
Research Article
Austin J Surg. 2019; 6(15): 1200.
Evaluation of Gram-Positive and Gram-Negative Bacterial Adherence on Four Different Vascular Prosthetic Grafts In Vitro
Ruemke S1,2*, Rubalskii E1,2, Mashaqi B1, Burgwitz K1,2, Haverich A1,2, Salmoukas C1,2 and Kuehn C1,2
¹Department of Cardiac, Thoracic, Transplantation and Vascular Surgery, Hannover Medical School, Germany
²Lower Saxony Centre for Biomedical Engineering, Implant Research and Development, Germany
*Corresponding author: Ruemke S, Department of Cardiac, Thoracic, Transplantation and Vascular Surgery, Hannover Medical School, Carl-Neuberg-Str. 1, D-30625 Hannover, Germany
Received: June 13, 2019; Accepted: July 19, 2019; Published: July 26, 2019
Abstract
Objectives: The aim of this study was to evaluate the adherence capability of clinically used vascular grafts (Uni-Graft® KD V, Silver Graft, Gelweave™ and Gore® Propaten®) against Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa and to assess efficacy of the graft impregnation with vancomycin/gentamicin solution.
Methods: Each graft type was cut into 1cm2 samples and was separately contaminated with suspensions of bacteria mentioned above. Experimental group of grafts was tested after antibiotic impregnation; the controls remained without any impregnation. After 24h incubation OD600nm measurement of the supernatant solution and CFU count of the adhered bacteria were performed.
Results: Antibiotic impregnation led to a significantly reduced OD600nm after incubation for all grafts and bacteria. Untreated grafts contained on their surface more than 105 CFU/cm2. Antibiotic treatment completely prevented all grafts from S. epidermidis adhesion.
S. aureus: After antibiotic impregnation Uni-Graft® KD V, Gelweave™ and Silver Graft were sterile. Gore® Propaten® showed no significant reduction of adhesion.
E. coli: After previous impregnation of Silver Graft and Uni-Graft® KD V remained sterile, whereas the Gelweave™ and Gore® Propaten® showed a nonsignificant reduction of bacterial count.
P. aeruginosa: Antibiotic impregnation led to significant reduction of adhered bacteria for Uni-Graft® KD V and Silver Graft. Gelweave™ and Gore® Propaten® showed no significant differences in CFU count.
Conclusion: This study showed that the antibiotic impregnation of vascular grafts with vancomycin/gentamicin solution is a feasible and easy to implement prophylactic measure to prevent Gram-positive and Gram-negative bacterial growth and adhesion to cardiovascular prosthetic materials.
Keywords: Vascular graft; Antibiotic impregnation; Bacterial adherence
Abbreviations
CFU: Colony Forming Units; OD: Optical Density; SD: Standard Deviation; CI: Confidence Interval; PET: Polyethylene Terephtalat; ePTFE: Expanded Polytetrafluorethylene; TSB: Tryptic Soy Broth; TSA: Tryptic Soy Agar
Introduction
Bacterial colonization and infection of implanted devices, especially vascular prosthetic grafts, is a feared complication due to the life-threatening consequences, significantly increasing patient mortality and morbidity. The incidence of prosthetic vascular graft infection ranges from 1-6%, depending on the location and type of the graft [1-3].
Infections can be divided into early and late vascular graft infections. In early vascular graft infection, S. aureus and coagulasenegative staphylococci (e.g. S. epidermidis) account for approximately 60-70% of VGI cases, while Gram-negative species like Pseudomonas aeruginosa and Escherichia coli are also common [4]. An early infection on a medical implant is initiated by bacterial adhesion on the graft surface. A major hindrance to treatment of graft infections is bacterial biofilm formation on the graft surface. Biofilms are a community of bacteria that adhere to each other and are embedded in a self-produced extracellular matrix that is highly resistant to host immune defenses and the penetration of antibiotics [5,6].
For this in vitro study, we tested four of the most common Gram-positive and Gram-negative bacteria involved in vascular graft infection on four different, clinically used vascular prosthetic grafts, in order to investigate possible differences in the amount of bacterial adherence on the different graft surfaces and to provide recommendations for the individual clinical use.
Furthermore we investigated, if an antibiotic impregnation of the grafts is a suitable method in preventing bacterial adherence to the graft materials.
Materials and Methods
Antibiotic agents
An antibiotic solution consisting of vancomycin and gentamicin was prepared. For this purpose, 1 g of vancomycin (Hikma Farmaceutica S.A., Terrugem, Portugal) was dissolved in 125 ml of 0.9% NaCl (B. Braun, Melsungen, Germany) and thus a solution with the concentration of 8 mg/ml vancomycin was prepared. Three ampoules of gentamicin (Ratiopharm, Ulm, Germany) (240 mg/6 ml) were mixed with 94 ml of 0.9% NaCl and thus reached a concentration of 2.4 mg/ml. Subsequently, both solutions were mixed in the ratio 1:1 and reached a final concentration of 4 mg/ml vancomycin and 1.2 mg/ml gentamicin. The solution was made under sterile conditions and according to the manufacturer’s instructions.
Bacterial strains
The bacterial strains used in this study were 20231DSMZ for Staphylococcus aureus, 20044DSMZ for Staphylococcus epidermidis, 1103DSMZ for Escherichia coli and 19880DSMZ for Pseudomonas aeruginosa. The bacteria were sub-cultured on tryptic soy agar (Oxoid, Hampshire, UK) according to the recommendations of the German Collection of Microorganisms and Cell Cultures GmbH (www.dsmz.de). Dilution of the overnight cultures was performed with tryptic soy broth (TSB). The optical density (OD600nm) used for graft contamination was 0.2. This OD corresponds to approximately 1 × 108 colony-forming units (CFUs) per millilitre for S. aureus, 1 × 106 CFUs per mL for S. epidermidis, 1 × 107 CFUs per mL for E. coli and 1 × 107 CFU per mL for P. aeruginosa.
Vascular grafts
The following four vascular graft prostheses were used (Table 1): Knitted double-velour vascular grafts made of fine polyester fibres [polyethylene terephthalate (PET; Dacron)] and impregnated with absorbable bovine gelatine (Uni-Graft®, B. Braun/Aesculap, Tuttlingen, Germany); double-velour vascular grafts made of fine polyester fibres (PET), impregnated with absorbable bovine gelatine (polygeline), coated with 0.07 – 0.16 mg/cm² silver on the outer graft surface (Silver Graft, B. Braun/Aesculap, Tuttlingen, Germany); gelatine sealed, woven polyester grafts (Gelweave™, Vascutek Terumo, Inchinnan, Renfrewshire, Scotland UK) and Gore® Propaten® grafts, made of expanded polytetrafluoroethylene (ePTFE) coated with heparin on the luminar surface (W. L. Gore & Associates, Inc., Flagstaff, Arizona, USA). All grafts were processed to 1 × 1 cm pieces under aseptic conditions.
Material
Special properties
Uni-Graft® KD V
Knitted double velour prosthesis made of PET fibers
Bovine gelatine coating
Gelweave™
woven PET prosthesis
Gelatine sealant
Silver Graft
Knitted double velour prosthesis made of PET fibers
Polygelin coating, Silver coating (0.07-0.16 mg/cm²)
Gore® Propaten®
ePTFE prosthesis
Intraluminal heparine surface
Table 1: Overview over the tested graft materials.
Methods
The first step involved the determination of an antibiotic solution suitable for the impregnation of the grafts. Various antibiotics and antibiotic mixtures were evaluated for this purpose (data not shown). The antibiotic impregnation should be effective against both Grampositive and Gram-negative pathogens. A solution of Vancomycin and Gentamicin was chosen, as it showed the required antimicrobial properties.
Antibiotic impregnation was performed by bathing the graft materials for 20 minutes in the prescribed Vancomycin/Gentamicin solution.
The amount of antibiotic solution taken up was determined for each graft material. Therefore 5 graft pieces of each graft material (n=20) were weighed before bathing for 20 minutes in the Vancomycin/Gentamicin solution. After that procedure the grafts were weighed again and weight difference was calculated.
Sample cultivation and bacterial count
After inoculation with 4 mL of the bacterial suspension (optical density (OD600nm): 0.2 ± 0.02), grafts were incubated at 37°C for 24h. OD600nm of the solution was measured afterwards. In the next step all grafts were separately harvested in sterile 5 mL phosphate buffered saline (PBS) in 15 mL falcon tubes and underwent sonication (Elma, Singen, Germany) for 20 minutes at 37°C to release viable adherent bacteria. Afterwards, decimal dilution steps (4 steps for S. epidermidis, S. aureus and E. coli and 5 for P. aeruginosa) were preformed and 100 ml of PBS–bacteria-solution were plated on TSA-covered Petri dishes. Subsequently incubation was done for 24 h at 37°C. CFUs were counted after that procedure (Figure 1).
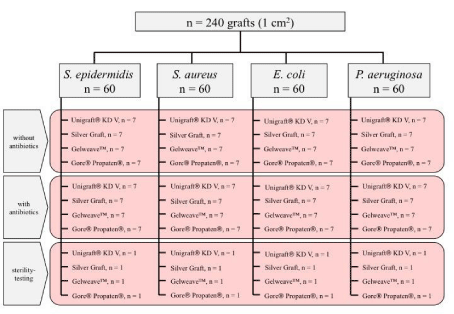
Figure 1: Schematic overview of our experimental study protocol.
Statistical analysis
All analyses were performed and graphs were created with Graphpad Prism 7 (Graphpad Software Inc. La Jolla, USA). Quantitative results are presented as arithmetic mean +/- standard deviation (SD). Additionally, 95% Confidence Intervals (CI) were calculated. Comparisons between the groups were performed using two-way ANOVA-test with Bonferroni correction. For comparison within one group one-way ANOVA-test with Bonferroni correction was used. Differences at p ‹ 0.05 were considered statistically significant.
Results and Discussion
Staphylococcus epidermidis
In the group without antibiotic impregnation (n=28) OD600nm of the TSB solution was measured (Figure 2) after 24h of incubation: 2.41 ± 0.534 for Uni-Graft® KD V, 2.45 ± 0.325 for Silver Graft, 2.44 ± 0.226 for Gelweave™ and 3.29 ± 0.564 for Gore® Propaten® The difference between Gore® Propaten® and the other three graft materials was significant (p‹0.01). After previous antibiotic impregnation OD600nm decreased for all graft types significantly (p‹0.001). Within the group differences were not significant (Uni-Graft® KD V 0.05 ± 0.003; Silver Graft 0.05 ± 0.005; Gelweave™ 0.04 ± 0.013; Gore® Propaten® 0.11 ± 0.013).
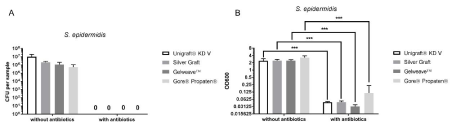
Figure 2: Number of CFU (A) and OD600nm (B) for the graft materials (n=56) tested with S. epidermidis. (***: p‹0.001).
In the group without antibiotic pretreatment (n=28) Uni-Graft® KD V showed significantly more CFU (Figure 2) than the other materials (p‹0.01). The differences between the other graft materials were not significant. After antibiotic impregnation, all samples remained sterile.
Staphylococcus aureus
Optical density (OD600nm) of the TSB - S. aureus solution without antibiotic (Figure 3) pretreatment after 24h of incubation: Uni-Graft® KD V 3,.69 ± 0.317, Silver Graft 4.05 ± 0.092, Gelweave™ 4.09 ±0.196, Gore® Propaten® 4.17 ± 0.551. Only the difference between Uni-Graft® KD V and Gore® Propaten® was significant (p<0.05).
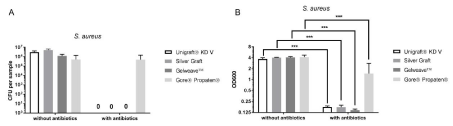
Figure 1: Number of CFU (A) and OD600nm (B) for the graft materials (n=56) tested with S aureus. (***: p‹0.001).
After previous impregnation Uni-Graft® KD V (0.18 ± 0.012), Silver Graft (0.17 ± 0.023) and Gelweave™ (0.15 ± 0.009) prosthesis types showed comparable results, while for Gore® Propaten® (1.47 ± 1.343) a significantly higher (p = 0.001) OD600nm was measured. Compared to Gelweave™ the difference was also significant (p‹0.001).
CFU counting showed the following results (without antibiotic impregnation): most CFU were found in the samples of the Silver Graft prosthesis (Figure 3). The number of CFU differed significantly from the number of CFU counted for Uni-Graft® KD V (p = 0.01). Uni-Graft® KD V showed the second highest value, followed by Gelweave™ and Gore® Propaten®. The difference between Silver Graft and Gelweave™, as well as the difference between Silver Graft and Gore® Propaten®, was highly significant (p ‹0.001). The difference between Gore® Propaten® and Uni-graft® KD V was also significant (p‹0.01).
After previous antibiotic impregnation all samples remained sterile, except for the samples from Gore® Propaten® (p‹0.001). For Gore® Propaten® antibitotic impregnation led to no significant reduction of CFU (p›0.99).
Escherichia coli
OD600nm in the group without antibiotic impregnation (Figure 4) after 24h of incubation: Uni-Graft® KD V 9.99 ± 0.700, Silver Graft 12.19 ± 0.763, Gelweave™ 11.90 ± 0.199, Gore® Propaten® 7.29 ± 0,171. For Gore® Propaten® in comparison to the other three materials a significant lower optical density was measured (p‹0.001).
Figure 4: Number of CFU (A) and OD600nm (B) for the graft materials (n=56) tested with E.coli (***: p‹0.001).
OD600nm after antibiotic impregnation and 24h of incubation: Uni-Graft® KD V 0.47 ± 0.09, Silver Graft 0.28 ± 0.03, Gelweave™ 0,69 ± 0,25, Gore® Propaten® 3.23 ± 0.71. The OD600nm was significantly lower (p‹0.001) after previous impregnation for all materials. The comparison between the materials however showed a significant higher (p‹0.001) OD600nm for Gore® Propaten® compared to the other three materials.
Without antibiotic impregnation Silver Graft and Uni-Graft® KD V showed the most CFU, whereas Gelweave™ showed the least number of CFUs (Figure 4). The antibiotic-treated samples of Unigraft ® KD V and Silver Graft remained sterile after incubation. Gore® Propaten® and Gelweave™ showed a significantly higher CFU count (p‹0.001).
Pseudomonas aeruginosa
Optical density of the TSB – P.aeruginosa solution (Figure 5) without antibiotic impregnation: Uni-Graft® KD V 9.62 ± 0.657, Silver Graft 10.57 ± 0.581, Gelweave™ 10.93 ± 1.745, Gore® Propaten® 8.49 ± 0.714. Differences between Gore® Propaten® and Silver Graft (p‹0.01) as well as Gore® Propaten® and Gelweave™ (p‹0.001) were significant.
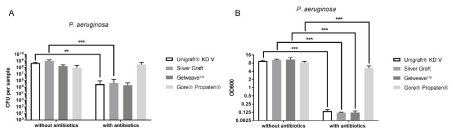
Figure 5: Number of CFU (A) and OD600nm (B) for the graft materials (n=56) tested with P.aeruginosa (**: p= 0.002; ***: p‹0.001).
After previous impregnation the following OD600nm was measured: Uni-Graft® KD V 0.14 ± 0.016, Silver Graft 0.12 ± 0.004, Gelweave™ 0.12 ± 0.018 and Gore® Propaten® 5.32 ± 1.028. The difference between Gore® Propaten® and the other graft materials was significant (p‹0.001), but antibiotic impregnation reduced OD600nm for all materials significantly.
Without antibiotic impregnation most CFU (Figure 5) were found in the samples of Silver graft and Uni-graft® KD V. The number of CFU counted for Silver graft was significantly higher than the CFU number counted for Uni-graft® KD V (p=0.001). Gelweave™ and Gore® Propaten® had significantly less CFU (p‹0.001) than Silver graft. In absolute terms, Gore® Propaten® produced the least CFU.
Antibiotic impregnation reduced CFU number significantly for Uni-graft® KD V (p=0.002) and Silver Graft (p‹0.001). However a non-significant reduction of CFU was observed for Gelweave™. For the Gore® Propaten® samples no reduction of CFU was observed.
Amount of absorbed antibiotic solution
The highest measured weight difference was calculated for the Unigraft® KD fragments (n=5) (51.7 ± 9.5 mg), followed by Gelweave™ (n=5) (47.0 ± 4.1 mg) and Silver Graft (n=5) (40.2 ± 10.4 mg). The Gore® Propaten® fragments (n=5) (14.3 ± 9.2 mg) showed the lowest weight difference. The difference between Gore® Propaten® and Unigraft® KD V was significant (p ‹0.001). The differences between Gelweave ™ and Gore® Propaten® (p ‹0.001), and between Silver graft and Gore® Propaten® (p ‹0.01) were also significant.
A broad spectrum of bacterial pathogens is causally involved in the development of vascular prosthesis infections. Both a number of Gram-positive bacteria, in particular Staphylococci, and Gram-negative pathogens are able to colonize various prosthetic surfaces [4].
In 1987, Anthony G. Gristina used the term “Race to the Surface” to describe the competition between tissue cells and bacteria around the surfaces of implants. The colonization of an implant with tissue cells leads to a sufficient immune defence and thus complicates a possible colonization by bacteria.
The result of this competition depends largely on the surface and material composition of the respective implant [7]. This fact led to the consideration that bacterial adhesion to vascular prosthesis materials is affected by two major factors: the nature of the prosthetic material and the adhesive properties of the bacteria.
One aim of this work was to investigate this interaction between bacteria and vascular graft materials in vitro. For this purpose, an in vitro model was established, which allowed the quantitative determination of the adherent bacteria on various prosthesis materials.
As a number of previous studies have already demonstrated the efficacy of antibiotic pre-treatment of prosthetic materials [8- 10], it was tested to what extent an antibiotic pre-treatment with vancomycin/gentamcin solution is able to reduce the number of adherent bacteria. In preliminary testing’s, the combination of vancomycin and gentamicin showed excellent antimicrobial properties against Gram-negative and Gram-positive bacteria and was therefore selected as impregnation solution.
Bacterial adhesion to the four types of prosthesis was studied in two subgroups, the first without antibiotic impregnation and the second with antibiotic impregnation. The quantitative bacterial adhesion to the prosthetic fragments was determined and compared. This was done by counting of the CFUs. In addition, after 24 hours of incubation of the prosthesis fragments in TSB bacteria suspension, the optical density (OD600nm) of the suspension was measured. The increase in optical density allowed conclusions to be drawn about the quantitative increase of bacteria during the 24-hour incubation interval.
Significantly lower OD600nm measurements were obtained for all samples after previous antibiotic impregnation of the prostheses. The impregnation proved to be effective against both Gram-positive and Gram-negative bacteria. Comparing the three PET prostheses with the PTFE prosthesis, the samples of the PET prostheses showed lower optical density values. At the same time, the measured weight differences (prior and after impregnation) were higher for PET prostheses than for PTFE prosthesis. Consequently, the PET materials absorbed more vancomycin/gentamicin solution than the PTFE prosthesis. Due to their woven or knitted fibre structure, the PET prostheses have better absorbing properties than the PTFE-prosthesis made of membranes. The amount of vancomycin/gentamicin solution absorbed before incubation is related to the subsequently measured optical density after completion of the incubation.
For the non-impregnated prosthesis fragments, measurements of the optical density of the bacterial TSB suspension after completion of the incubation interval showed comparable values. The differences between the individual materials were not significant. This fact supports the idea, that the materials themselves have no significant influence on the growth of the bacteria. Noteworthy is that an inhibition of bacterial growth by the Silver graft prosthesis, as previously described by Ueberrueck et al. [11] was not detectable in our testings. The CFU count for the impregnated prostheses did not show any significant differences between the tested materials in all the bacteria tested. In the case of S.epidermidis, Unigraft® KD V showed the most CFU, while Gore® Propaten® had the least CFU. A similar tendency was also observed for S. aureus. This lower adhesion of S.aureus to PTFE materials compared to PET materials was already previously described by Rosenman et al. [12].
The counting of CFU for the Gram-negative bacteria E.coli and P.aeruginosa showed an equivalent CFU number for all materials. This shows that the examined Gram-positive cocci adhere worse to smooth PTFE surfaces than P. aeruginosa and E. coli.
Among the tested non-impregnated PET grafts, Gelweave™ showed the lowest CFU counts. Although these differences were not significant, the structural design of the Gelweave™ prosthesis could have little effect on the number of CFU. The prosthesis, in contrast to the other two PET prostheses, consists of woven fibres, while Unigraft® KD V and Silver Graft are manufactured in a knitting process. The same observation was made by Schmitt et al. [13] who also noted a difference in bacterial adhesion between woven and knitted PET prostheses.
The result of the CFU counting after vancomycin/gentamicin impregnation impressively demonstrated the efficacy of this prophylactic measure. Thus, in the case of S. epidermidis, all materials remained free from adherent bacteria. A similar picture emerged when looking at the results of S.aureus. Again, the previously impregnated PET prostheses showed no CFU. Gore® Propaten®, on the other hand, showed a nearly identical value compared to the sample without impregnation. This fact also supports the assumption that the lack of absorbing properties of Gore® Propaten® results in insufficient antibiotic impregnation. Comparing the previously measured optical density values with the finally counted CFU, both data sets support this hypothesis.
The counting of CFU for E. coli showed sterile samples for Unigraft® KD V and Silver graft, while Gore® Propaten® and Gelweave™ showed significantly (p ‹0.001) more CFU. While in Gore® Propaten® a lack of uptake of the vancomycin/gentamicin solution could be responsible for the high CFU count, this theory is not transferable to Gelweave™. The Gelweave™ prosthesis received a comparable amount of antibiotic solution as Unigraft® KD V. As previously suggested, the different manufacturing process or the gelatin-coated prosthetic surface could be responsible for this effect.
The Analysis of CFU of P.aeruginosa after impregnation resulted in a significant change for only two out of the four investigated materials. Thus, the CFU numbers of Silver graft (p ‹0.001) and Unigraft® KD V (p = 0.002) were significantly reduced. The differences were not significant for Gelweave™ and Gore® Propaten® compared to their non-impregnated equivalent. Although the reduction in CFU was significant for two of the four prosthetic materials, none of the samples remained sterile. This may be indicative for limited efficacy of the antibiotic solution against P. aeruginosa.
Conclusion
In the present study, it could be shown that the antibiotic impregnation with vancomycin/ gentamicin solution is a good and easy to implement prophylactic measure to prevent bacterial growth and bacterial adhesion to cardiovascular prosthetic materials in vitro. It should also be mentioned that some graft materials are harder to impregnate than others because of their composition. This was particularly evident in the case of Gore® Propaten®. Since this prosthesis material is mainly used for prostheses of smaller calibre and therefore has to have a smooth, low thrombogenic surface, this fact is not surprising. It also turned out that Gram-negative pathogens, in particular P. aeruginosa, respond worse to the antibiotic combination of vancomycin/gentamicin. Gram-positive pathogens that cause the vast majority of vascular prosthesis infections, however, have been very successfully reduced by the impregnation. This work forms the basis for further in vivo studies that should be performed to improve prophylactic measures to reduce the incidence of vascular prosthesis infections.
Acknowledgement
We thank Mrs. Karin Burgwitz for help with the microbiological procedures.
References
- Teebken OE, Pichlmaier MA, Brand S, Haverich A. Cryopreserved arterial allo- grafts for in situ reconstruction of infected arterial vessels. Eur J Vasc Endovasc Surg. 2004; 27: 597-602.
- Batt M, Magne JL, Alric P, Muzi A, Ruotolo C, Ljungstrom KG, et al. In-situ revascularization with silver-coated polyester grafts to treat aortic infection: early and mid-term results. J Vasc Surg. 2003; 38: 983-989.
- Bisdas T, Bredt M, Pichlmaier M, Aper T, Wilhelmi M, Bisdas S, et al. Eight-year experience with cryopreserved arterial homografts for the in-situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010; 52: 323- 330.
- Wilson WR, Bower TC, Creager MA, Amin-Hanjani S, O’Gara PT, Lockhart PB, et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Peripheral Vascular Disease; and Stroke Council. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016; 134: e412-e460.
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat.Rev.Microbiol. 2004; 2: 95-108.
- Gandelman G, Frishman WH, Wiese C, Green-Gastwirth V, Hong S, Aronow WS, et al. Intravascular device infections: epidemiology, diagnosis, and management. Cardiol Rev. 2007; 15:13-23.
- Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987; 237: 1588-1595.
- Bisdas T, Beckmann E, Marsch G, Burgwitz K., Wilhelmi M, Kuehn C, et al. Prevention of vascular graft infections with antibiotic graft impregnation prior to implantation: in vitro comparison between daptomycin, rifampin and nebacetin. Eur J Vasc Endovasc Surg. 2012; 43: 448-456. doi: 10.1016/j. ejvs.2011.12.029. Epub 2012 Jan 20.
- Haverich A, Hirt S, Karck M, Siclari F, Wahlig H. Prevention of graft infection by bonding of gentamycin to Dacron prostheses. J Vasc Surg. 1992; 15: 187- 193.
- White JV, Benvenisty AI, Reemtsma K, Voorhees AB, Fox CL, Modak S, et al. Simple methods for direct antibiotic protection of synthetic vascular grafts. J Vasc Surg. 1984; 1: 372-380.
- Ueberrueck T, Zippel R, Tautenhahn J, Gastinger I, Lippert H, Wahlers T. Vascular graft infections: in vitro and in vivo investigations of a new vascular graft with long-term protection. Biomed Mater Res B Appl Biomater. 2005; 74: 601-607.
- Rosenman JF, Pearce WH, Kempczinski RF. Bacterial adherence to vascular grafts after in vitro bacteremia. Journal of Surgical Research 1985; 38: 648- 655.
- Schmitt DD, Bandyk DP, Pequet AJ, Towne JB. Bacterial adherence to vascular prostheses - A determinant of graft infectivity. J Vasc Surg. 1986; 3: 732-740.