
Research Article
Austin J Surg. 2018; 5(2): 1126.
Group Milleri Streptococci in Surgical Patients: A Review of 367 Patients from a Single Center
Bonatti H¹* and Stelzmueller I²
¹Unversity of Maryland Community Medical Group Surgical Care, Easton, USA
²Praxis DOZ. DR. Ingrid STELZMüLLER, Salzburg, Austria
*Corresponding author: Hugo Bonatti, Unversity of Maryland Community Medical Group Surgical Care, USA
Received: December 13, 2017; Accepted: January 12, 2018; Published: February 09, 2018
Abstract
Background: Group Milleri streptococci (GMS) also known as Group anginosus streptococci comprise a heterogeneous subgroup of Gram-positive cocci, which are capable to cause severe purulent infections and abscesses.
Patients and Methods: This is a retrospective study of infections due to 637 consecutive GMS isolates in 448 patients during a four year period. Eight patient subgroups were created: primary non surgical (18%), ENT/dental (16%), upper GI tract (5%), lower GI tract diseases (8%), appendicitis (9%), anorectal (10%), biliary (27%), thoracic (3%) and miscellaneous infections (4%).
Results: Overall, 346 patients had a single isolate, 71 had two, 18 had three and 21 had four or more isolates. Median patient age was 51.1 years (range 0.1-94), 58.6% were male. Significant co-morbidities were present in 19.2% of patients, 17.9% were immunocompromised, 11.6% had cancer and 10.9% were solid organ recipients. Swabs accounted for 51.8% of isolates, bile for 19%, aspirates for 13.7%, blood cultures for 7.8% and the remaining specimens were from miscellaneous sites. Mixed infections were found in 48% with E. coli and Bacteroides fragilis being the predominant secondary pathogens. Of the surgically treated patients, 70% also received antibiotics [mean duration of treatment was 6 days). Recurrent infections developed in 5.8% and persistent infection requiring repeat surgery and/or prolonged antibiotic therapy in 11.2%. During the study period, 20 patients died, GMS infection accounted for a 2.5% mortality rate. Resistance rates to penicillin G, piperacillin/tazobactam, carbapenems and clindamycin was <5%, for cephalosporins and quinolones resistance ranged between 5% and 40%.
Conclusion: GMS are significant surgical pathogens. They should be distinguished from viridans streptococci. Most GMS infections require combined surgical/interventional treatment and antimicrobial chemotherapy.
Keywords: Streptococcus group Milleri; Streptococcus group anginosus; Sepsis; Abscess; Antibiotic; Surgical infection, Immunosuppression
Introduction
Pyogenic infections may be caused by a multitude of different microorganisms, may affect everybody site [1-3] and are commonly polymicrobial [2,4,5]. Some infections only require antibiotics and many can be treated with drainage alone but for most pyogenic infections treatment includes drainage, source control and antimicrobial therapy [6,7].
Until the 1970s viridans streptococci were rarely associated with purulent infections except endocarditis [8]. Viridans streptococci including Group Milleri Streptococci (GMS) can cause purulent infections of various internal organs [8-11]. GMS - in the US referred as to Group anginosus streptococci - comprise a heterogeneous group of streptococci including the species Streptococcus anginosus, Streptococcus constellatus, Streptococcus intermedius and certain β-haemolytic streptococci from Lancefield groups C, F and G [1,12]. The relevance of GMS within viridans streptococci with regard to surgical infections may have been underestimated. GMS have a propensity to cause purulent infections and form deep seated abscesses, are frequently isolated as part of polymicrobial infections and have a high recurrence rate if not treated properly [13-19]. In 1956, Guthof first described Streptococcus milleri in abscesses of the oral cavity [20]; subsequently, GMS were isolated from liver and central nervous system abscesses and in patients suffering from appendicitis, peritonitis, pleural empyema, Ear-Nose-Throat (ENT) and dental infections [20-25]. They also may cause endocarditis and bacteraemia [10,26,27]. Very recently, worldwide an increasing interest in GMS can be observed with multiple new reports being available [28-36]. Some of these reports focus on previously described GMS infections others emphasize the significance of GMS in thus far poorly studied patient populations such as those with pulmonary disease or GMS in the setting of vascular graft infection [30,36].
In this retrospective study the significance of GMS in a cohort of patients from a single center was analyzed (637 consecutive GMS isolates in 448 patients).
Patients and Methods
Patient identification, data collection, statistical analysis
For identification of patients the computerized database of the Institute for Hygiene and Microbiology was screened for GMS isolates. Clinical data were retrieved from electronic and paper charts and entered into an MS EXCEL database. The work was conducted according to the rules of the local ethics committee; subsets of this series have been reported previously.
Infection was assumed if GMS was isolated from otherwise sterile body sites together with clinical signs of infection such as fever, cough or purulent discharge and/or radiological signs such as abscess formation and/or laboratory findings indicative for acute infection including leucocytosis and/or elevated C-reactive protein.
Eight patient subgroups were created: primary non surgical (18%), ENT/dental (16%), upper GI tract (5%) and lower GI tract diseases (8%), appendicitis (9%), anorectal (10%), biliary (27%), thoracic (3%) and miscellaneous infections (4%).
For non surgical patients only limited clinical data could be obtained. Data are reported as total numbers and percentage for discreet and median/mean with range for continuous parameters. Statistical analysis was performed using SPSS. A p-value of <0.05 was considered statistically significant.
Microbiological analysis
The microbiology database was queried for all isolates of GMS from a collection of approximately 430.000 processed samples during a four year period. Isolation and identification of GMS were performed according to the Clinical and Laboratory Standards Institute (CLSI). Aerobic and anaerobic testing was performed. Specimens were incubated on Columbia agar containing 5% sheep red blood cells and incubated in a CO2-enriched atmosphere for 48 hours. Streptococci were identified by morphology, colony size, and hemolysis pattern. GMS were identified using the API 20 Strep Bio Merieux® (Marcyl ´Etoile, France) assay. Antimicrobial susceptibility testing was performed using the disc diffusion assay according to CLSI guidelines (CLSI 2004 Performance Standards for Antimicrobial Susceptibility Testing; Fourteenth Informational Supplement CLSI, Wayne, Pa). Subsets of isolates were subtyped; Streptococcus intermedius and constellatus were identified but not Streptococcus anginosus.
Results
Clinical Data
A total of 637 GMS isolates in 448 patients could be identified. The majority of patients (65%) were treated at various surgical services. Twenty percent of isolates originated from non-surgical services including internal medicine (12%), gynecology and urology (4%), neurology (2%), dermatology and pediatrics (1% each). Figure 1a shows distribution within surgical services. Approximately 80% of all isolates were isolated from general surgical and transplant patients. Figure 1b displays distribution of GMS isolates according to the site of infection. The biliary tract was the most common infection site with 27% followed by ENT/dental infections with 16%; all other infections accounted for <10% of cases.
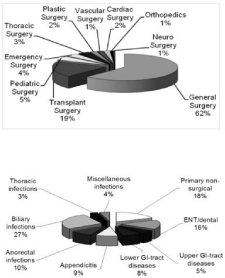
Figure 1: Study population: 1a: Distribution of surgical patients; 1b:
Distribution according to infection site for all patients.
The median age of the surgical cohort was 50.3 years. The youngest patient was a seven-day-old baby with necrotizing enterocolitis; the oldest was a 95 year old woman with perforated appendicitis. Overall, 57.7% of patients were male and 42.3% female. Median age of females was 54.9 years as compared to 50.6 years for the male cohort, p=0.01. Patients with appendicitis had the lowest median age (38 years) followed by patients with ENT/dental and anorectal infections (39 years). Patients with thoracic infections and infections associated with perforated lower gastrointestinal tract were significantly older. Significant co-morbidities could be identified in 18% of patients, 17% were immunocompromised (HIV, Crohn´s disease, chronic steroid treatment, diabetes mellitus), 16.5% had undergone solid organ transplantation (40 liver, one renal, five pancreatic, three intestinal recipients) and 12% had malignancies.
Microbiological data
The majority of the samples were obtained from swabs (n=328, 51%), followed by bile (n=125, 20%), aspirates (n=85, 13%), miscellaneous specimens (n=53, 8%) and blood cultures [n=46, 7%). When comparing surgical and non surgical patients, no differences with regard to type of specimen was found with the exception of blood cultures, which accounted for only 2.5% of surgical isolates (n=26 in 14 patients) but 18.7% of non-surgical isolates (n=20 in 12 patients), p<0.0001. There was an equal number of patients who had multiple GMS isolates (23.1% surgical vs. 25.3 % non-surgical patients, p=0.376). Within surgically treated patients, those with pleural empyma had the highest percentage of multiple isolates. Surgical patients had significantly more mixed infections when compared to non-surgical patients (52.7% vs. 19.8%, p<0.0001). Mixed infection was found in >80% of appendicitis, 50-60% in thoracic, hepatobiliary, anorectal and upper/lower gastrointestinal tract infections and <30% in soft tissue as well as ENT/dental infections (p=0.001).
GMS subtyping was performed in 268 cases (42%) including 193 from surgical patients, of which 29 isolates (6.2%) were Streptococcus constellatus and 164 specimens (35.5%) were Streptococcus intermedius. Out of the 637 isolates, GMS was the single pathogen in 331 cases (52%), whereas polymicrobial infection was found in 48% (306 isolates); for surgical patients mixed infection was present in 58% of cases. In total, 392 additional microorganisms were identified including 102 Gram positive bacteria, 181 Gram negative bacteria, 90 anaerobes and 19 fungi (Figure 2). Escherichia coli was the single most common coinfecting pathogen (n=79) followed by Bacteroides fragilis (n=74) and enterococci (n=40); there were 31 isolates of Klebsiella spp. and 28 of coagulase negative staphylococci (CNS), all other pathogens were cultured in <20 cases. Table 1displays the spectrum of the most common secondary pathogens according to the infection site. Staphylococcus aureus was a coinfecting pathogen in 3.3% with no major differences between the different infection sites. CNS was predominantly found in chest infections, Enterococcus spp originated mainly from the biliary tract. E. coli and Bacteroides fragilis were most frequently cultured from patients with gastrointestinal perforation. Pseudomonas aeruginosa was predominantly isolated in infections of the upper and lower gastrointestinal tract and Candida albicans was mainly found in upper gastrointestinal tract and chest infections.
Soft
non
ENT/
Appendi
Ano
Hepato
tissue/
total # of
surgical
dental
Upper GI
Lower GI
citis
rectal
biliary
Thoracic
various
p-value
isolates
Staphylococcus
aureus
4.4
4.3
4.8
0
0
0
0.8
7.7
0
0.257
10
Coagulase negative
staphylococci
3.3
5.7
4.8
11.8
0
0
9.2
23.1
8.7
0.026
28
Enterococcus spp.
4.4
1.4
9.5
14.7
4.9
0
21
0
4.3
<0.0001
40
Escherichia coli
1.1
1.4
28.6
38.2
61
27.9
16
7.7
4.3
<0.0001
79
Klebsiella spp.
1.1
0
23.8
11.8
4.9
0
13.4
7.7
8.7
<0.0001
31
Enterobacter spp.
1.1
2.9
4.8
8.8
2.4
2.3
5.9
7.7
4.3
0.588
18
Pseudomonas
aeruginosa
1.1
4.3
14.3
14.7
4.9
2.3
1.7
0
0
0.004
17
Bacteroides fragilis
2.2
0
9.5
55.9
46.3
37.2
11.8
0
8.7
<0.0001
74
Candida albicans
1.1
4.3
9.5
2.9
0
0
9.2
7.7
0
0.036
19
Table 1: Common co-infecting organisms according to infection site.

Figure 2: Overall spectrum of co infecting pathogens.
Sensitivity testing revealed resistance rates to penicillin G, piperacillin/tazobactam, carbapenems ranging from 1 – 2%. Clindamycin showed resistance rates of up to 9%, macrolides 6% and for cephalosporins and fluoroquinolones resistance ranged between 1 - 40%. All strains were resistant to aminoglycosides.
Therapy and outcome
Median hospitalization for the entire cohort was 9 (range 0-212) days; patients with appendicitis and dental infections were young, had few comorbidities or were immunocompromised and accordingly had a short hospitalization and few complications. Patients with gastrointestinal perforation and with thoracic infections had more commonly significant comorbidities and malignancies or were immunocompromised; accordingly they had more complications, higher recurrence rates and longer hospital stays and a higher rate of re-hospitalisation.
For the surgical patient populations, in all cases surgical/ interventional treatment was performed and 77% of patients additionally received antimicrobial chemotherapy. In 30.4% GMS was isolated from intraoperative cultures taken during surgical procedures. GMS was isolated from bile in 13% during Endoscopic Retrograde Cholangio-Pancreatography (ERCP). In all other cases, GMS infection was known at the time of surgery/interventional therapy.
The mean duration of antibiotic treatment was 6 days (range 0 to 48 days; 0 representing single shot prophylaxis). Preferred antibiotics were aminopenicillin, ureidopenicillin/β-lactamase-inhibitor combinations and 1st/2nd generation cephalosporins. For the surgical patients, death related to GMS infection occurred in 1.1% (two patients following infection of the upper and one patient following infection of the lower gastrointestinal tract). Surgical site infection was noticed in 6.1%, GMS positive blood cultures were found in 5.8%.
Discussion
Our study highlights the importance of GMS in surgical infections. The vast majority of GMS isolates were isolated from surgical sites involving various organs commonly as part of a polymicrobial infection. Individuals of all ages may be affected by these pathogens; men were more commonly affected than women. GMS infections are common in the immunocompromised host including solid organ recipients [37]. In most cases treatment included surgical source control together with antibiotics such as betalactams and clindamycin. To the best of our knowledge this is the largest such study on GMS ever performed.
GMS gained more interest in surgical disciplines due to their propensity to cause complicated abcsesses and deep seated infections [15,18,19,38]. In our series biliary tree and liver were the most common sites of infection. Corredoira et al. found a recent increase in GMS isolated from hepatic abscesses and Salavert et al. emphasized the relevant association between GMS and biliary sepsis [26,39]. In our series, liver transplant recipients with chronic cholangiopathy had a high yield of GMS [37] and GMS were also common isolates in non-transplant patients with cholangitis and cholecystitis.
GMS are “typical surgical” pathogens [16,17,19,22,23,40]. Various virulence factors have been identified including production of tissue toxic substances such as hyaluronidase, collagenase and various toxins that exhibit immunosuppressive properties [41-43]. The composition of the capsule prevents GMS from phagocytosis and phagocytic killing [41,44]. Wanahita et collegues showed resistance of GMS against the killing by Polymorphonuclear Leukocytes (PMNL), a phenomenon indicative for abscess forming pathogens [45]. Inhibition of chemotaxis by GMS also contributes to their virulence [45]. The exotoxins of GMS may play a particularly important role in mixed infections allowing other pathogens to escape the immune system and likewise, other pathogens may increase the virulence of GMS [46].
Host factors also predispose to GMS infections. Diabetes mellitus, hypertension, COPD and an immunocompromised status (HIV, inflammatory bowels disease), solid organ transplantation and malignancies have been associated with GMS infections [13,14,37,39].
Abscesses containing GMS are commonly polymicrobial [4,11,14,15,46]. In our study we observed 48% mixed GMS infections with Bacteroides spp and Escherichia coli being the dominant pathogens. It is not clear if GMS alone or polymicrobial infection including GMS is responsible for the high abscess rate [46]. Whereas polymicrobial infections dominated in intraabdominal infections, anorectal infections and thoracic infections, oral infections were mainly of monomicrobial nature [14,19,24,47]. Gram-negative rods such as Eschericha coli together with Bacteroides fragilis are dominating pathogens in appendicitis, lower gastrointestinal tract and anorectal infections [17,19,22]. CNS, Enterococcus spp and Candida albicans dominated mixed cultures from the hepato-biliary tract as well as in our thoracic infections.
Clarridge J E et al. found that S. intermedius was more frequently recovered in pure culture when compared to S. constellatus and S. anginosus [15]. In their series of nine intraabdominal and perirectal GMS isolates, eight were S. constellatus and one was S. anginosus without any cases of S. intermedius. In our cohort, S. intermedius was the predominant GMS subspecies with 35.5% of isolates mainly found in intraabdominal, hepatobiliary and perirectal infections. S. anginosus was not routinely tested for. S. anginosus may be cancerogenic as DNA-sequences have been identified in specimens from head, neck, oral, esophageal and gastric cancer; however, the exact pathogenesis is poorly understood [48-51].
All our surgical patients underwent at least one procedure including radiological guided drainage, surgical or endoscopic intervention [9,13,18,25]. GMS can be treated with betalactams, clindamycin, macrolides, some fluoroquinolones and tetracyclins but resistance needs to be considered [52,53]. GMS are not covered by aminoglycosides [53-55].
Drawbacks of our single centre study are the retrospective nature and the lack of a comparison group.
GMS have been recognized to be common and important surgical pathogens. Until 1980 only sporadic reports were available [8,10,20-23,41] but from 1985 to 2000 every year approximately 20 articles on GMS were published [9,11,13,17,18,26,39,40,42,43,46,4 7,49,51,55,56]. Since 2004 this number has doubled and every year up to 60 papers focusing on GMS become available in PubMed (Figure 3) [1,12,19,24,25,27-38,44,52,57]. Due to the often dramatic clinical presentation of GMS infections, many of these articles are case reports or small case series and only a limited number of large epidemiological studies such as ours are available [10,11,15,26,27,56]. Our work confirms that GMS are capable of causing severe and lifethreatening infections and therefore, early recognition and treatment including source control supported by antibiotics is necessary.
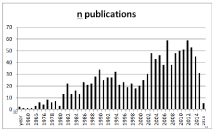
Figure 3: Annual reports on GMS in PubMed (https://www.ncbi.nlm.nih.gov/
pubmed).
References
- Petti C, Stratton C. Bacterial Diseases: Streptococcus anginosus group. In: Gerald L. Mandell JEB, Raphael Dolin editor. Mandell, Douglas and Bennett´s Principles and Practice of Infectious Disease. 7 edn. London: Churchill Livingstone. 2009; 194-250.
- Qadan M, Cheadle WG. Common microbial pathogens in surgical practice. Surg Clin North Am. 2009; 89: 295-310.
- Wolcott RD, Gontcharova V, Sun Y, Zischakau A, Dowd SE. Bacterial diversity in surgical site infections: not just aerobic cocci any more. J Wound Care. 2009; 18: 317-323.
- Brook I. Microbiology of polymicrobial abscesses and implications for therapy. J Antimicrob Chemother. 2002; 50: 805-810.
- Patel NP, Malangoni MA. Antimicrobial agents for surgical infections. Surg Clin North Am. 2009; 89: 327-347.
- Zimmerman LH, Tyburski JG, Stoffan A, Baylor AE, Dolman HS, Brinks LM, et al. Twelve hundred abscesses operatively drained: an antibiotic conundrum?. Surgery. 2009; 146: 794-798.
- Swenson BR, Metzger R, Hedrick TL, McElearney ST, Evans HL, Smith RL, et al. Choosing antibiotics for intra-abdominal infections: what do we mean by “high risk”?. Surg Infect (Larchmt). 2009; 10: 29-39.
- Facklam RR. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977; 5: 184-201.
- Molina JM, Leport C, Bure A, Wolff M, Michon C, Vilde JL. Clinical and bacterial features of infections caused by Streptococcus milleri. Scand J Infect Dis. 1991; 23: 659-666.
- Murray HW, Gross KC, Masur H, Roberts RB. Serious infections caused by Streptococcus milleri. Am J Med. 1978; 64: 759-764.
- Tresadern JC, Farrand RJ, Irving MH. Streptococcus milleri and surgical sepsis. Ann R Coll Surg Engl. 1983; 65: 78-79.
- Jensen A, Hoshino T, Kilian M. Taxonomy of the Anginosus group of the genus Streptococcus and description of Streptococcus anginosus subsp. whileyi subsp. nov. and Streptococcus constellatus subsp. viborgensis subsp. nov. Int J Syst Evol Microbiol. 2013; 63: 2506-2519.
- Admon D, Ephros MA, Gavish D, Raz R. Infection with Streptococcus milleri. J Infect. 1987; 14: 55-60.
- Belko J, Goldmann DA, Macone A, Zaidi AK. Clinically significant infections with organisms of the Streptococcus milleri group. Pediatr Infect Dis J. 2002; 21: 715-723.
- Claridge JE 3rd, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001; 32: 1511-1515.
- Han JK, Kerschner JE. Streptococcus milleri: an organism for head and neck infections and abscess. Arch Otolaryngol Head Neck Surg. 2001; 127: 650- 654.
- Piscitelli SC, Shwed J, Schreckenberger P, Danziger LH. Streptococcus milleri group: renewed interest in an elusive pathogen. Eur J Clin Microbiol Infect Dis. 1992; 11: 491-498.
- Porta G, Rodriguez-Carballeira M, Gomez L, Salavert M, Freixas N, Xercavins M, et al. Thoracic infection caused by Streptococcus milleri. Eur Respir J. 1998; 12: 357-362.
- Stelzmueller I, Aigner F, Albright J, Margreiter R, Fille M, Swenson BR, et al. Group Milleri Streptococci in Perianal Infections. Colorectal Dis. 2010; 12: e121-127.
- Guthof O. [Pathogenic strains of Streptococcus viridans; streptocci found in dental abscesses and infiltrates in the region of the oral cavity]. Zentralbl Bakteriol Orig. 1956; 166: 553-564.
- Bateman NT, Eykyn SJ, Phillips I. Pyogenic liver abscess caused by Streptococcus milleri. Lancet. 1975; 1: 657-659.
- Poole PM, Wilson G. Streptococcus milleri in the appendix. J Clin Pathol. 1977; 30: 937-942.
- Poole PM, Wilson G. Occurrence and cultural features of Streptococcus milleri in various body sites. J Clin Pathol. 1979; 32: 764-748.
- Stelzmueller I, Biebl M, Berger N, Eller M, Mendez J, Fille M, et al. Relevance of group Milleri streptococci in thoracic surgery: a clinical update. Am Surg. 2007; 73: 492-497.
- Salavert M, Gomez L, Rodriguez-Carballeira M, Xercavins M, Freixas N, Garau J. Seven-year review of bacteremia caused by Streptococcus milleri and other viridans streptococci. Eur J Clin Microbiol Infect Dis. 1996; 15: 365- 371.
- Siegman-Igra Y, Azmon Y, Schwartz D. Milleri group streptococcus--a stepchild in the viridans family. Eur J Clin Microbiol Infect Dis. 2012; 31: 2453-2459.
- Duquenne C, Dernis E, Zehrouni A, Bizon A, Duquenne M. [Streptococcus milleri: An unusual cause of skull extensive osteomyelitis in an immunocompetent patient]. Rev Med Interne. 2017; 38: 628-632.
- Akuzawa N, Hatori T, Kitahara Y, Kurabayashi M. Multiple liver abscesses and bacteremia caused by Streptococcus constellatus infection: a case report. Clin Case Rep. 2017; 5: 69-74.
- Reyes Valdivia A, Duque Santos A, Gallo Gonzalez P, Peromingo Fresneda R, Ocana Guaita J, Gandarias Zuniga C. Late aortic silver graft re-infection due to Streptococcus milleri group (Streptococcus anginosus). Case report and literature review. Rev Esp Quimioter. 2017; 30: 52-54.
- Che Rahim MJ, Mohammad N, Wan Ghazali WS. Pyopneumothorax secondary to Streptococcus milleri infection. BMJ Case Rep. 2016.
- Hindi Z. Rare Purulent Cardiac Tamponade Caused by Streptococcus Constellatus in a Young Immunocompetent Patient: Case Report and Review of the Literature. Am J Case Rep. 2016; 17: 855-859.
- Senol O, Suslu HT, Tatarli N, Tiryaki M, Guclu B. Thalamic abscess caused by a rare pathogen: streptococcus constellatus. Pan Afr Med J. 2016; 24: 256.
- Kaye ID, Protopsaltis TS. Cervical Facet Joint Infection and Associated Epidural Abscess with Streptococcus intermedius from a Dental Infection Origin A Case Report and Review. Bull Hosp Jt Dis (2013). 2016; 74: 237- 243.
- Gana TM, Awolaran O, Akhtar S. Streptococcus milleri and Recurrent Intra- Abdominal Abscesses: A Case Report and Literature Review. Case Rep Surg. 2016; 2016: 6297953.
- Navratilova L, Bardon J, Novotny R, Zatloukal J, Jakubec P, Kolek V, et al. The Streptococcus milleri group in chronic obstructive pulmonary disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016; 160: 378- 384.
- Stelzmueller I, Berger N, Wiesmayr S, Eller M, Tabarelli W, Fille M, et al. Group milleri streptococci: significant pathogens in solid organ recipients. Transpl Int. 2007; 20: 51-56.
- Bonnet EP, Arista S, Archambaud M, Boot B, Clave D, Massip P, et al. Streptococcus milleri group infection associated with digestive fistula in patients with vascular graft: report of seven cases and review. Infection. 2007; 35:182-185.
- Corredoira J, Casariego E, Moreno C, Villanueva L, Lopez, Varela J, et al. Prospective study of Streptococcus milleri hepatic abscess. Eur J Clin Microbiol Infect Dis. 1998; 17: 556-560.
- Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988; 10: 257-285.
- Arala-Chaves MP, Higerd TB, Porto MT, Munoz J, Goust JM, Fudenberg HH, et al. Evidence for the synthesis and release of strongly immunosuppressive, noncytotoxic substances by Streptococcus intermedius. J Clin Invest. 1979; 64: 871-883.
- Ruoff KL, Ferraro MJ. Hydrolytic enzymes of “Streptococcus milleri”. J Clin Microbiol. 1987; 25: 1645-1647.
- Unsworth PF. Hyaluronidase production in Streptococcus milleri in relation to infection. J Clin Pathol. 1989; 42: 506-510.
- Kanamori S, Kusano N, Shinzato T, Saito A. The role of the capsule of the Streptococcus milleri group in its pathogenicity. J Infect Chemother. 2004; 10: 105-109.
- Wanahita A, Goldsmith EA, Musher DM, Clarridge JE 3rd, Rubio J, Krishnan B, et al. Interaction between human polymorphonuclear leukocytes and Streptococcus milleri group bacteria. J Infect Dis. 2002; 185: 85-90.
- Shinzato T, Saito A. A mechanism of pathogenicity of “Streptococcus milleri group” in pulmonary infection: synergy with an anaerobe. J Med Microbiol. 1994; 40: 118-123.
- Kuriyama T, Nakagawa K, Kawashiri S, Yamamoto E, Nakamura S, Karasawa T. The virulence of mixed infection with Streptococcus constellatus and Fusobacterium nucleatum in a murine orofacial infection model. Microbes Infect. 2000; 2: 1425-1430.
- Morita E, Narikiyo M, Yano A, Nishimura E, Igaki H, Sasaki H, et al. Different frequencies of Streptococcus anginosus infection in oral cancer and esophageal cancer. Cancer Sci. 2003; 94: 492-496.
- Sasaki H, Ishizuka T, Muto M, Nezu M, Nakanishi Y, Inagaki Y, et al. Presence of Streptococcus anginosus DNA in esophageal cancer, dysplasia of esophagus, and gastric cancer. Cancer Res. 1998; 58: 2991-2995.
- Shiga K, Tateda M, Saijo S, Hori T, Sato I, Tateno H, et al. Presence of Streptococcus infection in extra-oropharyngeal head and neck squamous cell carcinoma and its implication in carcinogenesis. Oncol Rep. 2001; 8: 245- 248.
- Tateda M, Shiga K, Saijo S, Sone M, Hori T, Yokoyama J, et al. Streptococcus anginosus in head and neck squamous cell carcinoma: implication in carcinogenesis. Int J Mol Med. 2000; 6: 699-703.
- Obszanska K, Kern-Zdanowicz I, Kozinska A, Machura K, Stefaniuk E, Hryniewicz W, et al. Streptococcus anginosus (milleri) Group Strains Isolated in Poland (1996-2012) and their Antibiotic Resistance Patterns. Pol J Microbiol. 2016; 65: 33-41.
- Tracy M, Wanahita A, Shuhatovich Y, Goldsmith EA, Clarridge JE 3rd, Musher DM. Antibiotic susceptibilities of genetically characterized Streptococcus milleri group strains. Antimicrob Agents Chemother. 2001; 45: 1511-1514.
- Yamamoto N, Kubota T, Tohyama M, Kanamori S, Shinzato T, Higa F, et al. Trends in antimicrobial susceptibility of the Streptococcus milleri group. J Infect Chemother. 2002; 8: 134-7.
- Jacobs JA, Stobberingh EE. In-vitro antimicrobial susceptibility of the “Streptococcus milleri’ group (Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius). J Antimicrob Chemother. 1996; 37: 371-375.
- Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998; 27: 385-387.
- Stelzmueller I, Pfausler B, Fille M, Dossett LA, Bonatti H. Streptococcus milleri group isolates from blood cultures: consider surgical sepsis. Surg Infect (Larchmt). 2009; 10: 259-263.