
Special Article – Surgery Case Reports
Austin J Surg. 2018; 5(9): 1156.
Extensive Subcutaneous Emphysema after Laparoscopic Cholecystectomy, Two Cases Reports
Elsaady A¹*, Elsherbeny R² and Elzaeem A²
¹Department of General Surgery, Kafr Elshikh General Hospital, Egypt
²Department of General Surgery, Balteem Hospital, Egypt
*Corresponding author: Ahmed Elsaady, Department of general surgery, Kafr Elshikh General Hospital, Egypt
Received: November 05, 2018; Accepted: December 19, 2018; Published: December 26, 2018
Abstract
Laparoscopic surgery has expanded its horizon tremendously. It has been the preferred approach in many operations. Massive subcutaneous emphysema is a rare unique complication of laparoscopic surgery. Here, we report two cases that developed progressive extensive subcutaneous emphysema after laparoscopic cholecystectomy. On reviewing the literature, we found that the incidence ranges from 0.43% to 2.34%. There are many risk factors that have been implicated for its development including; pneumo-peritoneum of more than 200 minutes, and insufflation of CO2 at pressure more than 15mm Hg, & PETCO2 more than 50 mmHg. Clinically, subcutaneous emphysema produces an unusual crackling sensation on palpation and graded into four grades according to the severity.
The patients should be monitored closely for any cardio-respiratory changes and positive pressure ventilation should be continued until normocarbia is established and signs of respiratory distress & upper airway obstruction are absent. Although conservative supportive measures and close follow up are the only needed strategy in most of cases, however surgical drainage may be beneficial in some case. This achieved either incisions (infraclavicular or submandibular) or tube drainage through different techniques.
Keywords: Subcutaneous emphysema; Laparoscopic cholecystectomy; Complications of laparoscopy
Introduction
Laparoscopic surgery has expanded its horizon tremendously [1]. It has been the preferred approach in many operations [2]. Massive subcutaneous emphysema is a rare unique complication of laparoscopic surgery [3], that terrifies the patient& surgeon especially if the surgeon doesn`t have any experience in similar cases. We report here such a complication after laparoscopic cholecystectomy in two cases. Because of the increased rate of occurrence & their dangers, it is important that the surgeons should be familiar with these complications, their natural history, and their management [4].
Case Report
Case report no (1)
Female patient aged 32 years, presented with chronic calcular cholecyctitis, then laprascopic cholecystectomy was decided and done 1.5 years ago. The operation was done via four ports with feasible trocar introduction. Although the dissection and removable of GB was passed smoothly, however straining of the patient and increased intraabdominal pressure to 20 mmhg occurred and persisted for 10 minutes till it return to 15 mmhg again. The surgeon put a drain as a routine, closed the ports sites as usual, and finished the procedure in about eighty minutes. One hour after recovery from anesthesia progressive subcutaneous emphysema developed on the chest that rapidly progressed to the face with bilateral pre-orbital edema closing the eyes within several hours. Dyspnea with increasing pulse reaching 120b/m, as well as blood pressure (150/80mmHg) and wide pulse pressure occurred with O2 saturation 92% at room. The patient admitted at ICU with O2 supply in semi-setting position, and follow up. Chest X ray was normal. ABG was normal but actually done one day later. Then the condition settled and improved after three days of close follow up and discharged to room then to outpatient clinic after five postoperative days.
Case report no (2)
Female patient aged 38 years, presented for laparoscopic cholecystectomy for cholelethiasis 1month ago. The procedure passed smoothly via 4 trocars with dissection & removal of GB and closure of the ports after putting a drain within about one hundered minutes. Nothing unusual reported during the operation except trouble in the anesthesia machine making then anesthesiologist changed it as well as the insufflator, where the pressure was noted to be high more than 18 mmhg and persisted for sometimes. The patient immediately postoperative during recovery developed extensive progressive subcutaneous emphysema involving the whole chest and neck to the orbit without pneumothorax. Tachycardia (100 b/m), Dyspnea, were developed, Blood pressure was 130/80 mmhg & O2 saturation was 94% at room. Chest x ray excluded pneumothorax. Close follow up with O2 supply in semi setting position was done. Bilateral infraclavicular incisions were done which gave temporary improvement. Then subcutaneous tube drainage from chest wall with massage and intermittent suction were also tried. The emphysema decreased on the third day and completely disappeared on the sixth day and discharged one day later on.
Discussion
Epidemiology
Laparoscopy has surpassed and even replacing the open technique in many operations, with gaining benefits from their many advantages in terms of rapid recovery, less adhesions, less pain & length of hospital stays. However, post-operative subcutaneous emphysema is a unique complication, that particular to laparoscopy not to open surgery [5]. Although massive subcutaneous emphysema is a rare complication of laparoscopy, but it is quite annoying one [6]. It was reported in both in intra and extra-peritoneal laparoscopy such as renal and colorectal surgery [7]. The incidence ranges from 0.43% to 2.34% [3]. However, it is thought to be more if carefully assessed. The incidence might be as high as 34% if assessed by chest X-ray & up to 56% by computed tomography [5]. Other authors reported as much as 77% of laparoscopy patients have grossly undetectable subcutaneous emphysema & 20% have findings on postoperative chest radiograph of pneumomediastinum [8]. Murdock et al. reported incidence rates of 5.5% for hypercarbia, 2.3% for subcutaneous emphysema, and 1.9% for pneumothorax /pneumomediastinum following laparoscopy [4]. Pneumothorax can be developed by extension of insufflated gas through congenital diaphragmatic channels into the pleural cavities, and is reported as 0.03% [2]. Subcutaneous emphysema was reported to appear in the intraoperative as well as early and late postoperative periods (in the third day) [8].
Risk factors
There are many risk factors that have been implicated for development of this complication [9] shown in (Table 1). These include; prolonged surgery & pneumo-peritoneum of more than 200 minutes [10], and insufflation of CO2 at pressure =15 mm Hg [11]. Insufflator settings for pressure and flow rate influence insufflation dynamics, the amount of gas absorption or extra-peritoneal extravasation with higher pressures, and flow rates contributing to the increased incidence of gas extravasation, noted as subcutaneous emphysema [12]. The total amount of gas used may or may not be related to the length of time of the procedure and may be more important than the length of time of the procedure [4]. Maximum positive end-tidal CO2 (PETCO2) more than 50 mmHg is one predictor for its development [3].
Insufflator (high gas flow and high gas pressure setting)
Intra-abdominal pressure >15 mm Hg
Positive end-tidal CO2 >50 mm Hg
Procedures lasting >3.5 hours
Use of >5 cannulas
Number of trocars >4
Size of trocars is =10 mm
Type of trocar (valveless trocar)
Multiple attempts at the abdominal entry
Veress needle or cannula not placed in the peritoneal cavity
Improper cannula placement, causing stressed angulation
Skin/fascial fit/seal around the cannulas is not snug
Soft tissue dissection and fascial extension
Gas dissection leading to more dissection
Torqueing with traumatic expansion of the fascia
Long arm of the laparoscope is a force multiplier
Laparoscope used as a lever
Cannula acting as a fulcrum
Structural weakness caused by repetitive movements
Tissue integrity compromised by repetitive movements
Age > 65years
Coexisting metabolic diseases
Lack of external visualization during robotic procedures
Lack of haptic feedback during robotic procedures
Increased mechanical advantage without recognition during robotic surgery
Intraoperative risk factors for hypercarbia including; Integrity of the anesthesia circuit, position and function of the endotracheal tube, inadequate respiratory exchange, underlying obstructive lung diseases …etc
Table 1: Risk factors for development of subcutaneous emphysema post laparoscopy.
Also, number of ports (six or more) [13], size and geometry of fascial incision to trocar size of entry site, snugness of fit between trocar and fascia, number of times the entry site is entered, amount of torqueing and pressure on entry sites, vectoring of the laparoscope, fulcrum effect between laparoscope and fascia, extra-peritoneal dissection and methods of laparoscopy (video assisted or robotic) were also incriminated. Type of trocar used also may be a risk factor [14]. Recent articles citing use of a valve less trocar and dynamic pressure system (AirSeal; SurgiQuest, Milford, CT) showed a 16.4% rate of subcutaneous emphysema over 6.3 times the reported rate, a 3.9% rate of pneumomediastinum 2 times the reported rate, and a 0.9% complication rate of masked pneumothorax a rate 2.3 times the reported rate for a 21.24% total overall complication rate or 4.3 times the total reported rate [4]. Another risk factor is the age where older patients (especially more than 65years) are more prone to develop it, due to decrease in natural subcutaneous resistance [11]. In addition, the patient BMI, coexisting metabolic diseases & tissue integrity can be real risk factors [15].
Pathophysiolgy
Dissection of the insufflated CO2 from the peritoneal cavity to the subcutaneous tissue may occur at the trocar site or through a defect in the diaphragm [16]. Intra-abdominal pressure of CO2 should therefore be monitored, since high pressures are associated with a higher incidence of subcutaneous emphysema. Following CO2 absorption into the blood, the gas is stored in visceral and muscle tissue, delaying prompt elimination via the lungs. Tracking of gas along fascial planes from port sites or through diaphragmatic defects occurred allowing increased absorption of carbon dioxide [2]. A latent pocket of carbon dioxide in the abdominal wall which disseminated only later, after mobilization of the patient; a gas leak from the anastomosis, later resealed; a small iatrogenic mucosal disruption, resulting in retroperitoneal air leak, settled spontaneously may also explain its development. On the other hand, an iatrogenic airway injury or a pneumothorax appears unlikely because of clinical and radiological findings [17].
Dissection of the gas through the tissue depends on its structure, integrity, composition, architecture, morphology, tensile strength, and adherence to underlying structures. The pressure settings, pressure drop, volume, duration, and resistance to gas flow affect these tissue characteristics. With multiple repetitive movements of the laparoscope acting through a cannula, peritoneal separation can occur. The cannula acts as a fulcrum for the laparoscope (lever arm) to act as a class-one lever and force multiplier. The pivot point is the fascial entry site. The original peritoneal penetration site, then mechanically extended allowing gas extravasation into planes outside of the abdomen [4]. Anatomical connection between the cervical fascia and retro-peritoneum was documented [17]. Notably, the soft tissues of the neck are divided into three main compartments by layers of cervical fascia. Two of these three spaces terminate at the upper thoracic spine; the visceral space, however, descends further, investing the trachea and esophagus from the neck into the chest. Inferiorly, the visceral space extends with the esophagus through the diaphragmatic hiatus into the retroperitoneal soft tissues [17]. Murdock notes an established link between preperitoneal insufflation and extensive dissection of retroperitoneal air [17]. Hypercarbia stimulates sympathetic nervous system, and hence the BP and HR increased. It also sensitizes the myocardium to catecholamines, predisposing to cardiac arrhythmias [18]. Later on, several cardiopulmonary complications may rapidly settle as in (Table 2).
Complication
Clinical Implication
1
Subcutaneous surgical emphysema
May be associated with airway obstruction, pulmonary edema and atelectasis resulting in hypercarbia and hypoxemia.
2
Periorbital edema
May or may not cause visual disturbance. Usually benign
3
Pneumothorax (capnothorax)
Capnothorax is usually benign, seldom requires chest tube placement if respiratory embarrassment is severe
4
Pneumomediastinum (capnomediastinum)
May cause cardio-respiratory changes due to impairment of compliance requiring high intrathoracic pressure to ventilate the patient.
5
Pneumopericardium (Capnopericardium)
May affect filling pressures and consequently cardiac output
6
Carbon dioxide gas embolism
Paradoxical embolism in patients with atrial septum defects, can affect cerebral oxygenation. Fatal if large amounts of CO2 are injected intravascularly causing a gas lock in the right ventricle
7
Direct effects of insufflation-induced hypercarbia
Tachycardia, vasoconstriction, hypertension, increased myocardial irritability, arrhythmias (brady and tachy)
8
cardiac arrest
Due to gas embolism or vasovagal response.
9
EEG changes
Severe hypercarbia can cause flattening of the EEG tracing
Table 2: Cardio-Respiratory Complications Associated with Carbon Dioxide Insufflation in Laparoscopic Surgery [4].
Clinical assessment
Post-laparoscopy subcutaneous emphysema varies from isolated area, confined in a small space to extravasation outside of the abdominal cavity extending into the labia, scrotum, legs, chest, head, and neck. Subcutaneous emphysema, pneumomediastinum, and retroperitoneal extravasation without pneumothorax have also been described during laparoscopic surgery and may be associated with prolonged hypercarbia [4]. Unilateral periorbital edema after laparoscopic radical prostatectomy was also reported [17]. Rarely, air progressing through the cervical fascia can extend along fascial planes to the face and periorbital region. Fortunately, periorbital emphysema subsides over the course of a few days without causing any visual disturbance [17].
Clinically, subcutaneous emphysema produces an unusual crackling sensation on palpation as the gas in pushed through the tissues [15]. Severity of emphysema can be graded into four point Grades as in (Table 3); Grade O; no subcutaneous emphysema, Grade (1) Mild emphysema with crepitus at trocar insertion site, Grade (2); Mild emphysema with crepitus extending to the abdomen and thighs and grade 3; massive emphysema extending to the chest, neck and face [15].
Grade (O)
no subcutaneous emphysema
Grade (1)
Mild emphysema with crepitus at trocar insertion site,
Grade (2)
Mild emphysema with crepitus extending to the abdomen and thighs and grade
Grade (3)
Massive emphysema extending to the chest, neck and face.
Table 3: Grading the severity of subcutaneous emphysema post laparoscopy [15].
Another grading system for subcutaneous emphesema was also reported by Aghajanzadeh who divided it into five grades 19 as in (Figure 1). Grade 1; involves the base of the neck only, grade (2) all of the neck area, grade (3) subpectoralis major area, grade (4) chest wall and all of the neck, while grade (5) involves chest wall, neck, orbit, scalp, abdominal wall, upper limbs, and scrotum) [19].
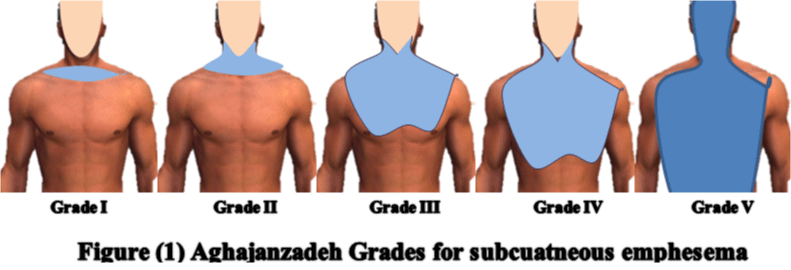
Figure 1: SE Classification proposed by Aghajanzadeh et al. We recorded demographics, management and outcome of each case.
Hypercarbia stimulates sympathetic nervous system, and hence the BP and HR increase. It also sensitizes the myocardium to catecholamines, predisposing to cardiac arrhythmias [18]. Cardiorespiratory complications may rapidly develop as in (Table 4).
Crepitus
Insufflation problems (flow and pressure),
Hypercarbia (monitor end-tidal CO2)
Acidosis (monitor partial pressure of CO2 in arterial blood and rule out malignant hyperthermia).
Change in lung compliance
Cardiac arrhythmias, sinus tachycardia, and hypertension
Intraoperative increase in partial pressure of end-tidal CO2 >50 mm Hg
Table 4: Recognizable Changes Seen with subcutaneous emphysema post laparascopy [15].
Management
Early intraoperative detection is very important [1]. Anesthesiologists must be aware of the risk factors to take precautions and detect emphysema in the early stages [1]. Anesthesia machines have option to measure VCO2. This parameter is useful to detect increases in CO2 production, which can be increased because of several conditions. These include inadequate ventilation, excessive CO2 production due to increased metabolism or release and external sources. During laparoscopic surgery, CO2 is absorbed by peritoneum; therefore, CO2 levels in blood are high. This is readily compensated by assisting expiration from the lungs as it has high aqueous solubility and diffusibility [20]. If alveolar ventilation is impaired, additional CO2 load is not cleared, resulting in hypercapnia and acidosis and EtCO2 elevation [20]. This is usually corrected by increasing minute volume as much as 30% [14]. If very high levels of Et CO2 are noticed, palpating the patients’ skin surface for emphysema and notifying the same to the surgeon is advisable. When subcutaneous emphysema appears, all the members of the team have to be notified and start the management as in (Table 5). A blood gas analysis is mandatory to evaluate the grade of acidosis, electrolytes disorders and gas interchange. Other potential events such as pneumothorax or air embolism must be ruled out. FiO2 of 100% must be delivered if oxygenation appears compromised [21]. The patient should be closely examined under the drapes intraoperatively even if the parameters of the ventilator are normal [21]. At the end of surgery, airway assessment before extubation must be performed. A direct laryngoscopy and a leak test should be considered as well as an airway exchange device has to be kept ready for a potential airway compromise [22]. Tracheal extubation should be postponed if unsure of airway patency post-extubation [23]. Treatment is usually aims at the underlined cause and respiratory support, while the air is gradually absorbed from interstitial tissues. The patients should be monitored closely for a longer duration in the postoperative period for any cardio-respiratory changes and positive pressure ventilation should be continued till normocarbia is established and signs of upper airway obstruction are absent [17].
Evaluate for a pneumothorax
Check end-tidal CO2 and arterial CO2
Increase ventilation rate and tidal volume
Increase oxygen to 100%
CO2 absorber in the circuit
Decreased IAP
Discontinue NO because it rapidly enters the area of tissue emphysema
Assess airway to ensure there is no compression before extubation
Table 5: Actions to Initiate when subcutaneous emphysema is suspected or noticed [4].
However, in extensive cases, surgical intervention may be needed hoping for drainage of subcutaneous gas [24]. The drainage may be achieved by either incisions or tube drainage. Several maneuvers have been reported [25]. Bilateral infra-clavicular incisions down to pectoralis fascia were recommended by Herlan as efficient procedure [26], while others recommended submandibular incisions [27]. On the other hands, many varieties for tube drainage have been reported. A trochar-type chest tube [26], wide angiocatheter (14G) & central venous catheter [28] are also reported to be efficient. Jackson-Pratt drain or 12Fr Seldinger drain administrated in the tract dissected, & then placed on gentle suction to ensure tube patency & air resorption was described [26]. Srinivas et al. [5] described a modified microcatheter & active compressive massage with the face downward & arms upward toward the catheter to facilitate the drainage [27]. Liposuction as efficient method for gas drainage was reported [28].
Unfortunately, there is no reliable comparison study, to identify the best method; neither have we had any large series study published about possible complications of each method [29]. Larger studies are needed to definitely come to safe conclusions [29].
However, the likelihood for development of subcutaneous emphysema in laparoscopy can be diminished by the following recommendations [4] shown in (Table 6); awareness of its potential; physician vigilance; attention to detail regarding abdominal entry; monitoring insufflator settings for pressure, flow rate, and volume of gas with alarm settings; quickness, but not rushing, to complete the procedure (because length of procedure and gas consumption relate to the condition); reduce the number of attempts to enter the abdomen; have a snug trocar skin condition; test for correct. Also, look at and feel the skin around the cannula insertion sites, perform the surgery quickly but not hurried, and, finally-attention to detail [4].
Awareness of its potential
Physician vigilance
Observation
Proper technique
Low gas flow and pressure settings
Low IAP
Cannulas that fit snugly
Gentle handling of trocars intraoperatively
CO2 gas monitoring (respiratory and blood)
Reduced torqueing and fulcrum effect
Performing quickly, but not rushing
Attention to detail, attention to detail, attention to detail
Table 6: Recommendations to prevent or minimize the possibility of development of subcutaneous emphysema in laparoscopy [4].
Review the two cases reports
We found that that the alarming factors in both cases were the increased intra-abdominal pressure more than 15 mmHg and absence of the awareness and experience of the operative team with this complication. In the two cases the progress of the emphysema is outmost in the first 24 hours and decreased on the third day, while completely relived after 1 week. Surgical drainage either by infraclavicular incision or by tube provided only temporary improvement. Awareness & attention to detail seem to be important factor especially in prevention, early detection and proper management as well as minimizing the panic feeling of the patients and their relatives with massive subcutaneous emphysema.
Conclusion
Massive subcutaneous surgical emphysema is a rare complication after laparoscopic surgery. Many risk factors have been incriminated including; pneumo-peritoneum of > 200 minutes 10, and insufflation of CO2 at pressure =15 mm Hg, maximum positive end-tidal CO2 (PETCO2) > 50 mmHg. Most of these factors are preventable early detection is important. The patients should be monitored closely for any cardio-respiratory changes and positive pressure ventilation should be continued until normocarbia is established and signs of respiratory distress & upper airway obstruction are absent. Although conservative supportive measures and close follow up are the only needed strategy in most of cases, however surgical drainage may be beneficial in some case. This is achieved either incisions or tube drainage through different techniques. The superiority of each technique is debatable, need more studies.
References
- Sood J. Advancing frontiers in anaesthesiology with laparoscopy. World J Gastroenterol. 2014; 20: 14308-14314.
- Saqib Saeed, Ofikwu Godwin, Albert K Adu, Alexius Ramcharan. Pneumomediastinum and subcutaneous emphysema after successful laparoscopic supra-cervical hysterectomy. J Surg Case Rep. 2017; 2017: 146.
- Critchley LA, Ho AM. Critchley Surgical emphysema as a cause of severe hypercapnia during laparoscopic surgery Anaesth Intensive Care. 2010; 38: 1094-1100.
- Douglas E. Ott, Subcutaneous Emphysema-Beyond the Pneumoperitoneum. JSLS. 2014; 18: 1-7.
- Angharad Jones, Umberto Pisano, Sherif Elsobky, Angus JM. Watson Grossly delayed massive subcutaneous emphysema following laparoscopic left hemicolectomy: A case report. Int J Surg Case Rep. 2015; 6: 277-279.
- Baron PW, Ben-Youssef R, Ojogho ON, Kore A, Baldwin DD. Morbidity of 200 consecutive cases of hand-assisted laparoscopic living donor nephrectomies: A single-center experience. J Transplant. 2012; 2012: 121523.
- Nakajima K, Kai Y, Yasumasa K, Nishida T, Ito T, Nezu R. Subcutaneous emphysema along cutaneous striae after laparoscopic surgery: a unique complication. Surg Laparosc Endosc Percutan Tech. 2006; 16: 119-121.
- Angel Blanco Coronil, Alfonso Moreno Sanchez-Canete, Ashish A Bartakke, Javier Garcia Fernandez, Ana Garcia. Life-threatening subcutaneous emphysema due to laparoscopy Indian Journal of anaesthesia. 2016: 60: 4: 286-288.
- Herati A, Atalla M, Rais-Bahrami S, Andonian S, Vira M, Kavoussi L. A new valve-less trocar for urologic laparoscopy: initial evaluation. J Endourlol. 2009; 23: 1535-1539.
- Horstmann M, Horton K, Kurz M, Padevit C, John H. Prospective comparison between the AirSeal System valve-less Trocar and a standard Versaport Plus V2 Trocar in robotic-assisted radical prostatectomy. J Endourol. 2013; 27: 579-582.
- Saggar VR, Singhal A, Singh K, Sharma B, Sarangi R. Factors influencing development of subcutaneous carbon dioxide emphysema in laparoscopic totally extraperitoneal inguinal hernia repair. J Laparoendosc Adv Surg Tech A. 2008; 18: 213-216.
- Murdock CM, Wolff AJ, Geem TV. Risk factors for hyper- carbia, subcutaneous emphysema, pneumothorax and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000; 95: 704-709.
- Giorgakis E, Fernandez-Diaz S. Diffuse Subcutaneous Emphysema after Transperitoneal Laparoscopic Donor Nephrectomy. Hippokratia. 2013; 17: 94.
- Tan PL, Lee TL, Tweed WA. Carbon dioxide absorption and gas exchange during pelvic laparoscopy. Can J Anaesth. 1992; 39: 677-681.
- Suman Sain, Nidhi Agrawal. Massive subcutaneous emphysema following laparoscopic nephroureterectomy: An unusual presentation. Indian J Anaesth. 2015; 59: 389-390.
- ASM Moosa, M Baharul Islam, Shahina Akther, M Latifur Rahman, Nazim Uddin Ahmed. Massive Subcutaneous Emphysema during Laparoscopic Cholecystectomy. TAJ. 2008; 21: 77-79.
- Jaydev Sarma. Unilateral Periorbital and Cervical Subcutaneous Emphysema Following Extraperitoneal Laparoscopic Radical Prostatectomy. The Open Anesthesiology Journal. 2011; 5: 1-4.
- Srivastava A, Niranjan A. Secrets of safe laparoscopic surgery: Anaesthetic and surgical considerations. J Minim Access Surg. 2010; 6: 91-94.
- Aghajanzadeh M, Dehnadi A, Ebrahimi H, Fallah Karkan M, Khajeh Jahromi S. Classification And Management Of Subcutaneous Emphysema: A 10-Year Experience. Indian J Surg. 2015; 77: 673-677.
- Valenza F, Chevallard G, Fossali T, Salice V, Pizzocri M, Gattinoni L. Management of mechanical ventilation during laparoscopic surgery. Best Pract Res Clin Anaesthesiol. 2010; 24: 227-241.
- Manchanda G, Bhalotra AR, Bhadoria P, Jain A, Goyal P, Arya M. Capnothorax during laparoscopic cholecystectomy. Indian J Anaesth. 2007; 51: 231-233.
- Yagihashi Y, Okinami T, Fukuzawa S. Case of pharyngeal emphysema with airway obstruction during retroperitoneal laparoscopic nephroureterectomy. Nihon Hinyokika Gakkai Zasshi. 2009; 100: 540-544.
- Chandra A, Clarke R, Shawkat H. Intraoperative hypercarbia and massive surgical emphysema secondary to transanal endoscopic microsurgery (TEMS). BMJ Case Rep. 2014; 2014.
- Sucena M, Coelho F, Almeida T, Gouveia A, Hespanhol V. Massive subcutaneous emphysema - Management using subcutaneous drains. Rev Port Pneumol. 2010; 16: 321-329.
- Peter O’Reilly, Hua Kiat Chen, Rachel Wiseman. Management of extensive subcutaneous emphysema with a subcutaneous drain. Respirology Case Reports. 2013; 1: 28-30.
- Srinivas R, Singh N, Agarwal R, Aggarwal AN. Management of extensive subcutaneous emphysema and pneumomediastinum by micro-drainage: Time for a re-think?. Singapore Med J. 2008; 48: e323-e326.
- Vijayalakshmi AM, Vel MT. Subcutaneous Emphysema. Indian Pediatr. 2003; 11: 1092-1093.
- Lloyd MS, Jankowksi S. Treatment of Life-Threatening Surgical Emphysema with liposuction. Plast Reconstr Surg. 2009; 2: 77e-78e.
- Aslanidis Theodoros, Kontos Athanasios. Surgical Treatment of Subcutaneous Emphysema. In Which Cases?. Journal of Surgery and Emergency Medicine. 2017; 1: 12.