S, Grün J, Bauer AT, Pilarsky C, Gaiser T, Grützmann R, et al. CCC- 5, a new primary cholangiocellular cell line. Int J Clin Exp Pathol. 2017; 10: 2451-2460.
- Ruckert F, Aust D, Bohme I, Werner K, Brandt A, Diamandis EP, et al. Five primary human pancreatic adenocarcinoma cell lines established by the outgrowth method. J Surg Res. 2011; 172: 29-39.
- Rückert F, Werner K, Aust DE, Hering S, Saeger HD, Grützmann R, et al. Immunohistological Analysis of Six New Primary Pancreatic Adenocarcinoma Cell Lines. Global Journal of Biochemistry. 2012; 3: 11.
- Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS One. 2008; 3: e1662.
- Feuer R, Vlaic S, Arlt J, Sawodny O, Dahmen U, Zanger UM, et al. LEMming: A Linear Error Model to Normalize Parallel Quantitative Real-Time PCR (qPCR) Data as an Alternative to Reference Gene Based Methods. PLoS One. 2015; 10: e0135852.
- Ruckert F, Hennig M, Petraki CD, Wehrum D, Distler M, Denz A, et al. Coexpression of KLK6 and KLK10 as prognostic factors for survival in pancreatic ductal adenocarcinoma. Br J Cancer. 2008; 99: 1484-1492.
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, et al. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995; 270: 7304- 7310.
- Ruckert F, Grutzmann R, Pilarsky C. Feedback within the inter-cellular communication and tumorigenesis in carcinomas. PLoS One. 2012; 7: e36719.
- Ruckert F, Dawelbait G, Winter C, Hartmann A, Denz A, Ammerpohl O, et al. Examination of apoptosis signaling in pancreatic cancer by computational signal transduction analysis. PLoS One. 2010; 5: e12243.
- Li Y, Turpin CP, Wang S. Role of thrombospondin 1 in liver diseases. Hepatol Res. 2017; 47: 186-193.
- Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004; 65: 459-468.
- Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014; 37: 83-91.