
Special Article – Surgery Case Reports
Austin J Surg. 2019; 6(15): 1201.
Resuscitation of Penetrating Placental Hemorrhage by Massive Transfusion
Guangyi L#, Hengjing Z#, Mingyong H, Min J, Zuofeng W, Yanhong L and Shan O*
Department of Anesthesiology, Chengdu First People’s Hospital, China
#Lai Guangyi and Zou Hengjing contributed equally to this study
*Corresponding author: Ou Shan, Department of Anesthesiology, Chengdu First People’s Hospital, No.18 Wanxiang North Road, Chengdu 610041, China
Received: June 17, 2019; Accepted: July 23, 2019; Published: July 30, 2019
Abstract
Background: Placenta implantation is one of the common causes of obstetric hemorrhage. Prior to the effective hemostasis by obstetricians, the main measures for treating obstetric hemorrhage are blood transfusion and prevention of Disseminated Intravascular Coagulation (DIC). How to reasonably use blood products to maintain effective circulation and coagulation function is the key and difficult point of treatment.
Method: We reported one patient with central placenta previa combined with penetrating placenta implanted into the bladder, with total blood loss exceeding 10,000 ml during the operation.
Results: We successfully saved the life of the patient and treated DIC by autologous blood transfusion and transfusion of blood products.
Conclusion: In the process of treating penetrating placental hemorrhage, blood products, especially plasma, should be transfused as soon as possible to delay the occurrence and development of DIC. In addition, autologous blood recovery technology is used to reduce the transfusion volume of allogeneic blood and to save more time for salvage.
Keywords: Obstetric hemorrhage; Penetrating placenta; Autologous blood transfusion; Massive transfusion
Abbreviation
DIC: Disseminated Intravascular Coagulation; Hb: Hemoglobin; Hct: Hematokrit; Plt: Blood Platelet; Fbg: Fibrinogen; PT: Prothrombin Time; APTT: Activated Partial Thromboplastin time; Glu: Blood Glucose; Lac: Lactic acid; BE: Base Excess; AG: Anion Gap
Case Presentation
The patient was a Chinese female, 31-year-old, admitted to the hospital due to “menopause for 34 weeks + 1 day, vaginal bleeding with lower abdomen tightness for 2 hours”. Admitting diagnosis: (1) dangerous placenta previa with hemorrhage; (2) placenta implantation: penetrating placenta implanted in the bladder; (3) G6P2+3 34+6 weeks’ intrauterine pregnancy LS live-birth threatened premature labor; (4) scarred uterus; (5) abnormal thyroid function during pregnancy. Physical examination showed symmetrical breathing of two lungs, clear breath sounds, regular heart rhythm, no heart murmurs, abdominal circumference of 95 cm, fundal height of 32 cm, LS of the fetus, fetal heart rate of 135 beats/min, irregular uterine contraction, with no vulva abnormality. Color Doppler ultrasound suggested placenta thickening, placenta implanted the anterior wall of the bladder, placenta previa, considering placenta implantation because of the placenta echo changes. MRI indicated total placenta previa, unclear border between the placenta and local uterine, and possibility of local adhesions of placenta and placenta implantation (Figure 1). Preoperative laboratory results: hemoglobin (Hb) 104 g/L, hematokrit (Hct) 30.1%, blood platelet (Plt) 116×109/L, fibrinogen (Fbg) 3.044, prothrombin time (PT) 10.1 s, activated partial thromboplastin time (APTT) 25.3 s. The patient refused abdominal aortic balloon occlusion and the elective cesarean section was arranged. Blood group screening and cross blood matching were performed before surgery, and 8 U of red blood cell suspension and 300 ml of fresh frozen plasma were prepared.
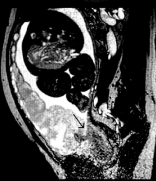
Figure 1: MRI (arrow) shows that the placenta completely covers the cervix
and is indistinct from local tissue.
After entering the operation room, the patient was routinely monitored for ECG, oxygen saturation and non-invasive blood pressure. Guided by B-ultrasound, bilateral transversus abdominis plane block was performed. A live baby girl was successfully delivered 8 minutes after skin incision. Upon omphalotomy, general anesthesia was induced by rapid induction of Ketamine and Scoline, and inhalation and intravenous general anesthesia was used to maintain the depth of anesthesia. During this period, the vital signs were stable, with blood pressure fluctuation around 150/90 mmHg and heart rate of 100 beats/min respectively. An autologous blood recovery device was used to collect blood after the fetus was delivered and the amniotic fluid was sucked out.
After 15 minutes of operation, massive hemorrhage occurred, with blood pressure decreasing to 55/35 mmHg, HR 105 beats/ min, SpO2 40% and CVP 7 mmHg within minutes. Laboratory test results: pH 7.196, K+ 3.04 mmol/L, Ca2+ 0.72 mmol/L, blood glucose (Glu) 13.4 mmol/L, lactic acid (Lac) 3.50 mmol/L, base excess (BE) -13.3 mmol/L, anion gap (AG) 6.9 mmol/L, Hb 51 g/L, Hct 15.6%, Plt 88×109/L. Within a few minutes, the estimated amount of blood loss was approximately 2 000 ml. The autologous blood was cleaned promptly for transfusion. Meanwhile, 8 U of red cell suspension and 300 ml of fresh frozen plasma were rapidly transfused. A continuous supply of red cell suspension and fresh frozen plasma at a ratio of 1:1. The results of the first coagulation after hemorrhage returned to PT 22.5 s, APTT 124.3 s, Fbg 0.539 g/L and Plt 51×109/L, indicating the coagulation dysfunction and disseminated intravascular coagulation (DIC). Since the placenta invaded the seromuscular layer of the posterior wall of the bladder, it was difficult to perform a hysterectomy due to uterine atony and uncontrolled bleeding. Twenty minutes later, the patient’s BP, HR, and SpO2 reduced progressively again. After several dozens of seconds of cardiopulmonary resuscitation, the patient’s vital signs returned to stable, with BP 90/70 mmHg, HR 115 beats/min, SpO2 92% and CVP 12 mmHg. The estimated amount of bleeding had reached 5,000 ml. The blood gas indicated pH 7.174, BE-15mmol/L, Hb 58g/L and Hct 17%. By that time, 8U of red cell suspension and 500 ml of fresh frozen plasma had been transfused. By monitoring blood routine, platelets dropped to 14×109/L at the lowest. After 2 hours, 20 U of cryoprecipitate and 1 therapeutic dose of platelets were transfused, the coagulation function showed PT 13.6 s, APTT 43.1 s and Fbg 2.137 g/L.
The entire operation lasted 4.5 hours with vital signs at the end of the operation: BP 126/84 mmHg, HR 84 beats/min, SpO2 100%, CVP 14 mmHg. Physical examination showed clear lung breath sounds, equal size of both pupils, reactive to light; blood gas: pH 7.315, Hb 82 g/L, Plt 56×109/L, K+ 4.3 mmol/L, Ca2+ 0.81 mmol/L, Glu 11.2 mmol/L, Lac 3.23 mmol/L, BE -5 mmol/L, AG 18 mmol/L. The total intraoperative urine volume was 1,350 ml. The amount of bleeding was about 15,000 ml. 26 U of red cell suspension, 3,050 ml of fresh frozen plasma, 3,150 ml of autologous blood, 20 U of cryoprecipitate, 1 therapeutic dose of platelet and 2 g of fibrinogen were transfused. 5,000 ml of crystalloid solutions and 1,500 ml of colloidal solutions were infused. The patient was sent to the ICU with tracheal tube, which was pulled out when the patient was awake on the following day. Since the indexes were basically returned to normal, the patient was transferred to the general ward and discharged one week later. Postoperative pathological examination revealed partial uterine wall defect and placental tissue attached to the uterine cavity in the lower uterine segment, which was consistent with the placenta previa. The placental lobular tissue was found in the interstitial tissue of the uterus, confirming that the patient had a placenta previa, accompanying with the penetrating placenta implanted into the bladder [1-5].
Discussion and Conclusion
Abnormal placenta, including placenta previa, placenta implantation, is one of the main causes of maternal hemorrhage [6]. The high proportion of women with a history of cesarean section giving birth leads to an increase of abnormal placenta and increased risk of perinatal hemorrhage. For patients with placenta previa and placenta implantation, hysterectomy is traditionally used to treat massive hemorrhage, with average blood loss of 3,000-5,000 ml. The interventional therapy of preventive intravascular balloon occlusion is used for patients with placenta previa or placenta. The strength is that technique of lower abdominal aorta balloon occlusion has also been found to reduce the intraoperative blood loss and has no obvious adverse effects on the mother and fetus [7-10]. The limitation is since this patient refused to accept any intervention, our perioperative preparation mainly focused on the rescue plan for massive hemorrhage.
The blood coagulation function needs to be monitored during continuous hemorrhage. The coagulation test results of traditional laboratories such as PT and APTT usually take 30-40 minutes, which cannot reflect the change of coagulation function in real time, and is difficult to guide further treatment in emergency situations. When severe blood loss occurs, it is not necessary to wait for the results of laboratory tests, but blood transfusion is performed as soon as possible to improve the success rate of rescue. Thromboelastography or TEG/ ROTEM can reflect the dynamic changes of blood coagulation, including the speed of fibrin formation, the thrombocytolytic state and the firmness and elasticity of coagulation, which can be used to confirm the presence of hypercoagulability during pregnancy and monitor the coagulation changes at bedside during obstetric hemorrhage [11]. Compared with the traditional coagulation function test, TEG/ROTEM can be more individualized to guild the transfusion timing and dosage of blood products and blood coagulation factor in obstetric hemorrhage [12]. In addition, fibrinogen is an independent predictor of severe bleeding in obstetric hemorrhage. Compared to PT and APTT, the reduction of platelets and fibrinogen may be more instructive for blood resuscitation and blood loss assessment in the operation [13-14]. Since the coagulation test result was delayed by about 40 minutes, we evaluated the treatment effect of the previous period based on the examination results and adjusted the treatment plan according to the bleeding during the operation, when the clinical experience of our doctors also played an important role. Guidelines of foreign countries also suggest that fresh frozen plasma can be transfused empirically when the results of coagulation cannot be obtained in time [15]. For the absence of TEG/ROTEM, we estimated the changes in coagulation function based on intraoperative bleeding and blood routine examination, supplemented blood components, and reexamined conventional coagulation function every 30 minutes during the operation.
Early resuscitation emphasizes goal-directed fluid resuscitation under volume monitoring. Lactated Ringer’s solution is preferred for volume expansion while colloids are used in subsequent treatment to maintain plasma osmolality and improve microcirculatory perfusion. Restrictive strategies for fluid resuscitation are used to avoid dilutive coagulation dysfunction caused by excessive fluids. The blood components are replenished quickly to increase oxygen carrying capacity and avoid tissue hypoxia and the blood coagulation factor is supplemented to prevent DIC [16]. Blood should be transfused immediately when a patient has lost more than 40% of his blood [17]. Due to the greater tendency of DIC in patients with major obstetric hemorrhage, many foreign studies believe that the transfusion ratio of fresh frozen plasma to red blood cell should be no less than 1, which will help improve postpartum hemorrhage survival rate [18,19]. During this operation, the ratio of transfused fresh frozen plasma to red blood cells was approximately 1.2 (because blood loss exceeded the patient’s own blood volume, autologous blood was not counted). Since the autologous blood was transfused during surgery, although fresh frozen plasma and red blood cells were transfused at a ratio of 1:1 after 5U red blood cells were transfused, the ratio of fresh frozen plasma to red blood cells was still greater than 1, indicating that the transfusion of autologous blood may reduce the need for allogeneic red blood cells. Royal College of Obstetricians and Gynaecologists believes that in order to maintain Plt >50×109/L during major obstetric hemorrhage, platelets should be transfused when Plt ‹75×109/L [20]. When the platelet was transfused, the main bleeding step was basically completed. Therefore, the platelet level was stable and >50×109/L after transfusion. Regardless of the ratio of blood transfusions, the therapeutic goal for obstetric hemorrhage is to maintain Hct ›24%, international normalized ratio ‹1.4, Plt ›50×109/L, Fbg›1 g/L, pH ›7.2, body temperature>35°C [21].
In a word, the key to the successful recovery of patients with major obstetric hemorrhage is the proper supplementation of blood components and the correction of coagulation function. In the process of treating penetrating placental hemorrhage, blood products, especially plasma, should be transfused as early as possible to delay the occurrence and development of DIC.
Declarations
Ethics approval and consent to participate
The case belonged to the process of treatment. The ethics approval has been obtained.
Consent for publication
The consent for publication has been obtained. Written consent to publish this information was obtained from study participants.
Availability of data and material
The data could be obtained in the Medical Record Room of Chengdu First People’s Hospital.
Funding
This work is supported by the grants from Foundation of Sichuan Medical Association (S15014); Sichuan Health and Family Planning Commission Research Project (16PJ062); Project of State Key Laboratory Fund of Trauma, Burns and Combined Injury (SKLKF201520).
Authors’ contributions
References
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006; 367: 1066- 1074.
- Collis RE CP. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015; 70: 78-86.
- Mhyre JM SA, Kuklina EV, Callaghan WM, Creanga AA, Kaminsky S, Bateman BT. Massive blood transfusion during hospitalization for delivery in New York State, 1998-2007. Obstet Gynecol. 2013; 122: 1288-1294.
- Perinatal Medicine Branch of Chinese Medical Association. A guide to placenta implantation diagnosis and treatment. Chin J Perinatal Med. 2015; 18: 481-485.
- Ducloy-Bouthors AS, Wong CA, Butwick A, Vallet B, Lockhart E. Medical advances in the treatment of postpartum hemorrhage. Anesth Analg. 2104; 119: 1140-1147.
- Maswime S, Buchmann EJ. Why women bleed and how they are saved: a cross-sectional study of caesarean section near-miss morbidity. BMC Pregnancy Childbirth. 2017; 17: 15.
- Sadashivaiah J, Wilson R, Thein A, McLure H, Hammond CJ, Lyons G. Role of prophylactic uterine artery balloon catheters in the management of women with suspected placenta accreta. Int J Obstet Anesth. 2011; 20: 282-287.
- Xie L, Wang Y, Luo FY, Man YC, Zhao XL. Prophylactic use of an infrarenal abdominal aorta balloon catheter in pregnancies complicated by placenta accreta. J Obstet Gynaecol. 2017; 37: 557-561.
- Manninen AL, Ojala K, Nieminen MT, Perälä J. Fetal radiation dose in prophylactic uterine arterial embolization. Cardiovasc Intervent Radiol. 2014; 37: 942-948.
- Semeraro V, Susac A, Morasca A, D’Antonio F, Belli AM. Foetal Radiation Dose during Prophylactic Occlusion Balloon Placement for Morbidly Adherent Placenta. Cardiovasc Intervent Radiol. 2015; 38: 1487-1493.
- Butwick AJ, Goodnough LT. Transfusion and coagulation management in major obstetric hemorrhage. Curr Opin Anaesthesiol. 2015; 28: 275-284.
- Guasch E GF. Massive obstetric hemorrhage: Current approach to management. Med Intensiva. 2016; 40: 298-310.
- Green L, Knight M, Seeney F, Hopkinson C, Collins PW, Collis RE. The haematological features and transfusion management of women who required massive transfusion for major obstetric haemorrhage in the UK: a population based study. Br J Haematol. 2016; 172: 616-624.
- Charbit B, Samain E, Baron G, Haddaoui B, Keita H, Sibony O, et al. de Prost D; PPH Study Group, The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007; 5: 266-273.
- Liumbruno GM, Lattanzio A, Piccoli P, Rossetti G. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party, Recommendations for the transfusion management of patients in the perioperative period. II. The intra-operative period. Blood Transfus. 2011; 9: 189- 217.
- Abdul-Kadir RMC, Ducloy AS, El-Refaey H, England A, Federici AB, Grotegut CA, et al. Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion. 2014; 54: 1756-1768.
- Blood transfusion status research group. Guidance for a large amount of blood transfusion programs. Chin J Blood Transfusion. 2012; 25: 617-621.
- Tanaka H, Matsunaga S, Yamashita T, Okutomi T, Sakurai A, Sekizawa A, et al. A systematic review of massive transfusion protocol in obstetrics. Taiwan J Obstet Gynecol. 2017; 56: 715-718.
- Matsunaga S, Seki H, Ono Y, Matsumura H, Murayama Y, Takai Y, et al. A retrospective analysis of transfusion management for obstetric hemorrhage in a Japanese obstetric center. ISRN Obstet Gynecol. 2012; 2012: 854064.
- Gynaecologists RCoOa. Postpartum Haemorrhage, Prevention and Management (Green-top 52). RCOG. 2009.
- Shields LE, Smalarz K, Reffigee L, Mugg S, Burdumy TJ, Propst M. Comprehensive maternal hemorrhage protocols improve patient safety and reduce utilization of blood products. Am J Obstet Gynecol. 2011; 205: 368 e1-8.