Research Article
Austin J Surg. 2018; 5(2): 1125.
Long-Term Survival Outcomes of Laparoscopy-Assisted Versus Open Gastrectomy for Advanced Gastric Cancer: A Meta-Analysis
Zhengyan Li*, Gang Ji, Jipeng Li, Bin Bai, Deliang Yu and Zhao Q
Department of Surgery, Xijing Hospital of Digestive Diseases, The Fourth Military Medical University
*Corresponding author: Zhengyan Li, Department of Surgery, Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, China
Received: December 11, 2017; Accepted: January 09, 2018; Published: February 08, 2018
Abstract
Background: Laparoscopy-Assisted Gastrectomy (LAG) has been accepted to treat early gastric cancer. The technical feasibility and favorable short-term surgical outcomes of laparoscopic gastrectomy for Advanced Gastric Cancer (AGC) have been confirmed by several meta-analyses, but the longterm survival outcome of LAG for AGC still remains controversial. This study aimed to compare the Overall Survival (OS) rate, Disease-Free Survival (DFS) rate and recurrence of LAG with OG for AGC.
Methods: Pub med, Embase, the ISI Web of Science and The Cochrane Library were searched (up to October 10, 2016) systematically. Prospective or retrospective studies comparing the clinical efficacy of LAG with OG for advanced gastric cancer were included to assess their survival and recurrence outcomes. Statistical analyses were carried out using RevMan 5.3 software.
Results: Ten studies with 2205 patients (1111 LAG, 1094 OG) were included in the analyses. There was no statistical difference in overall survival (OR=1.15, 95% confidence interval (CI):0.96, 1.38, P=0.13) and disease-free survival OR=1.15, 95% CI: 0.91, 1.44, P=0.25) between the two groups.
Conclusion: This meta-analysis demonstrated that LAG for AGC provided an acceptable prognosis with similar long-term survival outcomes and recurrence as OG.
Keywords: Laparo Scopic Gastrectomy; Advanced Gastric Cancer; Longterm Survival Meta-analysis
Introduction
Laparoscopy-Assisted Gastrectomy (LAG) has been accepted as one of best treatments for Early Gastric Cancer (EGC) since it was first reported by Kitano in 1994 [1]. The analysis of Randomized Clinical Trials (RCTS) and multiple high-qualities studies have described the safety of laparoscopic surgery for early gastric cancer [2-7]. In several medical centers, LAG gastrectomy for advanced gastric cancer has been carried out as a selection of operation for several years [8-13]. A growing number of reports and meta-analyses demonstrated that LAG was technically feasible and safety for locally advanced gastric cancer [14-18]. However, meta-analysis regarding long-term outcomes of AGC has-been seldom reported. This metaanalysis performs an updated evaluation of all the available highquality published trials aimed to compare the 5-year survival and recurrence outcomes of LAG with OG for AGC.
Methods
Literature search
A systematic literature search(up to October 10, 2016) was carried out using the Pub Med ,Embase, the ISI Web of Science and The Cochrane Library data base restricted to articles published in English, using the following search keywords:“gastric carcinoma Or gastric cancer”And“laparoscopy OR laparoscopic” And “gastrectomy”. In addition, the reference lists of all included studies were also searched. Three authors (Zheng-yan LI, Chuan LI and Zi-yanLuo) individually conducted the literature search and cross-checked their search results.
Study selection criteria
Studies were included if they meet the following criteria: (1) randomized or nonrandomized comparative studies were considered (2) The patients who underwent gastrectomy for advanced gastric cancer were acceptable; (3) the patients in the and OG groups were compared; (4) extent of lymp had enecto my from D1,D1+, D2 and D2+was acceptable; (5) all the potentially eligible studies should report at least one of the primary outcome measures, including the 5-year overall survival (OS), tumor recurrence and gastric cancer– related death rates. If there was an overlap between authors or centers we selected the higher quality or more recent literature. Studies cannot provide relevant data were excluded.
Data extraction and quality assessment
The following information was recorded for each eligible trial: authors’ names, study.
Design, the publication year, duration of follow-up, number of patients, general patient characteristics, 5-year overall survival (OS) rate, disease-free survival (DFS) rate and recurrence rate. The methodological quality of each included non-randomized control trial was assessed by the Newcastle-Ottawa scale (NOS). A score of 0 to 9 was assigned to each study.
Statistical analysis
This meta-analysis was carried out using the Review Manager (RevMan) software, version 5.3.Dichotomous variables was analyzed using Odds Ratios (OR) and 95% Confidence Interval (CI). We considered heterogeneity to be present if the I2 value was greater than 50% and a random effect model were applied. However, if I2 statistic was less than 50%, we used a fix effect model. The presence of reporting bias was evaluated according to the symmetry of the funnel plot. For all analyses, P<0.05 was considered statistically significant.
Results
Study characteristics
Finally, 10 retrospective non-randomized controlled studies [19-28] were included in the present meta-analysis. The flow chart of the selecting process of articles is shown in (Figure 1). In all, our meta-analysis include 2205cases (1111 patients underwent LAG, and 1094 patients underwent OG) with Advanced Gastric Cancer (AGC). The detailed characteristics of the selected articles are summarized in Table 1.
Study
Year
Region
Study type
Sample size
LN dissection
Surgical
extension
Number of
retrieved lymph node
Quality score
LG OG
Zhao
2011
China
RNT
346
313
D1 + a/β, D2
DG,
33.2(12.5)/32.8(15.6)
9
Chun HT
2012
Korea
RNT
52
67
D2
DG
39.1(15.2)/39.3(11.2)
9
Hamabe A
2012
Japan
RNT
66
101
D2
DG,TG
63.7(26.4)/ 44.0(18.9)
7
Kim KH
2012
Korea
RNT
88
88
D2
DG, TG
38.3(14.3)/41.8(15.3)
9
Siani
2012
Italy
RNT
25
25
D1 + a/β, D2
TG
35(18)/ 40(16)
8
2013
China
RNT
117
117
D2
TG
35.2(11.7)/37.4(13.2)
9
Gordon AC
2013
Japan
RNT
66
135
D1 + a/β, D2
DG
35.92(12.60)36.59(14.48)
7
Shinohara
2013
Japan
RNT
186
123
DG,PG,TG
45.3(16.9)/43.8(17.2)
9
Fang C
2014
China
RNT
87
87
D2
DG, TG
16.3/ 17.2
8
Zhang
2015
China
RNT
86
86
D2
DG
20(16-23)21(17-23)
8
Table 1: The detailed characteristics of the selected articles.
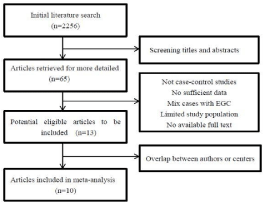
Figure 1:
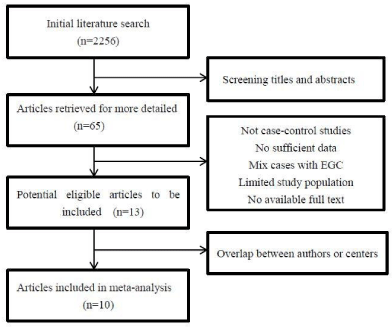
Flow Chart:
Five-year overall and survival
10 studies reported (1111cases in LAG group, and 1094 cases in OG group) 5-year overall survival outcomes. Visual inspection of the funnel plot revealed symmetry, indicating no serious publication bias, and none of the studies lay outside the limits of the 95% CI. (Figure 2).The result demonstrated that there was no significant difference between the LAG and OG groups (OR=1.15, 95% CI0.96, 1.38, P=0.13), and no heterogeneity was presented (Figure 3). 5 studies reported (632 cases in LAG group, and 599 cases in OG group) 5-year overall survival outcomes. Meta-analysis of these available data showed that the Disease-Free Survival (DFS) rate was not significantly different in participants who received LAG compared with OG, and no heterogeneity was presented (Figure 4).
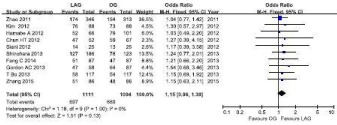
Figure 2:
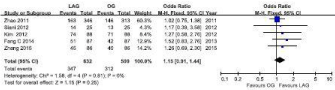
Figure 3:
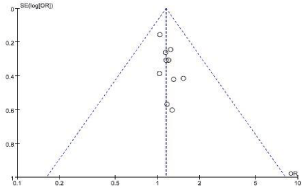
Figure 4:
Subgroup analyses
In order to remove several factors which may influence the results of survival results. We made the Subgroup analyses. All of the subsets are comparable between the two group, including the publication year, study region, sample size, surgery extension, lymp had enectomy, Number of retrieved lymph node and proportion of tumor stage (P<0.05). The 5-year overall survival outcomes of different subsets are summarized in Table 2.
Subgroup
Study No.
OR
(95% CI)
P
Model
I-Squared
P
Publication year
=2012
5
1.08
[0.84,1.40]
0.54
Fixed
0%
0.99
>2012
5
1.22
[0.95,1.58]
0.13
Fixed
0%
0.98
Region
Japan
3
1.24
[0.87, 1.79]
0.24
Fixed
0%
0.78
Korea
2
1.29
[0.66, 2.54]
0.46
Fixed
0%
0.98
China
4
1.10
[0.88, 1.37]
0.41
Fixed
0%
0.97
Sample size
<100
7
1.22
[0.91, 1.63]
0.18
Fixed
0%
1.00
=100
3
1.11
[0.88, 1.39]
0.38
Fixed
0%
0.83
Surgery extension
Distal only
4
1.11
[0.87, 1.43]
0.40
Fixed
0%
0.84
Total only
2
1.15
[0.72, 1.84]
0.55
Fixed
0%
0.97
Lymphadenectomy
D2/D2+only
7
1.19
[0.93, 1.50]
0.16
Fixed
0%
1.00
D1/D1+ (>20%)
2
1.40
[0.73, 2.69]
0.31
Fixed
0%
0.70
Nodes harvested
<30
2
1.18
[0.77, 1.81]
0.45
Fixed
0%
0.92
=30
8
1.14
[0.94, 1.39]
0.19
Fixed
0%
0.99
Tumor stage
I+II>50%
6
1.23
[0.93, 1.63]
0.15
Fixed
0%
0.99
I+II=50%
4
1.09
[0.87, 1.38]
0.45
Fixed
0%
0.97
RNT: Retrospective Non-Randomized Controlled Study; DG: Distal Gastrectomy; TG: Total Gastrectomy
Table 2: The 5-year overall survival outcomes of different subsets.
Recurrence and stage-specific survival analyses
6 studies reported (825 cases in LAG group, and 779 cases in OG group) recurrence during the follow-up period. The result suggested that there was no significant difference between the LAG and OG groups (OR=0.82, 95% CI0.65, 1.02, P=0.24). We made stage-specific analyses aim to make a precise analysis of survival outcomes. We also found that the results obtained are comparable between the two groups in stage II–III. Results of stage-specific analyses of 5-year overall survival outcomes are shown in Table 3.
Subgroup
Study No.
OR
(95% CI)
P
Model
I-Squared
P
Recurrence
6
0.82
[0.65,1.02]
0.07
Fixed
26%
0.24
Overall survival
10
1.15
[0.96,1.38]
0.13
Fixed
0%
0.51
Stage I
2
0.75
[0.18,3.09]
0.69
Fixed
0%
0.56
Stage II
2
2.07
[0.99,4.35]
0.05
Fixed
0%
0.81
2
1.32
[0.62,2.83]
0.48
Fixed
0%
0.84
Table 3: Five-Year Recurrence and Stage-Specific Analyses of the 5-Year Overall Survival.
Discussion
The long-term survival outcomes are key factor to evaluate the clinical efficacy and feasibility of an operation [7,29]. The present meta-analyses are a specific analysis of long-term survival outcomes and aim to make a precise analysis of relevant factors. The results indicate that the 5-year OS, recurrence is comparable between LAG and OG .Several factors such as the publication year, study region, sample size, Surgery extension, lymphadenectomy, Number of retrieved lymph node, and proportion of Tumor stage do not influence the results. What’s more, when we further study the OS outcomes by stage-specific analysis. We also found that the results obtained are comparable between the two groups. This updated metaanalysis confirms the results of several previous studies [30-33]. The results of 1 RCT conducted by Cai et al [11], show that the survival rate of the LAG group is slightly higher than that of the OG group though with no statistical significance. Zhao et al [19], reported the 5-year survival rate in the LG group was similar to the OG group which is consistent with the result of studies by Shinohara et al [26], and Bo T et al. [25]. A recent large-scale samples study conducted by Hao et al [31], reported that there was no significant difference in the stage-stratified survival rates between the two groups.
Perigastric lymphadenectomy is an important part of radical resection for gastric cancer patients [34-36]. Now, D2 has been acknowledged as the standard procedure for AGC by the European Society of Surgical Oncology (ESSO), the European Society for Medical Oncology (ESMO), and the European Society of Radiotherapy and Oncology (ESTRO) guidelines. Two meta-analyses reported that gastrectomy with D2 lymphadenectomy improves prognosis compared with D1.Our meta-analysis further evaluate the 5-year OS of different Lymphadenectomy subsets. 7 studies included in this meta-analysis were performed by gastrectomy with D2 or D2+lymphadenectomy. Mean while, Two studies included in this meta-analysis were performed by gastrectomy with D1/D1+>20% subset lymphadenectomy. No significant difference was shown with regard to the different extent of the dissection between the two groups.
The stage-specific analysis suggests that the long-term survival rates are comparable from Stage I to Stage III between the two groups. Moreover, we found that the studies in china present a higher recurrence rates than that in Japan and Korea. The primary reason for the differences is likely that over 80% of patients were diagnosed at advanced stage [19].Therefore, studies in china may include more stage III patients which have poor prognosis than that in stage I or stage II. Meanwhile the follow-up period of these studies are various which may lead to the difference of the recurrence rates. Regarding recurrence rates we found no significant difference between the two groups and these results suggest no inferiority of the long-term results for LAG compared with those for OG.
A multicenter retrospective study with lagre-scale cases suggest that the hematogenous recurrence pattern was most common in the LAG group, followed by the loco regional pattern [37]. The recurrence sites in these study are similar, included the peritoneum, liver, lymph node metastasis, and remnant stomach. Several studies stated that the peritoneum was the most common recurrence sites [23, 31,37]. Huang et al [38] reported port-site recurrence was occurred in one patient 10 month later after LG.
The majority of previous studies about LAG mainly included cases with T2 or T3 depth. Therefore, a general concern that laparoscopic surgery for AGC with serosa invasion (T4a ) may be lead an increased incidence of peritoneal seeding of malignant cells and port site recurrence. In previous studies, 25.9% patients (45/174) were diagnosed to have T4a depth of invasion. Recurrence was detected in 14 cases (16.1%) in the LAG group and 16 cases (18.4%) in the OG group which appeared to be not significantly different [27]. Till now, studies for patients with serous membrane invasion are rare and still needed to further investigate.
Some limitations of our present study need to be noted. First, no RCTs are included in the meta-analyses. Second, some subgroups such as stage-specific analyses of the 5-year OS only include the data of two studies which may limit the strength of the stage-specific analyses. What’s more, the survival outcomes may be influenced by learning curve, follow-up period and different baseline data of the two groups which is impossible to quantified.
Conclusion
In conclusion, our meta-analysis demonstrated that LAG for advanced gastric cancer provided an acceptable prognosis with similar long-term survival outcomes compared with OG. These findings support the acceptance and application of LAG for AGC. However, large-scale prospective RCT studies with long follow-up are still needed to further investigate the clinical outcomes of the two surgical procedures.
Authors’ Contributions
Zhengyan Li and Qingchuan Zhao designed the study. Zhengyan Li wrote the manuscript. Jipeng Li, Bin Bai and Deliang Yu performed the literature review, extraction of the data, and analysis of the pooled data. Gang Ji and Jipeng Li revised the manuscript. All authors read and approved the final manuscript.
Financial support: no
Ethics approval and consent to participate section: All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
References
- Kitano S IY, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994; 4: 146-148.
- Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005; 19: 1172- 1176.
- Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (Klass 01). J Korean Surg Soc .2013; 84: 123-30.
- Nakamura K, Katai H, Mizusawa J, Yoshikawa T, Ando M, Terashima M, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912). Jpn J Clin Oncol. 2013; 43: 324-327.
- Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg .2010; 251: 417-420.
- Yamashita K, Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Hosoda K, et al. Laparoscopic versus open distal gastrectomy for early gastric cancer in Japan: long-term clinical outcomes of a randomized clinical trial. Surgery Today. 2015; 46: 741-749.
- Lu W, Gao J, Yang J, Zhang Y, Lv W, Mu J, et al. Long-term clinical outcomes of laparoscopy-assisted distal gastrectomy versus open distal gastrectomy for early gastric cancer. Medicine. 2016; 95: e3986.
- Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016; 34: 1350-1357.
- Nakauchi M, Suda K, Kadoya S, Inaba K, Ishida Y, Uyama I, et al. Technical aspects and short- and long-term outcomes of totally laparoscopic total gastrectomy for advanced gastric cancer: a single-institution retrospective study. Surg Endosc. 2016; 30: 4632-4639.
- HOON HUR HMJ, Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years’ experience. J Surg Oncol. 2008; 98: 515-519.
- Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T, et al. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011; 28: 331-337.
- Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, et al. Laparoscopyassisted gastrectomy with D2 lymph node dissection for advanced gastric cancer without serosa invasion: a matched cohort study from South China. World J Surg Oncol. 2013; 11: 4-13.
- Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic Versus Open Subtotal Gastrectomy for Distal Gastric Cancer. Five-year results of a prospective trial. Annals of Surgery .2005; 241: 232- 237.
- Chen K, Xu XW, Mou YP, Pan Y, Zhou YC, Zhang RC, et al. Systematic review and meta-analysis of laparoscopic and open gastrectomy for advanced gastric cancer. World J Surg Oncol. 2013; 11: 182-194.
- Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015; 15: 355-362.
- Lu C, Zhou S, Peng Z, Chen L. Quality of D2 lymphadenectomy for advanced gastric cancer: is laparoscopic-assisted distal gastrectomy as effective as open distal gastrectomy? Surg Endosc. 2015; 29: 1537-1544.
- Choi YY, Bae JM, An JY, Hyung WJ, Noh SH. Laparoscopic gastrectomy for advanced gastric cancer: are the long-term results comparable with conventional open gastrectomy? A systematic review and meta-analysis. J Surg Oncol. 2013; 108: 550-556.
- Quan Y, Huang A, Ye M, Xu M, Zhuang B, Zhang P, et al. Comparison of laparoscopic versus open gastrectomy for advanced gastric cancer: an updated meta-analysis. Gastric Cancer. 2015; 19: 939-950.
- Zhao Y, Yu P, Hao Y, Qian F, Tang B, Shi Y, et al. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc. 2011; 25: 2960-2966.
- Chun H-T, Kim K-H, Kim M-C, Jung G-J. Comparative Study of Laparoscopy- Assisted versus Open Subtotal Gastrectomy for pT2 Gastric Cancer. Yonsei Medical Journal. 2012; 53: 952-959.
- Gordon AC, Kojima K, Inokuchi M, Kato K, Sugihara K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surgical Endoscopy. 2012; 27: 462-470.
- Hamabe A, Omori T, Tanaka K, Nishida T. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. 2012; 26: 1702-1709.
- Kim KH, Kim MC, Jung GJ, Choi HJ, Jang JS, Kwon HC, et al. Comparative analysis of five-year survival results of laparoscopy-assisted gastrectomy versus open gastrectomy for advanced gastric cancer: a case-control study using a propensity score method. Dig Surg. 2012; 29: 165-171.
- Siani LM FF, De Carlo A, Quintiliani A. Completely laparoscopic versus open total gastrectomy in stage I-III/C gastric cancer: safety, efficacy and five-year oncologic outcome. Minerva Chir. 2012; 67: 319-326.
- Bo T, Peiwu Y, Feng Q, Yongliang Z, Yan S, Yingxue H, et al. Laparoscopyassisted vs. open total gastrectomy for advanced gastric cancer: long-term outcomes and technical aspects of a case-control study. J Gastrointest Surg. 2013; 17: 1202-1208.
- Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013; 27: 286-294.
- Fang C, Hua J, Li J, Zhen J, Wang F, Zhao Q, et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am J Surg. 2014; 208: 391-396.
- Zhang Y, Qi F, Jiang Y, Zhai H, Ji Y. Long-term follow-up after laparoscopic versus open distal gastrectomy for advanced gastric cancer. Int J Clin Exp Med. 2015; 8: 13564-13570.
- Hiki N, Katai H, Mizusawa J, Nakamura K, Nakamori M, Yoshikawa T, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG0703). Gastric Cancer. 2018; 21: 155-161.
- Chen XZ, Wen L, Rui YY, Liu CX, Zhao QC, Zhou ZG, et al. Long-term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) .2015; 94: e454.
- Hao Y, Yu P, Qian F, Zhao Y, Shi Y, Tang B, et al. Comparison of laparoscopyassisted and open radical gastrectomy for advanced gastric cancer: A retrospective study in a single minimally invasive surgery center. Medicine (Baltimore) .2016; 95: e3936.
- Zou ZH, Zhao LY, Mou TY, Hu YF, Yu J, Liu H, et al. Laparoscopic vs open D2 gastrectomy for locally advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2014; 20: 16750-16764.
- MacLellan SJ, MacKay HJ, Ringash J, Jacks L, Kassam Z, Conrad T, et al. Laparoscopic gastrectomy for patients with advanced gastric cancer produces oncologic outcomes similar to those for open resection. Surg Endosc.2012; 26: 1813-1821.
- Tanizawa Y, Terashima M. Lymph node dissection in the resection of gastric cancer: review of existing evidence. Gastric Cancer.2010; 13: 137-48.
- Saka M, Morita S, Fukagawa T, Katai H. Present and Future Status of Gastric Cancer Surgery. Japanese Journal of Clinical Oncology. 2011; 41: 307-313.
- Shiraishi N, Yasuda K, Kitano S. Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric Cancer. 2006; 9: 167-176.
- Song J, Lee HJ, Cho GS, Han SU, Kim MC, Ryu SW, et al. Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol. 2010; 17: 1777- 1786.
- Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, et al. Laparoscopyassisted D2 radical distal gastrectomy for advanced gastric cancer. Dig Surg. 2010; 27: 291-296.