
Research Article
Austin J Surg. 2018; 5(5): 1142.
Minor Anorectal Conditions in Proctology
Weledji EP*
Department of Surgery, Faculty of Health Sciences, University of Buea, Cameroon
*Corresponding author: Elroy Patrick Weledji, Department of Surgery, Faculty of Health Sciences, University of Buea, S.W. Region, PO Box 126, Limbe, Cameroon
Received: February 16, 2018; Accepted: April 13, 2018; Published: April 20, 2018
Abstract
The article describes a guide to the clinical features, diagnosis and management of some common benign anorectal disorders. Many tests are available to investigate anorectal disorders, each only providing part of a patient’s assessment, so results should be considered together and alongside the clinical picture derived from a careful history and physical examination. Normal pelvic floor function relies on a complex interplay between various mechanisms. The indications for anorectal imaging may be divided into three broad clinical areas: sepsis and fistula disease, malignancy and faecal incontinence. A wide range of investigations are required in most anorectal disorders as there is usually more than one contributing factor. Anorectal pathology is a growing problem with a greater need for understanding sexually-transmitted diseases in the practice of proctology.
Keywords: Benign; Anorectal disease; Sexually-transmitted infections; Treatment
Introduction
Anatomy and physiology of the anal Canal
The adult anal canal is approximately 4 cm long and begins as the rectum narrows passing backwards between the levator ani muscles. It has the upper limit at the pelvic floor and the lower limit at the anus. The anorectal ring at the commencement of the anal canal can be accurately palpated on digital rectal examination because of the prominent fibres of the puborectalis sling. The muscles of the anal sphincter form a tube within a funnel. The sides of the upper part of the funnel are levator ani with the external sphincter making the stem of the funnel. The tube within the funnel includes the lower rectal muscularis propria and the internal sphincter. The circular muscle of the rectum becomes thicker (1-5mm) as it forms the internal anal sphincter. The conjoint longitudinal coat is a thin fibroelastic sheet that passes between the internal and external sphincters. It is formed by fusion of the outer longitudinal layer of rectal muscle with the fibrous components of puborectalis. It separates the two sphincters creating the intersphincteric space. The external anal sphincter, made of striated muscle is 6-10mm thick, innervated by the pudendal nerve (S2-4), and surrounded by the superficial fascia of the ischiorectal fossa and perianal subcutaneous tissue. It is attached to the coccyx posteriorly by the anococcygeal raphe and to the perineal body anteriorly [1-3]. The proximal canal is lined by simple columnar epithelium, changing to stratified squamous epithelium lower in the canal via an intermediate transition zone just above the dentate line. This zone plays a critical role in the sensory function of the anal canal and eliciting continence. Increasing rectal distension is associated with transient reflex relaxation of the internal anal sphincter and contraction of the external anal sphincter, known as the rectoanal inhibitory reflex [4]. This reflex may enable rectal contents to be sampled by the transition zone mucosa to enable the discrimination between solid, liquid and flatus [5]. Anal glands that secrete mucus empty into small pockets above the anal valves located in the dentate line called anal crypts. The glands are mostly submucosal and some penetrate into the internal sphincter. Infection within these glands may result in perianal or ischiorectal abscesses and fistulae in ano [1,2]. Beneath the mucosa is the subepithelial tissue, composed of connective tissue and smooth muscle. This layer increases in thickness throughout life and forms the basis of the vascular cushions thought to aid continence by accounting for up to 15% of resting anal pressure acting as an effective barrier against mucus and fecal material [2]. If this junction prolapses, as in patients with haemorrhoids, such that it comes to lie outside the highpressure zone, then this barrier function fails and patients experience faecal spotting [1-3]. The internal anal sphincter has an intrinsic nerve supply from my enteric plexus together with an additional supply from both the sympathetic (superior and inferior hypo gastric plexuses) and parasympathetic nervous systems (nervi erigentes- S2-4). Sympathetic activity enhances and parasympathetic reduces internal sphincter contraction. Anorectal physiological studies provide measurements of the resting and squeeze pressures along the canal and between 60% and 85% of resting anal pressure can be attributed to the action of the internal anal sphincter. The external anal sphincter and the puborectalis muscle generate maximal squeeze pressure [6]. Thus symptoms of passive anal leakage are attributed to internal sphincter dysfunction, whereas urge symptoms and frank incontinence of faeces are due to external sphincter problems [7,8]. As a result of this complex interplay between continence factors and faecal evacuation, and,as in most clinical situations of anorectal disorders there is more than one contributing factor a wide range of investigations may be needed for full assessment [9].
Clinical assessment
Anal disorders usually present with bleeding at the time of defaecation, pruritus (itching) ani, pain on defaecation, perianal swelling or discharge (faecal, mucus or pus). Clinical examination is an essential feature of assessment of any patient with symptoms attributable to the anal canal and the rectum and colon must always be examined to ensure that the underlying cause is not proximal. The causes of rectal bleeding as a symptom are shown in (Table 1). The patient is placed in the left lateral position and the examination comprises three components: inspection, palpation and endoscopy (proctoscopy, sigmoidoscopy or colonoscopy if necessary). If investigation is impossible in the outpatient department, it can be done under anaesthetic (EUA), particularly when pain and discomfort prevent digital palpation. The wide range of investigations is needed for full assessment as in most clinical situations of anorectal disease there is more than one contributing factor or there may be coexisting diseases. Sphincter function may be assessed using anal manometry and electrophysiology, whereas sphincter anatomy may be assessed using anal endosonography (AES) and MRI. The former being the standard for the diagnosis of sphincter trauma. Dynamic MRI and evacuation proctography are useful in the assessment of patients with evacuatory disorders. Pelvic MRI is the best imaging modality for anorectal sepsis and can predict recurrence of complex anal fistulas after surgery, although three-dimensional AES provides a useful alternative [10,11].
Haemorrhoids
Diverticular disease
Colorectal cancer
Colorectal polyps
Arteriovenous malformations
Ischaemia
Trauma
Colitis
Solitary rectal ulcer
Anal conditions: Fissure, Fistula, Thrombosis, Squamous carcinoma, Warts
Table 1: Causes of rectal bleeding.
Anorectal Disorders
Haemorrhoids
The anal (vascular) cushions function normally when they are fixed in their proper sites within the anal canal by sub mucosal smooth muscle and elastic fibres (Treitz’s muscle). These fibres may be fragmented by prolonged downward stress related to straining during defecation of hard stools. When the supporting sub mucosal fibres fragments, the anal cushions are no longer restrained from engorging excessively with blood and this results in bleeding and prolapse. Veins that traverse the anal sphincter are blocked whereas arterial inflow continues, leading to increasing haemorrhoidal congestion. The anal cushions may remain in their usual position in the anal canal (first degree), descend to involve the skin of the distal anal canal so that they prolapse on defaecation but reduce spontaneously (second degree) or become such a size that they are always partly outside the anal canal (third degree). Classsical positions are the left lateral, right posterior and right anterior positions, although secondary haemorrhoids can occur in between these anatomical sites [12]. Conditions related to (and which may be confused with) haemorrhoids are shown in (Table 2). Constipation and straining at the time of defaecation may be a factor in the development of haemorrhoids as more effort is required to evacuate small, hard stools [13]. Diarrhoea is also a potential risk factor and the tenesmus from diarrhoea causes straining that aggravates haemorrhoid [14]. Pregnancy is associated with a higher risk and aggravates pre-existing diseases. Other factors implicated include heredity, erect posture, and absence of valves within the haemorrhoidal plexus and draining veins, as well as impedance of venous return from raised intra-abdominal pressure. Portal hypertension may lead to engorgement of the porto-systemic venous anastomosis (varices) above the dentate line at the site of the haemorrhoidal plexus.
Condition
Position
Anal skin tags
Anal margin
Fibrous anal polyps
Line of the anal valves
Prolapse of rectum
Similar to haemorrhoids but circumferential
Thrombosis in perianal skin (perianal haematoma)
Distal to mucocutaneous junction
Fissure
Primarily at the mucocutaneous junction but may have a distal skin tag
Benign tumours of the rectum
Within the rectum at sigmoidoscopy
Varices (from portal hypertension)
Haemangioma
Rare, same site as haemorrhoids (above the dentate line) but almost impossible to distinguish
Rare congenital abnormality
Table 2: Conditions related to (and which may be confused with) haemorrhoids.
Clinical features: Bleeding -usually at defaecation- bright red in nature, dripping or spurting into the toilet pan or onto toilet paper may occur afterwards. This is painless and occurs commonly with grade1 piles. Minor faecal soiling or mucus leak is common along with pruritus, occasional discomfort and palpable swelling. External haemorrhoids comprise dilated vascular plexuses below the dentate line, covered by squamous epithelium. They may swell and cause some discomfort. Pain only occurs upon thrombosis or the development of coexistent anal fissure. Bleeding is unusual but quite severe pain can arise as a result of acute thrombosis. If left untreated, the thromboses external haemorrhoids will become external skin tags. Internal haemorrhoids are symptomatic arteriovenous channels sited above the dentate line and covered by transitional and columnar epithelium. In addition, sensory function may be impaired and this may partially account for the complaints of minor incontinence by some patients. On examination, haemorrhoids vary from a slight increase in the normal anal cushion size, visible only at proctoscopy, to large third degree internal/external haemorrhoids apparent on inspection of the anal verge.
Management: It must firstly be recognised that haemorrhoids may coexist with other conditions such as rectal cancer or inflammatory bowel disease and thus, patients with rectal bleeding; altered bowel habit, abdominal symptoms and family history of colorectal cancer should have further evaluation of the colon and rectum. Secondly, since the anal vascular cushions contribute to anal continence, treatment is reserved for symptomatic disease and the amount of haemorrhoidal tissue prolapsing beyond the anal verge.
Non-prolapsing or mildly prolapsing haemorrhoids
If the piles are not permanently prolapsed, non-operative measures should be attempted first in (Table 3). In some patients, improving bowel action with laxatives (fibre drink- Duphalac) may help to control the symptoms. Other forms of treatment that can/may give more immediate symptomatic relief include rubber band ligation [15-17] and injection sclerotherapy [18]. First-degree haemorrhoids commonly bleed and are adequately treated by injection of sclerosant agents such as phenol (5%) in almond oil or sodium tetradecyl sulphate into the submucosa around the pedicle, at the level of the ano-rectal ring. The correct plane is shown by elevation of the mucosa without blanching during injection. This technique is about 70% effective as apart from reducing blood flow into the haemorrhoid from the ensuing inflammation, causes fibrosis which draws minor prolapse back into the anal canal [18,19]. Inappropriately deep injections can cause perirectal fibrosis, infection, urethral irritation and prostatitis. Severe sepsis is not uncommon and such patients should be admitted for antibiotics and observation until completely well. Topical applications of phlebotonics such as Daflon 500 are popular with many patients who testify to relief from bleeding and pain but no clinical trials have demonstrated any benefit [20].
First-degree haemorrhoids(bleeding but no prolapse)
- Stool softeners, Sclerotherapy, Rubber band ligation
Second-degree haemorrhoids ( prolapse but spontaneously reducible)
- Rubber band ligation, Sclerotherapy, Electrocoagulation, Haemorrhoidectomy ( recurrent haemorrhoids after rubber band ligation)
Third-degree haemorrhoids (prolapse requiring manual reduction)
- Rubber band ligation, Sclerotherapy, Electrocoagulation, Haemorrhoidectomy
Fourth-degree haemorrhoids (irreducible prolapse)
- Haemorrhoidectomy
Table 3: Management of internal haemorrhoids.
Irreducible prolapsed haemorrhoids
When anal cushions have prolapsed or thrombosed, they no longer function effectively to maintain continence and surgery may then be needed (Table 3). Third- and fourth-degree haemorrhoids are appropriately treated by surgery, which is conventionally performed by excision of the three primary piles (Right anterior, posterior, left lateral) [12] or Park’s submucosal haemorrhoidectomy [21]. Minor variations in conventional excisional haemorrhoidectomy include leaving the wounds to granulate (Milligan-Morgan open haemorrhoidectomy) [22] or closed with sutures (Ferguson’s closed haemorrhoidectomy) [23]. Whether the pedicle is ligated, or whether the piles are excised with scissors or diathermy or ligasure devises for less bleeding, several randomized studies have shown that closure of the wounds provides none or little gain because of frequent breakdown of the suture line [24]. Stapled haemorrhoidectomy adequately addresses most circumferential prolapses with massive engorgement [25,26]. Conventional haemorrhoidectomy, however, deals with the symptoms (bleeding, pain, prolapse,) alone without correction of pathophysiology by fixation of the congested anal cushions [25]. After reduction of the prolapsed haemorrhoidal tissue stapled anopexy (haemorrhoidopexy) excises redundant lower rectal mucosa, fixing the prolapsed back into its proper place on the wall of the anal canal. This helps prevent subsequent re-dislodgement and recurrence. This concept of symptomatic haemorrhoids being a disease of mucoanal prolapsed was devised by Longo in 1998 in which the haemorrhoids are not excised but fixed to the staple line and there are no wounds of the anal canal [26]. Infact, failed conventional haemorrhoidectomy may be due to an unforeseen associated rectal mucosal prolapse [27]. Transanal Haemorrhoidal Dearterialisation (THD) was introduced by Sohn et al. in 2001 [28]. This technique consists of ligating the terminal haemorrhoidal branches of the superior rectal artery using specially designed proctoscope with a Doppler probe to locate the vessels. This results in reduction of blood flow and decongestion of the haemorrhoidal plexus. Dal Monte et al reported more than 90% resolution of symptoms with minimal complications [29]. Although there are variations to the haemorrhoidectomy technique (Milligan-Morgan, Park’s, Ferguson, Whitehead radical, stapled haemorrhoidectomy etc), the main postoperative problems encountered are similar although to different degrees. These are mainly postoperative pain, anal incontinence and haemorrhage. Post-operative pain is worse with the Milligan-Morgan open haemorrhoidectomy from the perineal wounds and least with the stapled procedure which avoids the perineal skin. However, the amount of pain after the latter is said to depend on the height of mucosectomy above the anal verge although haemorrhoidal thrombosis or developing sepsis below the staple line may also cause considerable pain. The use of botulinum toxin reduce pain towards the end of the first postoperative week, and 0.2% glyceryl trinitrate (GTN) ointment is also associated with decreased post- operative pain. Others have tried lateral sphincterotomy with some success [30] but not advocated because of potential permanent adverse effects. Metronidazole was shown to reduce pain on days 5-7 after open, largely day-case haemorrhoidectomy in a randomized trial by Carapeti et al. [31]. It was proposed that a reduction in bacterial colonisation was an important factor. Intravenous fluid administration during surgery is kept to a minimum to reduce the incidence of acute urinary retention of urine following perineal surgery [32] Gentle oozing from reactionary haemorrhage is usually due to a slipped ligature or small bleeding point in one of the anal wounds can be stopped by external pressure or submucosal adrenaline injection. Post-operative haemorrhage may also be secondary as a result of postoperative infection. Post-haemorrhoidectomy anal stricture is uncommon if adequate skin and mucosal bridges were maintained intra-operatively and close follow-up to detect stricture formation early. The stricture usually presents at 6 weeks postoperatively and up to two-thirds may be managed conservatively with stool bulking agents and local anaesthetic gels. The remaining one-third may require an anoplasty. Sepsis after either conservative or operative treatment is uncommon, but when it occurs delay in treatment can be catastrophic.
Thrombosed haemorrhoid
Acute thrombosis may develop within haemorrhoidal veins and commonly presents as a surgical emergency. The episode is best managed conservatively with the application of ice, non-constipating analgesics and lubricant laxatives. Resolution takes place over the following 7- 10 days. There is usually underlying sphincter tightness with superimposed spasm, so in the first 48 hours after thrombosis anal dilatation will give great relief of pain. If this is the first attack then lasting cure with dilatation can be expected, but if there is a long history of prolapse there is much to be said for an immediate haemorrhoidectomy. Urgent haemorrhoidectomy is occasionally used but runs the risk of a postoperative anal stenosis as a result of excessive perianal excision and not leaving sufficient mucosal skin bridges.
Acute perianal haematoma
Acute localised thrombosis may affect the external plexus, causing a perianal haematoma. This is often caused by straining but is not associated with any internal haemorrhoids. The lesion appears as a tense blue swelling on the anal margin. Evacuation under local anaesthetic will give immediate relief, or the haematoma can be left until it discharges spontaneously.
Fissure in ano
Anal fissures are common and most commonly affect patients aged between 30 and 50. They are equally common in men and women and represent up to 10% of referrals to colorectal clinics [32]. A fissure is a split in the lower half of the anal canal extending from the anal verge towards the dentate line. Most fissures are usually single and posterior in the 6 o’clock position, but anterior ones (2.5-10%) may be sited in the 12 o’clock position [33]. If fissures are multiple or eccentrically located, inflammatory bowel disease, tuberculosis, syphilis or Human Immunodeficiency Virus (HIV) infection must be considered. The cause is not clear (often idiopathic), but a fissure often starts after an attack of either diarrhoea or constipation (adults and children). Other risk factors include traumatic childbirth and anal intercourse. The constipation may also be a consequence of the patient’s unwillingness to defecate because of pain. Thus, a vicious cycle. The base of the fissure has somatic innervations, so pain can become intense after a bowel action as last as a dull pain for many hours. There may be associated fresh rectal bleeding noticed as blood-staining of toilet paper and patients may have a history of an abnormal bowel habit. Pruritus ani and a discharge of small quantities of pus may occur if associated abscess. Some anal fissures heal spontaneously but many become chronic (persisting for more than 6 weeks despite adequate medical therapy), causing months of misery. A chronic intersphincteric abscess can cause a similar sort of pain, but pain severe enough to warrant emergency admission to hospital must be assumed to be due to a high anorectal abscess. Reflex spasm of the external sphincter occurs whenever the background fissure is disturbed. There is also a background tightness of the internal sphincter, which may well be the primary fault because correction of this abnormality almost always cures the fissure.
Clinical assessment
If the history of an associated altered bowel habit is suspicious a proximal colonic lesion must first be excluded. Unless there is intense spasm, the lower end of a fissure will come into view with the patient in the left lateral position as the perianal skin gently retracted. When the pain is minimal, a gentle digital examination and proctoscopy may be done but, it is cruel to perform more than a visual examination in patients with severe pain due to an anal fissure. This will show a fibrotic ulcer with white transverse internal sphincter fibres exposed. There may be a hypertrophic papilla (sentinel pile) at the internal edge and fibrous polyps that often accompany chronic fissures. Indeed, the fissure itself may be missed if the sentinel pile is not retracted to reveal the fissure. The presence of rectal mucosal prolapse or haemorrhoids is not unusual.
Management
Medical treatment: For acute fissure, local anaesthetic gel and bulk laxatives may help. More recently, the recognition of nitric oxide as a neurotransmitter mediating the relaxation of the internal sphincter has led to the use of topical glyceryl trintrate 0.2% and the calcium channel blocker, diltiazem 2% ointment to treat acute and chronic fissures. 0.2% GTN achieves optimal healing in up to 70% of cases with minimal adverse effects (predominantly headaches) [34,35]. The mainstay of treatment for chronic anal fissure used to be surgery until recently when there was a shift towards medical therapy. It creates an effect of temporary or reversible sphincterotomy, decreasing the anal sphincter pressure thereby allowing healing of the fissure. Despite more favourable results from surgery (lateral sphincterotomy) in recent trials, the enthusiasm for local creams or botulinum A toxin (Botox) temporary chemical sphincterotomy [36] has not diminished mainly because of concerns of faecal incontinence, albeit usually mild after sphincterotomy. As medical therapy is safe and side-effects are not serious and are reversible with cessation of therapy surgery may be reserved for treatment failures. However, medical therapies are not as effective as surgery and late recurrence is also higher with medical therapy [37].
Surgical treatment: Patients who have failed medical treatment or who have features of chronicity such as sentinel piles or the fibrous polyp should be offered a tailored lateral sphincterotomy. In a tailored sphincterotomy, the internal sphincter is divided up to the highest point of the fissure only by either an open or closed method, each showing similar results [38,39]. Posterior midline sphincterotomy is not favoured as its results are not superior to lateral sphincterotomy and a gutter (keyhole) defect may lead to soiling. Nevertheless, it may lay open if it presents as a posterior intersphincteric fistula which is usually associated with an underlying intersphincteric abscess. Surgical excision of a sentinel pile with its associated pain and bleeding from trauma and the fibrous polyp with its associated tenesmus or soilage at the time of lateral sphincterotomy is an advantage of surgical treatment over medical treatment. Uncontrolled anal stretch or dilatation should no longer be performed [38]. An uncontrolled fracturing of the internal sphincter by this method although shown to have good rates of healing, results in an unacceptable high incidence of incontinence. Sphincterotomy by virtue of its division of the internal sphincter musculature, predisposes to incontinence to flatus and faecal soilage in up to 35% of patients (although the risk is usually far less than this) [40]. Surgical removal of the fissure (fissurectomy) with subsequent use of isosorbide dinitrate cream or Botox injection has been shown to be effective, with no recurrence and no internal sphincter defects on postoperative endosonography [41,42]. This relatively new technique is as yet unverified by a randomised controlled trial, although it presents a novel approach to an age-old problem.
Anorectal Sepsis
Anorectal sepsis is common, presenting as either an acute abscess or a chronic anal fistula (Table 4). An abscess and a fistula are respectively the acute and chronic sequelae of an infected anal gland. About 10 glands drain into the anal canal at the level of the dentate line. Some penetrate the internal sphincter to reach the intersphincteric space. The majority of chronic anal fistulas are preceded by an episode of acute anorectal sepsis, although acute sepsis does not inevitably lead to fistula formation. The majority of cases can be dealt with avoiding complication, but a small minority can present a major challenge to both patient and surgeon.
Condition
Usual finding
Non-specific infection
Acute abscess
Fistula in ano
Tuberculosis
Chronic infection, Occasionally fistula
Crohn’s disease
Chronic intractable infection, Complex fistula
Hidradenitis suppurativa
Skin abscesses. Anal canal rarely if ever involved.
Skin sepsis
Usually Staphylococcus aureus; Abscesses are often multiple
Trauma
Sepsis in developmental cysts
External: sexual intercourse, accidental injury.
Internal: foreign body (ingested bone)
Usually dermoid cysts
Intrapelvic sepsis
Malignant disease
Diverticular disease, Crohn’s disease
Sepsis is an uncommon complication
Table 4: Conditions associated with perianal sepsis.
Anorectal abscesses
Eisenhammer [43] considered all non-specific abscesses and fistulas to be the result of extension of sepsis from an intramuscular anal gland, the sepsis being unable to drain spontaneously into the anal lumen because of infective obstruction of its connecting duct across the internal sphincter. Spread of sepsis from an acutely infected anal gland may thus occur in any of three planes, vertical, horizontal or circumferential. Caudal spread is the simplest and most usual way to present acutely as a perianal abscess. Cephalad extension in the same space will result in a high intermuscular abscess or a supralevator pararectal abcess, depending on the relation to the longitudinal muscle layer. Lateral spread across the external sphincter will reach the ischiorectal fossa, where further caudal spread may point at the skin or upward spread may penetrate the levators to reach the supralevator pararectal space (Figure 1). Circumferential spread may occur in any of the three planes. Abscesses not considered to be of cryptoglandular origin include the submucous abscess (arising from an infected haemorrhoid, sclerotherapy or trauma), the mucocutaneous or marginal abscess (infected haematoma), the perianal abscess (follicular skin infection), some ischiorectal abscesses (primary infection or foreign body) and the pelvirectal supralevator abscess originating in pelvic disease [44]. Pilonidal infection, hidradenitis and perianal Crohn’s disease are usually fairly easy to recognise by history and examination. Patients with acute anorectal sepsis usually presents as an emergency. Patients with perianal sepsis tend to present early, 2 or 3 days after onset of symptoms, with pain and a palpable exquisitely w tender, well-defined lump close to the anal margin, and usually with no constitutional upset. Patients with ischiorectal abscesses tend to present later with more vague discomfort, and, because much more pus may accumulate in the large relatively a vascular loose areolar tissue of the ischiorectal fossa, they often have fever and constitutional upset. Examination may reveal tender in duration over the abscess. Sepsis higher up in the sphincter complex may present with rectal pain, and possible disturbance of micturition, and there may be no external signs of pathology. The rare submucosal abscess is revealed on digital examination of the anal canal as a distinct tender bulge, and the patient may have reported the passage of pus from the anal canal with relief of symptoms.
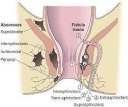
Figure 1: Anorectal sepsis and fistulae (with permission) [52].
Management of acute sepsis: The optimal management of acute sepsis should reside in an understanding of aetiology. Clues as to the aetiology of perineal sepsis may also be gleaned from the microbiology of the drained pus [45,46]. If skin organisms alone are cultured and the acute abscess and the acute adequately drained, recurrence should not occur and a fistula will not result. If gut organisms are cultured, however, it is probable but not inevitable that there is an underlying fistula. Fistulography is rarely used in general practice. A policy (in experienced hands) of simple incision when a fistula is not evident, and primary fistulotomy (laying open and allowing to heal by secondary intention) when a fistula is evident and low (or a draining loose seton placed if there is any doubt about the level or concern about incontinence) is sensible [47,48].
Anal fistulas
Patients with an established fistula usually give a history of intermittent pain and purulent discharge from an opening on the perineum, the pain building up until relief is felt when the pus escapes. Patients in whom the internal opening is rectal, and those with large internal openings irrespective of site, may pass flatus and stool through the opening(s).
Clinical assessment: A full history and examination including proctosigmoidoscopy are essential in all cases to exclude any associated conditions. Clinical assessment involves five essential points, enumerated by Goods all and Miles at the end of the 19th century [49]: 1) location of the internal opening; 2) location of the external opening; 3) course of the primary track; 4) presence of secondary extensions; 5) presence of other diseases complicating the fistula. The relative positions of the external and internal openings will indicate the likely course of the primary track, and the presence of any palpable in duration would indicate either a high primary tract or secondary extension in the roof of the ischiorectal fossa or supralevator space. If the tract is not palpable, it is probable that the fistula is not intersphincteric or low trans-sphincteric (Figure 1) [50- 52]. The distance of the external opening from the anal verge may assist in differentiating an intersphincteric from a trans-sphincteric fistula; the greater the distance, the greater the likelihood of a complex cephalad extension [47,50,51]. Goods all’s rule generally applies in that the likely site of the internal opening can be predicted by the position around the anal circumference of the external opening. It states that if the perianal skin opening is posterior to the transverse anal line, the fistulous tract will open into the anal canal in the midline posteriorly, sometimes taking a curvilinear (horseshoeshaped) course. A perianal skin opening anterior to the transverse anal line is usually associated with a radial fistulous tract following a simple, direct course (Figure 2) [49,51]. Exceptions to this rule include anteriorly located openings more than 3cm from the anal verge (which may be anterior extensions of posterior horseshoe fistulas) and fistulas associated with other diseases, especially Crohn’s and malignancy [44,51].
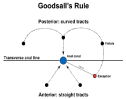
Figure 2: Schematic diagram of Goods all’s Rule.
Management: Successful surgical management of anal fistula depends upon accurate knowledge of anal sphincter anatomy and the fistula’s course through it. Failure to understand may result in fistula recurrence or incontinence. The crypto glandular hypothesis central to the classification of anal fistula, holds firstly that the majority of fistulas arise from an abscess in the intersphincteric plane and secondly that the relation of the primary track to the external sphincter is paramount in surgical management [50,51]. Four main groups exist (Figure 1): intersphincteric (45%); transsphincteric (29%); suprasphincteric fistulas (20%) run up to a level above puborectalis and then curl down through the levators and ischiorectal fossa to reach the skin; extrasphincteric fistulas (5%) run without relation to the sphincters and are classified according to their pathogenesis [50]. In addition to horizontal and vertical spread sepsis can spread circumferentially in any of the three spaces: intersphincteric, ischiorectal or pararectal. About 5% of fistulas have complex branching tracts (secondary extensions) passing high up through the sphincter complex, and initial attempts at surgery often fail because of inadequate exploration of tracts for fear of producing incontinence [44,47,50,51]. A Short Tau Inversion Recovery (STIR) sequence (a fat suppression technique) MRI, to highlight the presence of pus and granulation tissue without the need for any contrast media is the gold standard for imaging anal fistulas [53]. The potential advantages include the lack of ionising radiation, the ability to image in any plane and the high soft-tissue resolution. The multiplicity of techniques designed to preserve sphincter function and at the same time eradicate fistula pathology reflects their relative lack of success. The risk of incontinence associated with treatment ranges from 10% to 57% [48,51]. Because of individual fistula (and sphincter) variability and individual surgeon preference and skill, the use of adequately powered prospective randomised trials is perhaps unachievable. In addition to inadequate follow-up, a degree of caution and scepticism may be apportioned when assessing reported results of the various approaches towards complex fistula. Some authors advise bowel preparation before fistula surgery, although laid-open wounds in the perineum heal remarkably well despite the continual bacterial load. Most authors recommend parenteral antibiotics preoperatively and postoperatively for any of the more complex procedures [44,51].
Surgical treatment: A fistula has a primary track and may have secondary extensions. Complete eradication of both will lead to cure. Lay-open (fistulotomy) remains the surest way of eliminating an anal fistula. Lateral traction of an opened Eisenhammer retractor may reveal dimpling at the internal opening through its underlying fibrous inelasticity. Digital massage of the track may reveal the site of the internal opening as a bead of pus. The instillation of dilute hydrogen peroxide as opposed to methylene blue dye along the track via the external opening is the easiest way of locating the internal opening, as staining is avoided. If the internal and external openings are easily detected but a probe cannot easily traverse the path of the track, it is possible that there is a high extension and it would be difficult to negotiate probes around a horse-shoe posterior trans-sphincteric fistula. Persistence of granulation tissue after curettage during the operation is an indication of a secondary extension [44,51].
Fistulotomy: All lay-open procedures divide some of the internal sphincter, so patients should be warned of a 1 in 4 chance of flatus incontinence and mild mucus leakage. Fistulotomy is restricted to situations where a significant degree of incontinence would not result. High trans-sphincteric (especially anterior tracks in women-short sphincters) and suprasphincteric tracks should not be treated by onestage fistulotomy. Intersphincteric and low trans-sphincteric tracts are probably best treated by this method. Marsupialisation, (suturing the divided wound edge to the edges of the curetted fibrous track), results in smaller wound and faster healing [54]. Anterior fistulas in women are dangerous and should only rarely be laid open. With nonrecurrent complex fistulas, fistulotomy combined with immediate sphincter reconstitution and advancement flap repair yielded equivalent results in respect to healing and functional outcomes [55].
Fistulectomy: The technique of fistulectomy which excises the fistula track has been criticised on the basis that the greater tissue loss leads to delayed healing [56]. However, a core-out technique [57] may have the advantages of a) more accurately determining the precise course of the tract and avoiding the creation of false tracks with a probe, b) reduces the risk of missing secondary tracks, which are seen as transacted granulation tissue and which may be followed by same technique, c) ascertaining the relation of the primary track to the external anal sphincter before any sphincter muscle is divided and d) a complete specimen is available for histology.
LIFT: In 2007 Arun Rojanasakul et al. of Thailand [58], developed the technique of Ligation of the Intersphincteric Fistula Tract (LIFT) with impressive early outcome. The central idea of this procedure is that the excision and ligation of intersphincteric tract can occlude the entry of faecal particles in the fistula and, at the same time, eliminate the septic focus in the intersphincteric space. The external orifice was left open to heal by secondary intention whilst promoting good drainage. This could result in the cure of anal fistula. This procedure aim to maintain an intact anal sphincter and preserve continence postoperatively [59]. The initial study described the technique on 18 patients and provided a primary cure rate of 94.4%, with one patient undergoing a reoperation with the same technique. There was no report of incontinence but long-term follow-up and a larger study was required [60]. The main indication for LIFT is for trans-sphincteric fistulas in patients without previous surgery and with short fistula tracts. Patients with more complex fistulas, especially with multiple previous surgeries, should be considered for the Endorectal Advancement Flap (ERAF) or Video-Assisted Anal Fistula Treatment (VAAFT) procedures [61].
VAAFT: The main feature of the Video-Assisted Anal Fistula Treatment (VAAFT) technique is that the procedure is performed entirely under direct endoluminal vision. With this approach, the internal opening can be found in 80% of cases [62]. Diagnostic fistuloscopy under irrigation is followed by an operative phase of fulguration of the fistula tract, closure of the internal opening using a stapler or cutaneous-mucosal flap and suture reinforcement with cyanoacrylate. Moreover, fistuloscopy helps to identify any possible secondary tracts or chronic abscesses. The VAAFT technique is sphincter-saving, and the surgical wounds are extremely small. The preliminary results are very promising.
Setons: The loose set on (thread, loosely tied) is often used as a marker of a fistula track when its exact position and level in relation to the external sphincter is unclear at surgery, perhaps because of scarring from, previous surgery or because of the depth of sphincter muscle relaxation under anaesthesia. It can be used as a drain of acute sepsis, to allow subsidence of acute inflammatory changes and safer definitive fistula surgery. More specifically in the field of sphincter and continence preservation, the loose set on can be used in three ways: to preserve the entire external sphincter; to preserve part of the voluntary muscle; or as part of a staged fistulotomy in order to reduce the consequences of division of large amounts of muscle in one procedure. The key points of a staged fistulotomy are the amount of muscle divided at each stage and the time allowed for fibrosis to develop between the divided muscle edges before a further length of sphincter is divided [42]. The tight or cutting seton is similar to that of the staged fistulotomy technique, in that the divided muscle is not allowed to spring apart but there is supposed to be a gradual severance through the sphincter followed by fibrosis. It is recommended whenever the fistula encircles more than 30% of the sphincter complex and when local sepsis or fibrosis precludes the raising of an advancement flap [49]. The portion of the track outside the sphincters is laid open, although others in the USA have recommended Penrose drainage of horseshoe limbs. The elimination of acute sepsis and secondary extensions before sphincter division, and secondly the speed with which the seton cuts through the sphincter are the critical aspects of management by the cutting set on. projecting through the skin opening as a primary track. Many patients have secondary lateral openings 2-5cm from the midline pit. The skin opening and superficial portion of the track are lined with squamous epithelium, but the deep cavity and secondary extensions are lined with granulation tissue. Hairs are drilled or sucked into the cavity owing to friction with movement of the buttocks. Barbs on the hairs prevent their expulsion, so they become trapped, provoking a foreign body type reaction and infection [65]. Half of the affected patients present as emergencies with an acute pilonidal abscess; the remainder have fluctuating discomfort and chronic infection with a foul smelling discharge. Examination reveals the characteristic opening in the natal cleft, through which a turf of hair may be seen emerging.
Treatment
Many different treatments have been advocated with conflicting enthusiasm, suggesting that none are perfect. Some patients can be treated as an outpatient or day case basis e.g. simple curettage of small sinuses and phenol injection down the opening of the main tract but require meticulous and prolonged attention to the sinus if recurrence is to be avoided, while others require periods in hospital. A balance has to be struck between minimising inpatient treatment without compromising lasting healing. The principal surgical options include the excision and primary closure [66] or excision and lying open of a non-infected sinus, and the drainage and excision of an infected sinus or abscess allows healing by secondary intention [67]. Frequent changes of dressing and close supervision to prevent pocketing and premature closure or bridging of the skin edges over an incompletely healed cavity are necessary. Excision with primary suture and flattening of the natal cleft help to diminish the accumulation of hair and reduce friction with movement. The sinus is excised asymmetrically, with one flap being undermined and overlapped over the opposite flap [65,68,69] or alternatively a myocutaneous flap [70] or Z-plasty flap [71,72] can be used to close the wound. Recurrence is usually due to neglect of wound care, persisting poorly drained tracks, recurrent infection of hair follicles and midline scars. Sinus pits may be difficult to recognise in the presence of infection and oedema, but easily distinguished a week after primary drainage of abscess, when the oedema has resolved. Post-treatment shaving of the skin is necessary for 6 months as failure to do so is the commonest cause of recurrence.
Hidradenitis Suppurativa
The apocrine sweat glands, usually in the perineum, inguinal regions and axilla, become the site of mixed bacterial infection. Obesity, poor hygiene and hormonal imbalance are contributory factors. Various degrees of sepsis occur with tracks running widely in the subcutaneous tissue in the perianal region; these may be confused with anal fistula [73]. The treatment is to control the contributory factors and to lay open the septic areas. Long-term prophylactic antibiotics and occasionally plastic surgery procedures are necessary [74].
Pruritus ani
Pruritus ani is a common symptom which may be caused by inadequate hygiene, anal disorders, as part of a skin infection, local infection, infestation by parasites, or an allergic reaction to pharmaceuticals or as part of a generalised pruritus in obstructive jaundice or in faecal incontinence due to a sphincter defect. In many instances a primary cause cannot be found. Indeed, idiopathic pruritus ani is usually associated with a minor degree of faecal incontinence [8]. This may be due to a local pathology permitting stool to leak to the outside, such as a fissure, fistula or prolapsing haemorrhoid, or to a high fibre diet, leading to difficulty with anal cleaning and fragments of stool becoming trapped in the anal canal, only to seep out later and set up irritation. There may be internal sphincter dysfunction, or other contributory causes such as irritative foods (spices, alcohol, caffeine) [75]. The secondary causes of pruritus ani are listed in (Table 5). Itching in the perianal skin is the initial symptom and scratching with the applications of inappropriate topical creams (local anaesthetics as they are sensitive to skin; strong steroids as they lead to dependence of the skin) and excessive cleansing of the perianal skin exacerbate this condition exacerbate the condition. If the causative factor persists, soreness and pain even on walking occur [76]. The key to successful management is the accurate determination and treatment of the cause. The diagnosis is most often revealed by good history taking and physical examination alone, for causes of minor anal leakage. Important facts such as duration of symptoms, dietary habits, recent travel history and change in bowel habit should be elucidated. Physical examination should start with a general inspection of the patient for dermatological disease elsewhere on the body. Then the perineum and underclothes inspected for soil age. Perianal skin changes, particularly excoriation and ichthyosis indicate long- standing pruritus. Specific examination of the perineum includes a digital rectal examination for anal tone and squeeze. Palpation for polyps, malignancies and fistula tracts is required and patients examined while straining to exclude any prolapse. Proctoscopy should be performed and further examination using endoscopy, radiology or laboratory tests may be required in certain cases. Skin lesions should be biopsied and examined for fungal elements. Enterobius or threadworm may be seen on sigmoidoscopy, or placement of ‘Sellotape’ to the anus may reveal the diagnosis. The presence of ova on a glass microscopy slide is indicative of infection and treated with mebendazole or piperazine [76,77]. Treatment is based on the primary pathology. In primary pruritus ani, the aims of treatment are the reduction of leakage (antimotility agent or dietary modification), maintenance of good personal hygiene and the prevention of further injury to the perianal skin.
Neoplasia
- Rectal adenoma, Rectal adenocarcinoma, Anal squamous cell carcinoma, Malignant melanoma, Bowen’s disease, Extramammary Paget’s disease
Benign anorectal conditions
- Haemorrhoids, Fistula in ano, Anal fissure, Rectal prolapse, anal sphincter injury or dysfunction, faecal incontinence, Radiation proctitis, Ulcerative colitis
Infections
- Condyloma acuminatum, Herpes simplex virus, Candida albicans, syphilis, lymphogranuloma venereum
Dermatological
- Neurogenic dermatitis, Contact dermatitis, Lichen simplex, Lichen planus, Lichen atrophicus
Table 5: Secondary causes of pruritus ani.
Sexually Transmitted Diseases (STDs) and the Anorectum
A variety of infections may be transmitted during anal intercourse, which is predominantly a practice of homosexual men, but also may affect some heterosexuals. It is important to consider the possibility of sexually transmitted disease in patients presenting with anorectal disorders and symptoms of acute proctitis (pruritis ani, anal discharge, anal pain, rectal bleeding and diarrhoea). The common anorectal sexually transmitted infections are, gonococcus infection, chlamydia infection, herpes and primary syphilis.
Gonorrhoea
Gonorrhoea is caused by Neisseria gonorrhoea, a gram negative diplococcus. The organism is spread during anal intercourse and by auto contamination by the vagina in women. An incubation period of five to seven days is followed by proctitis and infection of the anal crypts. Patients with symptomatic disease have pruritus ani, mucopurulent discharge, tenesmus and bleeding. Most people with rectal gonorrhoea are asymptomatic but a quarter of homosexual men with gonorrhoea have anorectal involvement. Disseminated infections may be associated with systemic manifestations including joint pains. Proctoscopy shows proctitis, and mucopus can often be expressed from the anal crypts. The anal canal is usually spared. Culture of the mucopus is diagnostic. Penicillin G was the treatment of choice until the 1970s, when penicillinase producing N. gonorrhoea emerged. More recently, quinolone resistant N. gonorrhoea has been reported and increasing worldwide especially for homosexual males [78].
Anal syphilis
An anal chancre is a common manifestation of primary syphilis and three quarters of those infected are homosexual men. The chancre appears two to six weeks after exposure during anal intercourse. It may be confused with an anal fissure and when secondary bacterial infection supervenes can cause considerable pain at the anus [79]. Most patients with primary anal syphilis have painless inguinal lymphadenopathy, which is rare in patients with anal fissures. Early lesions are teeming with spirochaetes, which are readily shown by dark field microscopy. The fluorescent treponemal antibody test (FTA) is the first serological test to become positive, 4-6 weeks after infection [80]. The Venereal Disease Research Laboratory (VDRL) assay gives positive results in three quarters of patients with primary syphilis. The Treponema Pallidum Haematological Assay (TPHA) is also useful as a specific confirmatory test. Secondary syphilis appears usually 6-8 weeks after the primary lesion. Patients develop moist, smooth, warty masses around the anus (condylomata lata) and have a discharge and pruritus. The warts are less keratinised, smoother, flatter, and moister than anal papillomas. These lesions are highly infectious as spiorochaetes are abundant in the discharge and all serological tests for syphilis usually give positive results. Tertiary syphilis is now rare. Rectal gumma may be confused with malignancy and patients with tabes dorsalis may have severe perianal pain and functional problems due to paralysis of sphincters [81]. Patients with syphilis are treated with intramuscular penicillin and those allergic to penicillin are treated with tetracycline or erythromycin. Followup serological tests are repeated periodically for at least a year after treatment to confirm eradication of the infection [80].
Chlamydia infection
Chlamydia trachomatis infection is a cause of proctitis among those who practise anoreceptive intercourse and may be subclinical. There are two strains or biovars, chlamydia trachomatis and chlamydia lymphogranuloma venereum. Symptoms include a mucoid or blood stained discharge, pain, tenesmus and fever [82,83]. Those with lymphogranuloma venereum may have inguinal lymphadenopathy. The pathogen is intracellular and cell culture and direct immunofluorescence assay are the most sensitive tests for chlamydial infections. Treatment is with tetracycline or erythromycin or a prolonged course of vibramycin. Rectal stricture is a rare complication of lymphogranuloma venereum due to L1-3 serovars but, rarely requires surgical treatment [83,84].
Herpes simplex virus
Herpes simplex virus infection is common among homosexual men and is an important complication of HIV infection [85]. Chronic mucocutaneous herpes simplex virus infection in a patient infected with HIV is considered diagnostic of AIDS, particularly if it is of the ulcerative and persistent variety (more than a month’s duration). 90% of anal infections are due to herpes simplex virus type 2 and 10% to type 1 [86]. Symptoms develop one - three weeks after anal intercourse and include burning, mucoid or bloody discharge, and constitutional symptoms such as malaise and fever. Examination reveals vesicles, pustules, and shallow ulcers around the anus. Sigmoidoscopy shows proctitis and pronounced erythema. The lesions are usually extremely sore, precluding examination without an anaesthetic. Infection can be confirmed by viral culture of vesicular fluid. Patients are treated with oral or intravenous acyclovir depending on the severity of the illness. Treatment is continued until all the mucocutaneous surfaces have healed [87].
HIV
HIV/AIDS patients frequently present with proctological diseases and operations for anorectal pathology represent one of the most common indications for surgery in HIV positive patients [14,88]. These diseases can be divided into three categories: (1) proctological complaints common to the population at large (e.g; haemorrhoids, fissures, pruritus) are frequently seen in HIV/AIDS patients and may the primary reason for seeking medical help; (2) diseases associated with high risk behaviours such as anoreceptive intercourse. Included in this group are the STD’s which cause proctitis and anogenital ulcerations discussed above; (3) those illnesses associated with HIV infection such as HIV anal ulceration, unusual opportunistic infections (cytomegalovirus, cryptosporidiosis, isosporiasis, mycobacterium avium- intracellularre, other pathogens: Shigella spp, Campylobacter spp, E Histolytica) and opportunistic tumours (Kaposi’s sarcoma and lymphoma). It should be noted that squamous cell carcinoma may be mistaken for a small benign anal ulcer, Kaposi’s sarcoma may resemble an ulcerated haemorrhoid [89], non-Hodgkin’s lymphoma may resemble a perianal abscess [90] and anal tuberculosis may mimic anal carcinoma in HIV/AIDS [91]. The distribution of the most common anorectal pathologies reported in HIV patients include anal ulcer (29-32%), anal condyloma (32- 43%), anal fissure -6-33%), anal fistula (6-33%), perirectal abscess (3-25%) and haemorrhoids (4-14%) [92,93]. Anorectal pathology in HIV/AIDS –infected patients has not been impacted by highly active antiretroviral therapy [93]. The general approach to the HIVinfected patient with proctological complaints starts with a thorough history, which includes presenting symptoms, bowel and sphincter function, sexual practices and prior anorectal surgery. The current antiretroviral treatment the patient is taking as well as CD4 counts and viral load are determined. Most patients require just visual inspection and anoscopic or proctoscopic examination and abnormal lesions are biopsied [94]. Symptomatic improvement of the underlying anorectal pathology may make delayed wound healing in AIDS an acceptable complication in many instances. Idiopathic anal fissures in HIV-positive patients must be distinguished from HIV-associated ulcers and STDs that cause anogenital ulcers [92,94]. Clnically they both result in pain with defaecation, but AIDS ulcers are more likely to result in disabling pain unrelated to bowel movements. On examination AIDS ulcers are differentiated by their location proximal to the dentate line with a broad-based ulcer which may dissect between tissue planes [95] (Figure 3). The presence of a cavity contributes to stool and pus trapping, which may explain the severity of pain. Biopsy identifies treatable aetiologies of these ulcers, including HSV, CMV, Treponema pallidum, mycobacterium, Cryptococcus, Haemophilus ducreyi, chlamydia trichomatis and cancer. Surgical treatment consists of debridement, unroofing cavities (to eliminate trapping) and intralesional steroid injection (80-160mg methyl prednisolone acetate in 1ml of 0.25% bupivacaine) after excluding an underlying viral aetiology or oral acyclovir if herpes related. The goal is pain relief as ulcer healing is not common [96]. Treatment of idiopathic anal fissures in HIV-positive patients is similar to that of the HIV-negative patients starting with conservative measures such as warm soaks, stool softeners and topical diltiazem/GTN or botox injection into the internal anal sphincter. Anoreceptive intercourse is discouraged. Lateral internal anal sphincterotomy is appropriate for patients who fail conservative measures but do not have chronic diarrhoea or preexisting incontinence. Alternatively, a cutaneous advancement flap is preferred in those with a contraindication to sphincterotomy [88,97].

Figure 3: HIV ulcer (with permission) [95].
Anal papillomas
Anal papillomas are relatively common. They are of viral origin, being caused by infection with human papilloma virus, notably types 6, 16, and 11. High risk (oncogenic) serotypes, HPV 16 and 18, are strongly associated with anal and cervical cancer [98,99]. There is an increased incidence among homosexual men who practise anoreceptive intercourse, suggesting a venereal mode of transmission and with the immunosuppression of HIV/AIDS. However, anal papillomas also arise in the in the absence of any anal sexual contact among heterosexual men and women [100,101]. The papillomas appear as white, pink, or grey lesions around the anus and perineum and inside the anal canal. There may be associated lesions on the penis and vulva. They vary greatly in number and extent from a few scattered papillomas to bulky lesions without discernible intervening skin. The symptoms vary accordingly; they include itching, discomfort, discharge and bleeding although many are asymptomatic. They can be self- limiting and resolve spontaneously after several years, perhaps owing to an effective host immune response. Although the lesions are usually obvious on inspection of the perineum, proctoscopy should be performed systematically to identify lesions within the anal canal and rectum which may require treatment. All patients should be fully screened for other STDs. Being of viral origin surgical treatment is for local control only and not cure. Several different methods of treatment have been described but should be adapted to the individual patient. Repeated application of chemical agents such as the cytotoxic resin, podophyllin is suitable for small numbers of polyps outside the anal canal and the local immunomodulator, imiquimod for HPV in the mucocutaneous surfaces [102,103]. Persistent and more extensive warts are treated by surgical excision or ablation by diathermy. Excision has the benefit of providing tissue for histopathology but excision of intralesionaly raised lesions as opposed to wide excisions would avoid anal stenosis during healing. A quadrivalent HPV vaccine against HPV 6, 11, 16 and 18 in early adolescence is now available as a prophylactic against the infection and its predisposition to cervical and anal cancer [104]. It therefore plays no role in the treatment of visible anal condyloma.
Conclusion
Amongst the benign anorectal disorders, haemorrhoidal disease is common but other life-threatening diseases must first be excluded. Treatment allow for symptomatic relief but stapled haemorrhoidectomy for prolapsing piles as opposed to simple excision gives a better functional outcome. Anal stenosis has many aetiologies but the commonest is a result of anal surgery and treatments range from anal dilatation to flap procedures. Anal fissures are common and their aetiology is multifactorial. Although chemical sphincterotomies have good results, lateral sphincterotomy remains the gold standard for chronic anal fissures. It is important to distinguish the idiopathic anal fissure from HIV-associated ulcers and STDs that cause anogenital ulcers as the treatments differ. Lay-open of anal fistulas is the most certain treatment where it is possible and when the risks have been properly explained and accepted. Meticulous hygiene and shaving until the wound has healed are important for all forms of treatment of pilonidal sinus disease in order to prevent recurrence. Pruritus ani may result from many anorectal or dermatological conditions and remains a difficult problem to manage and treat although reduction of anal leakage and good personal hygiene remain important aspects of treatment. With the increasing prevalence and variety of STDs including HIV, the knowledge of sexually transmitted diseases is important to the colorectal surgeon in the evaluation, proper diagnosis and treatment of their associated anorectal disorders.
References
- Goligher JC, Leacock AG, Brossy JJ. The surgical anatomy of the anal canal. Br J Surg. 1955; 43: 51-61.
- Milligan ETC, Morgan CN. Surgical anatomy of the anal canal with special reference to anorectal fistulae. Lancet. 1934; 2: 1150-1156.
- Dalley AF 2nd. The riddle of the sphincters. Am Surg. 1987; 53: 298-306.
- Duthie HL, Gairns FW. Sensory nerve-endings and sensation of the anal region of man. Br J Surg. 1960; 47: 585-595.
- Fenger C. The anal transitional zone. Acta Pathol Microbiol Immunol Scand Suppl. 1987; 289: 1-42.
- Lestar B, Pennickx F, Kerremans R. The composition of anal basal pressure. Int J Colorect Dis. 1989; 4: 118-122.
- Kamm MA, Lennard-Jones JE. Rectal mucosal electrosensory testingevidence for a rectal sensory neuropathy in idiopathic constipation. Dis Colon Rectum. 1990; 33: 419-423.
- Eyers AA, Thompson JP. Pruritus ani: is anal sphincter dysfunction important in aetiology?. Br Med J. 1979; 2:1549-1551.
- Parks AG, Porter NH, Melzak J. Experimental study of the reflex mechanism controlling the muscles of the pelvic floor. Dis Colon Rectum. 1962; 5: 407- 414.
- Williams AB, Bartram CI, Halligan S, Marshall MM, Nicholls RJ, Kmiot WA. Endosonographic anatomy of the normal anal canal compared with endocoil magnetic resonance imaging. Dis Colon Rectum. 2002; 45: 176-183.
- Stoker J, Rociu E, Zwamborn AW, Ruud Schouten W, Johan S. Laméris. Endoluminal MR imaging of the rectum and anus: techniques, application, and pitfalls. Radiographics. 1999; 19: 383-398.
- Loder PB, Kamm MA, Nicholls RJ, Phillips RKS. Haemorrhoids: pathology, pathophysiology and aetiology. Br J Surg. 1994; 81: 946-954.
- Ambroze WL, Pemberton JH, Bell AM, Brown ML, Zinsmeister AR. The effect of stool consistency on rectal and neorectal emptying. Dis Colon Rectum. 1991; 34: 1-7.
- Weledji EP. Human Immunodeficiency virus and the anorectum. Alexandria J Med. 2013; 49: 163-167.
- Barron J. Office ligation of internal haemorrhoids. Am J Surg. 1963; 105:563- 570.
- Shanmugam V, Thaha MA, Rabindranath KS, Campbell KL, Steele RJ, Loudon MA. Rubber band ligation versus excisional haemorrhoidectomy for haemorrhoids. Cochrane Database Syst Rev 2005; 1.
- Griffith CD, Morris DL, Wherry DC, Hardcastle JD. Out-patient treatment of haemorrhoids: a randomized trial comparing contact bipolar diathermy with rubber band ligation. Coloproctology. 1987; 6: 322-334.
- Khoury GA, Lake SP, Lewis MCA, Lewis AAM. A randomised trial to compare single with multiple phenol injection treatment for haemorrhoids. Br J Surg. 1985; 72: 741-742.
- Senapati A, Nicholls RJ. A randomised trial to compare the results of injection sclerotherapy with a bulk laxative alone in the treatment of bleeding haemorrhoids. Int J Colorectal Dis. 1988; 3: 124-126.
- Copsite M. Double- blind versus placebo evaluation of clinical activity and safety of Daflon 500mg in the treatment of acute haemorrhoids. Angiology. 1994; 6:566-73.
- Parks AG. The surgical treatment of haemorrhoids. Br J Surg. 1956; 43: 337-351.
- Milligan ETC, Morgan C, Naughton Jones LF, Officer R. Surgical anatomy of the anal canal and the operative treatment of haemosshoids. Lancet. 1937; 2: 1119-1124.
- Ferguson JA, Heaton JR. Closed haemorrhoidectomy. Dis Colon Rectum. 1959; 2:176-179.
- Ho YH, Seow- Choen F, Tan M, Leong AF. Randomised trial of open and closed haemorrhoidectomy. Br J Surg. 1997; 84: 1729-1730.
- Seow- Choen F. Surgery for haemorrhoids: ablation or correction. Asian J Surg. 2002; 25: 265- 266.
- Lloyd D, Ho KS, Seow-Choen F. Modified Longo’s haemorrhoidectomy. Dis Colon Rectum. 2002; 45: 416-417.
- Weledji EP, Enow-Orock G, Aminde L. Massive prolapsed haemorrhoid managed by ablation and correction in a poor resourced area. J Surg Case Rep. 2013.
- Sohn N, Aronoff JS, Cohen FS, Weinstein MA. Tranasanal haemorrhoidal dearterialization is an alternative to operative haemorrhoidectomy. Am J Surgery. 2001; 182: 515-519.
- Dal Monte PP, Tagariello C, Giodano P, Sarago M, Shafi A, Cudazzo E, et al. Trans anal haemorrhoidal dearterialisation: non excisional surgery for the treatment of haemorrhoidal disease. Tech Coloproctol. 2007; 11: 338-339.
- Marthai V, Ong BC, Ho YH. Randomized controlled trial of lateral internal sphincterotomy with haemorrhoidectomy. Br J Surg. 1996; 83: 380- 382.
- Carapeti EA, Kamm MA, McDonald PJ, Phillips RK. Double- blind randomized controlled trial of effect of metronidazole on pain after day-case haemorrhoidectomy. Lancet. 1998; 351: 169-172.
- Smith LE. Anal fissure. Neth J Med. 1990; 37: S33.
- Leong AFPK, Seow- Choen F. lateral sphincterotomy compared with anal advancement flap for chronic anal fissure. Dis Colon Rectum. 1995; 38: 69- 71.
- Lund JN, Scholfield JH. A randomized, prospective, double-blind, placebocontrolled trial of glycerine trinitrate ointment in the treatment of anal fissure. Lancet. 1997; 349: 11-14.
- Carapeti EA, Kamm MA, McDonald PJ, Chadwick SJ, Melville D, Phillips RK. Randomized control trial shows that glyceryl trinitrate heals anal fissures, higher doses are not more effective, and there is a high recurrence rate. Gut. 1999; 44: 727-730.
- Maria G, Sganga G, Civello IM, Brisinda G. Botulinum neurotoxin and other treatments for fissure-in-ano and pelvic floor disorders. Br J Surg. 2002; 89: 950-961.
- Nelson R. Non- surgical therapy for anal fissure. Cochrane Database Syst Rev. 2006; 4.
- Nelson R. Operative procedures for fissure-in-ano (metaanalysis). Cochrane library. 2003; 3.
- Littlejohn DR, Newstead GL. Tailored lateral sphincterotomy for anal fissure. Dis Colon Rectum. 1997; 40: 1439- 1442.
- Garcia-Aguilar J, Belmonte C, Wong WD, Lowry AC, Madoff RD. Open vs closed sphincterotomy for chronic anal fissure: long-term results. Dis Colon Rectum. 1996; 39: 440-443.
- Engel AF, Eijsbouts QA, Balk AG. Fissurectomy and isosorbide dinitrite for chronic fissure-in-ano not responding to conservative treatment. Br J Surg. 2002; 89: 79-83.
- Scholz T, Hetzer FH, Dindo D, Demartines N, Clavien PA, Hahnloser D. Long-term follow-up after combined fissurectomy and Botox injection for chronic anal fissures. In J Colorectal Dis. 2007; 22: 1077-1081.
- Eisenhammer S. The final evaluation and classification of the surgical treatment of the primary anorectal, cryptoglandular intermuscular (intersphincteric) fistulous abscess and fistula. Dis Colon Rectum. 1978; 21: 237-254.
- Phillips RKS, Lunniss PJ. Anorectal sepsis. In: Nicholls RJ, Dozois RR (eds) Surgery of the colon and rectum. New York: Churchill Livingstone. 1997; 255-284.
- Grace RH, Harper IA, Thompson RG. Anorectal sepsis: microbiology in relation to fistula-in-ano. Br J Surg. 1982; 69: 401-403.
- Toyonga T, Matsushima M, Tanaka Y, Shimojima Y, Matsumura N, Kannyama H, et al. Microbiological analysis and endoanal ultrasonography for diagnosis of anal fistula in acute anorectal sepsis. Int J Colorect Dis. 2007; 22: 209-213.
- Lunniss PJ, Phillips RKS. Surgical assessment of acute anorectal sepsis is a better predictor of fistula than microbiological analysis. Br J Surg. 1994; 81: 368-369.
- Quah HM, Tang CL, Eu KW, Chan SY, Samuel M. Meta-analysis of randomized clinical trials comparing drainage alone vs primary sphinctercutting procedures for anorectal abscess-fistula. Int J colorectal Dis. 2006; 21: 602-609.
- Goodsall DH, Miles WE. In Diseases of the Anus and Rectum. Longmans, Green, London. 1900: 92.
- Parks AG, Gordon PH, Hardcastle JD. A classification of fistula- in -ano. Br J Surg. 1976; 63: 1-12.
- Phillips RKS. Operative management of low cryptoglandular fistula-in-ano. Operat Tech Gen Surg. 2001; 3: 134-141.
- Burgess BE. Anorectal Disorders. Chp 88; In: Tintinalli JE, Stapczynski JS, Cline DM, Ma OJ, Cydulka RK, Meckler GD, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York: McGraw-Hill. 2011.
- Lunniss PJ, Barker PG, Sultan AH, Armstrong P, Reznek RH, Bartram CI, et al. Magnetic resonance imaging of fistula-in-ano. Dis Colon Rectum. 1994; 37: 708-718.
- Pescatori M, Ayabaca SM, Cafaro D, Iannello A, Magrini S. Marsupialization of fistulotomy and fistulectomy wounds improves healing and decreases bleeding: a randomized controlled trial. Colorectal Dis. 2006; 8: 11-14.
- Perez F, Arroyo A, Serrano P, Sánchez A, Candela F, Perez MT, et al. Randomized clinical and manometric study of advancement flap versus fistulotomy with sphincter reconstruction in the management of complex fistula-in-ano. Am J Surg. 2006; 192: 34-40.
- Kronborg O. To lay open or excise a fistula-in-ano: a randomised trial. Br J Surg. 1985; 72: 970.
- Lewis A. Excision of fistula-in-ano. Int J Colorectal Dis. 1986; 1: 265-267.
- Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula- in- ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007; 90: 581-586.
- Rojanasakul A. LIFT Procedure: a simplified technique for fistula in ano. Tech coloproctol. 2009; 13: 237-240.
- Huda T, Ashok M. Lift Technique for Fistula in ANO with Redefined Criteria - A Step towards Better Outcome. IOSR Journal. 2013; 11: 61-63.
- Vergara - Fernandes O, Espino - Urbina LA. Ligation of intersphincteric fistula tract: What is the evidence in a review?. World J Gastroenterol. 2013; 19: 6805-6813.
- Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011; 15: 417-422.
- Lord PH. Etiology of pilonidal sinus. Dis Colon Rectum. 1975; 18: 661-664.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980; 87: 567-572.
- Karyadakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust NZ J Surg. 1992; 62: 385-389.
- Bascom J. Pilonidal disease: long-term results of follicle removal. Dis Colon Rectum. 1983; 26: 800-807.
- Marks J, Harding KG, Hughes LE, Ribiero CD. Pilonidal sinus excisionhealing by open granulation. Br J Surg. 1985; 72: 637-640.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987; 154: 118-122.
- Kitchen PRB. Pilonidal sinus: excision and primary closure with lateralized wound- the Karydakis operation. Aust NZ J Surg. 1982; 52: 302-305.
- Perez-Gurri JA, Temple WJ, Ketcham AS. Gluteus maximus myocutaneous flap for the treatment of recalcitrant pilonidal disease. Dis Colon Rectum. 1984; 27: 262-264.
- Toubanakis G. Treatment of pilonidal sinus disease with the Z-plasty procedure (modified). Am Surg. 1986; 52: 611-612.
- Monro RS, McDermott FT. The elimination of casual factors in pilonidal sinus treated by Z-plasty. Br J Surg. 1965; 52: 177-181.
- Shelley WB, Cahn MM. The pathogenesis of hidradenitis suppurativa in man. Arch Dermatol. 1995; 72: 562- 565.
- Williams ST, Busby RC, Demuth RJ, Nelson H. Perineal hidradenitis suppurativa: presentation of two unusual complications and a review. Ann Plast Surg. 1991; 26: 456-462.
- Daniel GL, Longo WE, Vernava AM. Pruritus ani, causes and concerns. Dis Colon Rectum. 1994; 37: 670-674.
- Wexner SD, Dailey TH. Pruritus ani: diagnosis and management. Curr Concepts Skin care. 1986; 7: 5-9.
- Matsen JM, Turner JA. Reinfection in enterobiaiasis (pin worm infection). Simultaneous treatment of family members. Am J Dis Child. 1969; 118: 576- 581.
- Fenton KA, Ison CA, Johnson AP, Rudd E, Soltani M, Martin I, et al. Ciprofloxacin resistance in Neiserria gonorrhoeae in England and Wales in 2002. Lancet. 2003; 361: 1867-1869.
- Marino AWM. Proctological lesions observed in male homosexuals. Dis Col Rectum. 1964; 7: 121-128.
- Mindel A, Tovey SJ, Timmins DJ, P Williams. Primary and secondary syphilis, 20 years’ experience. Clinical features. Genitourinary Med. 1989; 65: 1-3.
- Smith D. Infectious syphilis of the anal canal. Dis Colon Rectum. 1963; 6: 7-14.
- Ootani A, Mizuguchi M, Tsunada S, Toda S, Fujimoto k. Chlamydia trachomatis proctitis. Gastrointest Endosc. 2004; 60: 161-162.
- Stark D, van Hal S, Hillman R, J. Harkness, D. Marriott. Lympogranuloma venereum in Australia: anorectal chlamydia trachomatis serovar L2b in men who have sex with men. J Clin Microbiol. 2007; 45: 1029-1031.
- Carder C, Mercey D, Benn P. Chlamydia trachomatis. Sex Transm Infect. 2006; 82: S10-12.
- Krone MR, Wald A, Tabet SR, Paradise M, Corey L, Celum CL. Herpes simplex virus type 2 shedding in human immunodeficiency virus-negative men who have sex with men: frequency, patterns and risk factors. Clin Infect Dis. 2000; 30: 261-267.
- Goldmeier D. Proctitis and herpes simplex virus in homosexual men. Br J Venereal Dis. 1980; 56: 111-114.
- Goodell SE, Quin TC, Mkrtician E, Schuffler MD, Holmes KK, Corey L, et al. Herpes simplex virus proctitis in homosexual men: clinical, sigmoidoscopic and histopathological features. N Engl J Med. 1983; 308: 868-871.
- Weledji EP, Nsagha DS, Chichom AM, Enoworock G. Gastrointestinal surgery and the acquired immune deficiency syndrome. Ann Med Surg. 2015; 4: 36-40.
- Lorenz HP, Wilson W, Leigh B, Schecter WP. Kaposi’s sarcoma of the rectum in patients with the acquired immunodeficiency syndrome. Am J Surg. 1990; 160: 681-683.
- Kaplan LD, Abrams DL, Feigal E, McGrath M, Kahn J, Neville P, et al. AIDSassociated non-Hodgkin’s lymphoma in San Francisco. JAMA. 1989; 261: 719-724.
- Enow Orock. Anal tuberculosis mimicking anal carcinoma in HIV/AIDS.
- Brar HS, Gottesman L, Surawiwicz C. Anorectal pathology in in AIDS. Gastrointest Endosc Clin N Am. 1998; 8: 913-931.
- Gonzalez-Ruth C, Heartfield W, Briggs B, Vukasin P, Beart RW. Anorectal pathology in HIV/AIDS -infected patients has not been impacted by highly active antiretroviral therapy. Dis Colon Rectum 2004; 47: 1483-1486.
- Schmitt SL, Wexner SD, Nogueras JJ, JagelmanDG. Is aggressive management of perianal ulcers in homosexual HIV-seropositive men justified?. Dis Colon Rectum. 1993; 36: 240-246.
- Weledji EP. A case of HIV ulcer. JRSM Open. 2015; 6: 1-2.
- Modesto VL, Gottesman L. Surgical debridement and intralesional steroid injection in the treatment of idiopathic AIDS-related anal ulcerations. Am J Surg. 1997; 174: 439-441.
- Wexner SD, Smithy WB, Milsom JW, Dailey TH. The surgical management of anorectal diseases in AIDS and pre-AIDS patients. Dis Colon Rectum. 1986; 29: 719-723.
- Nielson CM, Harris RB, Dunne EF, Abrahamsen M, Papenfuss MR, Flores R, et al. Risk factors for anogenital human papillomavirus infection in men. J Infect Dis. 2007; 196: 1137-1145.
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007; 297: 813-819.
- Hernandez BY, McDuffe K, Zhu X, Wilkens LR, Killeen J, Kessel B, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev. 2005; 14: 2550-2556.
- Guiliano AR, Nelson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, et al. The optimal anatomotic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007; 196: 1146-1152.
- Maitland JF, Maw R. An audit of patients who have received imiquinod cream 5% for the treatment of anogenital warts. Int J STD AIDS. 2000; 11: 268-270.
- Kaspari M, Gutzmer R, Kaspari T, Kapp A, Brodersen JP. Application of imiquimod by suppositories (anal tampons) efficiently prevents recurrences after ablation of anal condylomata. Br J Derm. 2002; 147: 757-759.
- Palefsky J. Human papillomavirus in HIV-infected persons. Top HIV Med. 2007; 15: 130-133.