Research Article
Austin J Surg. 2019; 6(11): 1189.
AFP Response and mRECIST for Unrespectable Hepatocellular Carcinoma after Transarterial Chemoembolization Combined with Sorafenib
Tian M¹, Huang G², Li J³ and Zhang Y³*
¹Department of Radiology, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, China
²Digestive Disease Center, The Seventh Affiliated Hospital, Sun Yat-sen University, China
³Department of Interventional Oncology, The First Affiliated Hospital, Sun Yat-sen University, China
*Corresponding author: Yingqiang Zhang, Department of Interventional Radiology, The Seventh Affiliated Hospital, SunYat-sen University, No 628. Zhenyuan Road, Shenzhen, 518107, P.R. China
Received: April 08, 2019; Accepted: May 07, 2019; Published: May 14, 2019
Abstract
Background/Aims: The use of Alpha-fetoprotein (AFP) response in unresectable Hepatocellular Carcinoma (HCC) patients undergoing Transarterial Chemoembolization (TACE) combined with sorafenib has not been rigorously evaluated. We defined potential AFP criteria and compared them with modified Response Evaluation Criteria in Solid Tumors (mRECIST) aimed to: a) validate the prognostic role of AFP response, and b) determine the extent of agreement between AFP and imaging criteria.
Methods: In total, 203 unresectable HCC patients with baseline AFP (> 20ng/mL), who underwent combined TACE with sorafenib therapy, were retrospectively enrolled for AFP-imaging correlation analysis. AFP response was classified as complete response, normalization of AFP; partial response, > 50% decrease from baseline; stable disease, -50% to +30% change from baseline; or progressive disease, > 30% increase from baseline. AFP- and mRECIST- based Response Rate (RR) and Disease Control Rate (DCR) was compared, and associations between AFP response and Overall Survival (OS) were evaluated.
Results: The k value for agreement between AFP criteria and mRECIST was 0.47 (moderate), with RR and DCR were 43.3%, 69.0%, and 42.9%, 53.7% (P=0.920, P=0.002), respectively. A higher area under curve for AFP control was observed in receiver operating characteristic curve compared with mRECIST control (0.908 vs. 0.866). The AFP and mRECIST response or control significantly correlated with OS. Both AFP control (Hazard Ratio) [HR]=0.211; 95% CI: 0.132, 0.337; P‹0.001) and mRECIST control was confirmed by multivariate analysis.
Conclusion: The proposed AFP criteria provided accurate predictions in patients with unresectable HCC and positive AFP after TACE combined with sorafenib.
Keywords: Alpha-fetoprotein; Hepatocellular Carcinoma; Response; Sorafenib; Transarterial chemoembolization
Introduction
Hepatocellular Carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related death worldwide [1]. Liver resection, liver transplantation, and percutaneous ablation are the main radical treatments for HCC. However, only 30- 40% of early-stage patients are amenable for such curative therapies, and more than 50% of all HCCs are diagnosed at the unresectable stage [2,3]. Sorafenib, an oral inhibitor of multiple kinases involved in HCC proliferation and angiogenesis, and Transarterial Chemoembolization (TACE) are important and common treatments for most patients with unresectable stage HCC [4-7]. Recently, there has been an increasing focus on combining TACE with sorafenib to potentially improve the efficacy for patients with unresectable/ advanced HCC [8-13].
Conventionally, the treatment response of HCC tumors is assessed radiologically. The Response Evaluation Criteria in Solid
Tumors (RECIST) criteria focus on tumor size, has been widely used in the assessment of tumor response to systemic chemotherapy [14]. Whereas, because they include radiologically enhanced criteria, the European Association for the Study of the Liver (EASL) [15] and modified RECIST (mRECIST) [16] criteria can reliably predict the treatment response and survival in patients with HCC undergoing TACE [17-19]. Further, Liu et al concluded that EASL and mRECIST response were a better predictor for OS than RECIST response in HCC patients treated with combination TACE and sorafenib therapy [20]. Therefore, the enhanced radiologic criteria are widely used to assess the tumor response in patients with HCC.
Changes in serum tumor markers are also important for monitoring anticancer treatment response. Alpha-fetoprotein (AFP) is a universally recognized tumor marker for HCC [21]. The diagnostic and prognostic role of AFP in HCC patients has been confirmed [22- 28]. Memon et al [29] and Personeni et al [30] presented their analysis in Journal of Hepatology in which they conclude that AFP response is a reliable prognostic factor for treatment response and survival in HCC patients undergoing TACE or sorafenib alone, respectively. Recently, TACE combined with sorafenib, as a combination of local and systemic therapy, has been a commonly used treatment in multiple, advanced HCC tumors. Therefore, it is needed to identify whether the same holds true for the combination therapy. We present data to support this concept. Meanwhile, we defined potential AFP criteria and compared them with mRECIST to determine the extent of agreement between the tumor maker criteria and imaging criteria.
Patients and Methods
Patient selection
The study protocol was approved by the ethics committees of the institution. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. Patients with unresectable HCC who underwent TACE combined with sorafenib as initial treatment at our center, between January 2010 and December 2014, were retrospectively analysed. The HCC diagnosis was made according to the EASL guideline [3].
The inclusion criteria were: (a) age between 18-75 years; (b) Barcelona Clinic Liver Cancer stage B or C; (c) Child-Pugh class A or B liver function; (d) Eastern Cooperative Oncology Group (ECOG) performance scores = 2; (e) no previous treatments; (f) HCC with elevated baseline AFP (> 20 ng/mL); and (g) availability of radiologic imaging and serum AFP data. Patients were excluded for any of the following: (a) HCC with normal baseline serum AFP (< 20 ng/mL); (b) inadequate target lesion (diffuse pattern or largest lesion < 1 cm); (c) Child-Pugh class C liver function or massive ascites, esophageal gastric variceal bleeding, or hepatic encephalopathy; (d) obstructive jaundice; (e) secondary malignancy; (f) missing data.
Treatment protocol
The treatment protocol was performed according to our previous report [12,13]. Briefly, 10 to 20 mL lipiodol (Guerbet, Paris, France) was mixed with 20–40 mg epirubicin (Pfizer, New York, USA) to create an emulsion. Depending on the tumor size and liver function, 2–20 mL of the emulsion was infused into the liver tumor through a catheter. Subsequently, embolization using gelfoam was carried out. When blood flow slowed or a vascular cast was observed, the injection was stopped. sorafenib treatment was started 1–3 days after TACE. The initial dose of oral sorafenib was 400 mg given twice daily. Doses were modified depending on the toxicity according to National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) 3.0.
Follow-up
Standard follow-up evaluations protocol of treatment for HCC described previously [13,31] including dynamic contrast-enhanced CT scans and laboratory tests, were performed to evaluate the efficacy at 4-6 weeks after initiation of therapy and every 2 months thereafter. Laboratory tests included hematologic analyses, liver function test, serum AFP assay, and hepatitis serologic test. Complications and adverse events were recorded.
Assessments
Serum AFP levels were measured using a microparticle enzyme immunoassay (Abbott Laboratories, Chicago, IL). AFP response was classified as follows: Complete Response (CR), normalization of AFP; Partial Response (PR), >50% decrease from baseline; Stable Disease (SD), -50% to +30% change from baseline; or Progressive Disease (PD), >30% increase from baseline. Tumor response evaluation criteria were based on radiologic evaluation according to the mRECIST guideline [16] as: CR: disappearance of any intratumoral arterial enhancement in total lesions; PR: =30% decrease in tumor size; SD, neither PR nor PD; or PD: =20% increase in tumor size or the appearance of new lesion(s). The overall response assessment of target and nontarget lesions was determined based on the mRECIST criteria. All measurements were performed by an independent observer (.., who had >15 years of experience) who was blinded to clinical data to minimize the possibility of false categorizations. Whenever response categorization was not obvious, final classification was made by consensus (.. and .., who had >20 years of experience). The interval between AFP measurement and CT scan was up to 2 days.
Because the initial response is a robust predictor for favorable outcome, the concept was applied in the present study. The initial response was defined as the first assessment after initial therapy [32- 34]. Objective response was defined as sum of CR and PR, disease control was a sum of CR, PR and SD. whereas PD was defined nonresponse. The primary endpoint was Overall Survival (OS). OS was defined as the time from the date of treatment initiation until the date of death or last follow-up. The correlation of response and OS was analyzed.
Statistical analyses
All statistical analyses were performed using SPSS software (SPSS version 16.0, SPSS, Chicago, IL). For baseline characteristics, continuous variables are described as medians±standard deviations and categorical variables are expressed as frequencies and percentages. Intermethod agreement between the two methods was assessed using Cohen’s kappa (k) coefficient. A k coefficient >0.75 represented excellent intermethod agreement and a k coefficient of ‹0.21 represented poor intermethod agreement [35]. The Kaplan– Meier method and log-rank test were used to calculate and compare survival differences, respectively. Univariate analyses were performed using the log-rank test. Variables with a P value ‹0.1 were entered into a multivariate analysis using the Cox proportional hazards model to identify risk factors associated with OS. All statistical tests were two-sided, and P ‹0.05 was considered statistically significant.
Results
Study population
Among 577 consecutive newly diagnosed patients with unresectable HCC who underwent TACE plus sorafenib as initial treatment, 374 were excluded because they had normal serum AFP (n=139), diffuse HCC (n=8), Child-Pugh class C liver function (n=63) or massive ascites (n=16), esophageal gastric variceal bleeding (n=10), or hepatic encephalopathy (n=6), obstructive jaundice (n=11); or secondary malignancy (n=10). In addition, 112 patients were excluded because their data were not available. The analysis cohort included 203 patients. The baseline characteristics of all patients are detailed shown in Table 1. The mean duration of sorafenib treatment was 12.8 months (range 1-56 months). The mean follow-up duration was 15.7 months (range 3-63 months).
Characteristics
Number (%)
Age (years)*
51.4 ± 10.9
Sex
Male
197 (97.0)
Female
6 (3.0)
Etiology
Hepatitis B
195 (96.1)
Hepatitis C
1 (0.5)
Other
7 (3.4)
Cirrhosis
Present
161 (79.3)
Absent
42 (20.7)
No. of tumors
5-Jan
99 (48.8)
>5
104 (51.2)
Size of main tumor (cm)*
9.9 ± 3.9
=5
23 (11.3)
10-May
79 (38.9)
>10
101 (49.8)
ECOG
0
49 (24.1)
1
128 (63.1)
2
26 (12.8)
Child-Pugh class
A
191 (94.1)
B
12 (5.9)
BCLC stage
B
84 (41.4)
C
119 (58.6)
PVTT
94 (46.3)
Extrahepatic spread
47 (23.2)
AFP (ng/mL) (range)
24-999999
56 (27.6)
>400
147 (72.4)
*Data represent mean ± SD. AFP: Alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; PVTT: Portal Vein Tumor Thrombus.
Table 1: Baseline patient characteristics.
Response rates, disease control rates and inter method agreements between AFP criteria and mRECIST According to AFP criteria, 37 patients had a CR, 51 had a PR, 52 had SD, and 63 had PD. The AFP objective response rates (ORR) and disease control rates (DCR) were 43.3% and 69.0%. According to mRECIST, 15 patients had a CR, 72 had a PR, 22 had SD, and 94 had PD. The mRECIST ORR and DCR were 42.9% and 53.7%. The ORR between the two criteria were comparable (P=0.920), but the AFP DCR is higher than that in mRECIST (P=0.002). The AUC of AFP disease control was 0.908 (95% CI=0.869, 0.947) (Figure 1A), which was higher than mRECIST disease control (0.866, 95% CI=0.816, 0.917) (Figure 1B). Inter method agreements between the two methods are shown in Table 2. The k value between the AFP criteria and mRECIST was 0.47 (moderate).
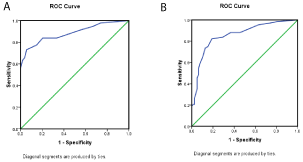
Figure 1: The Receiver Operating Characteristics (ROC) curve for overall survival in AFP disease control and mRECIST disease control. The under the curve
(AUC) was 0.908 for the AFP disease control (A) and 0.866 for the mRECIST disease control (B).
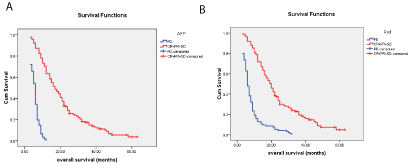
Figure 2: Kaplan-Meier curves showing Overall Survival (OS) for disease control and non-responder.
(A) The median OS was 17.0 months for AFP disease control and 6.0 months for AFP non-responders (P‹0.001).
(B) The median OS was 20.0 months for radiologic disease control and 6.0 months for radiologic non-responders (P‹0.001).
AFP response
CR
PR
SD
PD
Total
mRECIST
CR
12
3
0
0
15
PR
24
40
6
2
72
SD
1
1
16
4
22
PD
0
7
30
57
94
Total
37
51
52
63
203
The k value for agreement between AFP criteria and mRECIST criteria was 0.47. AFP: Alpha-fetoprotein; mRECIST: Modified Response Evaluation Criteria in Solid Tumors; CR: Complete Response; PR: Partial Response; SD: Stable Disease; PD: Progressive Disease.
Table 2: Inter method agreement between the AFP criteria and mRECIST criteria.
Independent risk factors for survival
On univariate analysis, the number of tumors, tumor size, AFP level, AFP control, mRECIST control and Barcelona Clinic Liver Cancer (BCLC) stage were significantly associated with OS. On multivariate analysis, AFP disease control (hazard ratio [HR]=0.211; 95% CI: 0.132, 0.337; P‹0.001). mRECIST disease control (HR=0.493; 95% CI: 0.323, 0.754; P=0.001), and BCLC stage (HR=2.018; 95% CI: 1.388, 2.933; P‹0.001) were independent prognostic factors for OS in Table 3.
Univariate Analysis
Multivariate Analysis
Variable
Median OS (mo)
95% CI
P
Hazard Ratio
95% CI
P
Tumor size
0.014
0.657
> 10 cm
8
6.7, 9.3
1
=10 cm
17
14.0, 20.0
0.932
0.686, 1.269
No. of Tumors
< 0.001
0.069
19
16.0, 22.0
1
> 5
8
6.4, 9.6
1.398
0.974, 2.005
AFP level (ng/mL)
0.001
0.992
>400
9
7.6, 10.4
1
= 400
22
17.6, 26.4
0.998
0.685, 1.455
BCLC stage
<0.001
<0.001
B
22
19.5, 24.5
1
C
8
6.6, 9.4
2.018
1.388, 2.933
AFP response
< 0.001
<0.001
Non-responder
6
5.2, 6.8
1
Disease control
17
14.1, 19.9
0.211
0.132, 0.337
mRECIST response
< 0.001
0.001
Non-responder
6
5.2, 6.7
1
Disease control
20
17.3, 22.7
0.493
0.323, 0.754
AFP: Alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; CI: Confidence Interval; mRECIST: modified Response Evaluation Criteria in Solid Tumors; OS: Overall Survival.
Table 3: Univariate and multivariate analysis of prognostic factors.
Discussion
For the assessment of HCC treatment, the size and enhancement criteria on radiological imaging are reliable predictors of therapy response and survival, and their role in selecting a treatment strategy is irreplaceable. However, the radiologic criteria present some limitations including inter observer subjectivity, variable enhancement, increased patient exposure to radiation, and misinterpretation because of regenerative or dysplastic nodules, or perfusion abnormalities, especially in multiple, advanced tumors [25]. The tumor biomarker AFP can provide an objective reflection of tumor activity [21-24]. Therefore, AFP-based criteria might offer a simple and potentially less subjective method of assessing tumor response.
In the present study, we propose the AFP criteria for assessing the efficacy of combination therapy in unrespectable HCC. First, we found that, in patients with unrespectable HCC undergoing TACE plus sorafeinb, the AFP response was highly associated with the mRECIST response (n=88 vs. 87), and was a good predictor of survival. These findings are congruent with those concluded in previous studies [25- 30]. Second, the inter method agreement is moderate between AFP criteria and mRECIST (k=0.47), which mainly account for 39.4% (n=37) patients with AFP PR (n=7) or SD (n=30) were assigned to radiologic PD (n=94). Most of those patients were appeared as a new lesion or pulmonary metastasis after therapy, while the primary tumor obtained a response. Another cause for the moderate agreement was 33.3% (n=24) patients with AFP CR were assigned to radiologic PR (n=72), which most of patients with AFP was less than 200 ng/ mL. Generally, lower AFP level associated with higher response and survival rate [36]. When radiologic partial response was detected, accordingly the AFP level was decreased to normalization easily. Therefore, this is not contradictory with that this therapy is effective for those patients. Third, we found the AFP control was higher than them in mRECIST (n=143 vs. 109). There are likely explanations for this phenomenon: 37 of 94 radiologic PD patients achieved AFP PR (n=7) or SD (n=30). Based on mRECIST criteria, if a new lesion or metastasis were detected, the overall radiological response was classified as PD, ignoring any primary tumor response. We know that the post-TACE may promote tumor growth and metastasis due to upregulation of circulating vascular endothelial growth factor [37- 39]. Although a new lesion or metastasis lesion might have appeared as a result of this therapy, the primary tumor might have shown a control response, therefore decreasing tumor burden, and, in parallel, decreasing or stabilizing serum AFP and such patients underwent subsequent therapy to treat any new lesions. Therefore, this therapy might prolong the survival time in those patients.
In our study, the cut-off values were selected based on previously published researches. We collected data from published studies that used AFP as a measure of response to therapy. In the present study, which examined response to combination therapy of TACE and sorafenib, we defined patients with a >50% decrease in baseline AFP as AFP responders. Using this cut-off as an exploratory value, the number of patients who were AFP responders was almost equivalent to the number of mRECIST responders (n=88 vs. 87). By contrast, if we had used a >20% decrease to define AFP response, there would have been more AFP responders than mRECIST responders would (n=105 vs.87). Because there have no published cut-off value for AFP progression, we explored using a 30% increase as an indicator of progression, which led us to identify a subgroup with a relatively better survival in terms of radiologic progression. Additionally, in the present study, we chose to use the mRECIST as reference criteria because they measure unidimensional enhanced lesions and are easy to use. Furthermore, previous studies reported that mRECIST had good accuracy for predicting survival in patients treated with TACE [19,34].
The present study has several limitations. First, it is a retrospective analysis and the data came from a single center. The reason we analyzed data from the single center is that the success of TACE strongly depends on the applicator’s experience. This center had the largest population of liver cancer and had considerable experience with the particular TACE procedure in the South China. However, HCC with positive AFP was occurred in a portion of patients, the appraisal of AFP criteria is accurate, and it can potentially apply to the majority of HCC patients.
In conclusion, this study suggests that the proposed AFP criteria provided accurate predictions in patients with unrespectable HCC and positive AFP after TACE combined with sorafenib. With the era of targets agents, which mainly demonstrate a stable disease, therefore, the AFP criteria defined by our team might identify a subgroup of patients with real and objective biological response. The AFP criteria warrant further prospective validation in large trails.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58: 71-96.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005; 42: 1208-1236.
- European Association for The Study of The Liver. European Organization for Research and Treatment of Cancer EASL-EORTC clinical practice guidelines: management ofhepatocellular carcinoma. J Hepatol. 2012; 56: 908-943.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359: 378-390.
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10: 25-34.
- Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarteriallipiodol chemoembolization forum resectable hepatocellular carcinoma. Hepatology. 2002; 35: 1164-1171.
- Llovet JM, Bruix J. Systematic review of randomized trials for unrespectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003; 37: 429-442.
- Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unrespectable hepatocellular carcinoma. J Hepatol. 2012; 56: 1336-1342.
- Cabrera R, Pannu DS, Caridi J, Firpi RJ, Soldevila-Pico C, Morelli G, et al. The combination of sorafenib with transarterialchemoembolisation for hepatocellular carcinoma. Alimen Paharmacol Ther. 2011; 34: 205-213.
- Zhao Y, Wang WJ, Guan S, Li HL, Xu RC, Wu JB, et al. Sorafenib combined with transarterialchemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patients. Ann Oncol. 2013; 24: 1786-1792.
- Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014; 272: 284-293.
- Zhang Y, Fan W, Wang Y, Lu L, Fu S, Yang J, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: A retrospective analysis. Oncologist. 2015; 20: 1417-1424.
- Li J, Zhang F, Yang J, Zhang Y, Wang Y, Fan W, et al. Combination of individualized local control and target-specific agent to improve unresectable liver cancer managements: a matched case control study. Target Oncol. 2015; 10: 287-295.
- Therasse P, ARbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92: 205-216.
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001; 35: 421-430.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30: 52-60.
- Jung ES, Kim HJ, Yoon EL, Lee HJ, Lee SJ, Suh S, et al. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Hepatol. 2013; 58: 1181-1187.
- Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST response are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial chemoembolization. J Hepatol. 2011; 55: 1309-1316.
- Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012; 262: 708-718.
- Liu L, Wang W, Chen H, Zhao Y, Bai W, Yin Z, et al. EASL- and mRECIST-Evaluated responses to combination therapy of sorafenib with transarterial chemoembolization predict survival in patients with hepatocellular carcinoma. Clin Cancer Res. 2014; 20: 1623-1631.
- Mclntire KR, Vogel CL, Princler GL, Patel IR. Serum alpha-fetoprotein as a biochemical marker for hepatocellular carcinoma. Cancer Res. 1972; 32: 1941-1946.
- Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J ClinGastroenterol. 2000; 31: 302-308.
- Baig JA, Alam JM, Mahmood SR, Baig M, Shaheen R, Sultana I, et al. Hepatocellular carcinoma (HCC) and diagnostic significance of alpha-fetoprotein (AFP). J Ayub Med Coll Abbottabad. 2009; 21: 72-75.
- Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J SurgOncol. 2013; 11: 212.
- Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial alpha-fetoprotein measurement in prediting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009; 27: 446-452.
- Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009; 27: 5734-5742.
- Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan ST, et al. The significance of early Alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist. 2011; 16: 1270-1279.
- Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010; 116: 4590-4596.
- Memon K, Kulik L, Lewandowski RJ, Wang E, Ryu RK, Riaz A, et al. Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol. 2012; 56: 1112-1120.
- Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, et al. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012; 57: 101-107.
- Zhang Y, Huang G, Wang Y, Liang L, Peng B, Fan W, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients down staged by transarterial chemoembolizarion? Ten years of experience. Oncologist. 2016; 21: 1442-1449.
- National Cancer Institute. Common terminology criteria for adverse events, version 3.0.
- Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Heaptol. 2015; 62: 1304-1310.
- Kim CJ, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Radiologic response to transcatheter hepatic arterial chemoembolization and clinical outcomes in patients with hepatocellular carcinoma. Liver Int. 2014; 34: 305-312.
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005; 37: 360-363.
- Xu L, Peng ZW, Chen MS, Shi M, Zhang YJ, Guo RP, et al. Prognostic nomogram for patients with unrespectable hepatocellular carcinoma after transcatheter arterial Chemoembolization. J Hepatol. 2015; 63: 122-130.
- Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, et al. Association between increment of serum VEGF level and prognosis after trans catheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008; 99: 2037-2044.
- Jeong Y, Yoon SM, Han S, Shim JH, Kim KM, Lim YS, et al. Propensity score matching analysis of changes in alpha-fetoprotein levels after combined radiotherapy and trans arterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus. PLoS One. 2015; 10: e0135298.
- Liu L, Zhao Y, Jia J, Chen H, Bai W, Yang M, et al. The Prognostic Value of Alpha-Fetoprotein Response for Advanced-Stage Hepatocellular Carcinoma Treated with Sorafenib Combined with Trans arterial Chemoembolization. Sci Rep. 2016; 6: 19851.