
Special Article – Surgery Case Reports
Austin J Surg. 2019; 6(16): 1204.
Necrotizing Fasciitis – A Case Report of GBS Infection in a Previously Healthy Woman
Kochan P1*, Rajca L2, Samet A3, Heczko PB1 and Brzychczy-Wloch M1
1Chair of Microbiology, Jagiellonian University Medical College, Poland
2Kociewskie Centrum Zdrowia, Starogard Gdanski, Poland
3Clinical Microbiology Laboratory, Clinical University Centre, Poland
*Corresponding author: Piotr Kochan, Chair of Microbiology, Jagiellonian University Medical College, 18 Czysta Street, 31-121 Cracow, Poland
Received: July 18, 2019; Accepted: August 08, 2019; Published: August 15, 2019
Abstract
This article describes a case of necrotising fasciitis following a procedure of total hysterectomy caused by group B streptococcus in a previously healthy 50 year-old female patient. The patient was readmitted to the hospital in the 7th post-surgical day with a necrotic surgical site infection. Microbiological cultures were collected and Streptococcus agalactiae isolate was further characterized by molecular techniques. Besides of the invasive infection, the patient also developed an abscess in the lumbar region. The treatment consisted of surgical debridement, incision and drainage, a combination of antibiotic courses and hyperbaric therapy. The patient made a full recovery. This was her second episode of postsurgical complications, with the first one occurring in 2011. This is possibly the first report of necrotizing fasciitis in a previously healthy patient caused by GBS in Poland. We would like to open a discussion whether GBS screening before gynaecological procedures should be a standard, since invasive GBS infections play a role not only in Europe but also in the USA.
Keywords: Streptococcus agalactiae (GBS); Invasive infection; Necrotizing fasciitis; Multilocus sequence typing (MLST)
Introduction
Necrotising fasciitis is a severe, invasive and life-threatening bacterial infection. Such invasive infections may occur as a complication of surgical procedures or as posttraumatic infections and often involve patients with underlying chronic medical conditions including diabetes, immunocompromise, obesity, alcoholism, peripheral vascular disease cancer, intravenous drug use and malignancies. There are 6 general types of necrotising fasciitis as per etiological agents: (i) caused by streptococci (mainly group A); (ii) caused by Clostridium sp.; (iii) caused mixed aerobic and anaerobic microbes; (iv) caused by CA-MRSA; (v) caused by K. pneumoniae and (vi) Vibrio vulnificus [1,2]. The infection described here could be characterized as (i) and (iii), as above.
Streptococcus agalactiae, also referred to as Lancefield group B Streptococcus (GBS), is an important pathogen in neonates and adults with predisposing conditions in the USA and Europe [3,4]. GBS cause important infections in neonates, encompassing respiratory tract infections, meningitis and sepsis, especially in prematurely born. It colonises the genitourinary and lower gastrointestinal tract of 10 to 40% of women. Even 1/3 of pregnant women may be colonized with group B streptococcus [4]. S. agalactiae is a genetically diverse organism. Therefore, nowadays, a combination of several molecular typing methods should be considered to gain a better understanding of the pathogenesis and epidemiology of GBS isolates [5-9]. In many countries, there are guidelines for 3rd trimester GBS vaginal screeening during pregnancy and intrapartum antibiotic prophylaxis [4].
Patient Description
Female patient, aged 50, was admitted to the Department of Gynaecology in Kociewskie Centrum Zdrowia in Starogard Gdanski, Poland, 7 days post surgery owing to Surgical Site Infection (SSI). She underwent total hysterectomy due to a left ovarian tumour on 28.11.2012. Upon admission her chief complaint was elevated body temperature of 38°C for two days, oedema and erythema around the surgical site. Looking back into the medical history, her immediate perioperative course showed no complications. The first few days after surgery showed no complications either. The patient discharged herself against medical advice on the 3rd day post-surgery.
On readmission (05.12.2012), the patient presented in moderately bad general status, with body temperature of 38°C, BP 80/40 mmHg but her cardiovascular and respiratory status was stable. Proximity of the surgical site was oedematous, erythematous and warm with grey margins. After removal of stiches, wound dehiscence occurred along the whole length of the surgical site. Large necrotic changes were observed which extended to the subcutaneous tissue and fascia, and a lot of dark-coloured foul-smelling fluid was present (Figures 1 & 2). In the region of the left margin of the surgical site there was inflammatory infiltration penetrating to the wing of ilium. Microbiological material was collected by swabbing and aspiration from the wound. A semi quantitative scale was used to describe the initially cultured organisms (05.12.2012), for details please see (Table 1). There were more samples collected along the course of the infection and the wound aspirates on 06.12.2012 and 07.12.2012 did not show the growth of GBS anymore. Also the control cultures (14.12.2012) from the throat, nose, rectum, vagina, inguinal region, axilla were negative for GBS. The follow-up swabs collected on 14, 21, 24, 30 December 2012 as well as 6 January 2013 were also negative for GBS.
Samples collectedMicroorganisms culturedWound aspirateWound swabVaginal swabRectal swabS. agalactiae cMLSB
Ear swabS. agalactiae cMLSB
Other materials:throat and nasal swabs, urine, bloodNegative
Legend: + less numerous growth; ++ numerous growth; +++ abundant growth.
Table 1: Initial culture results from samples collected upon readmission (05.12.2012).
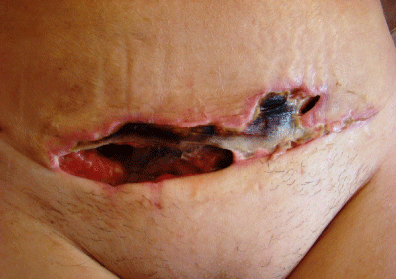
Figure 1: Photograph showing an example of a surgical site infection upon
readmission. Visible are the necrotic changes and inflammatory signs.
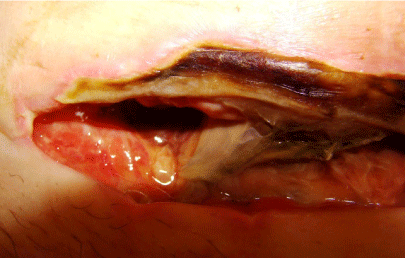
Figure 2: Up-close photograph of a surgical site infection with necrosis.
On 5 December 2012 the wound was thoroughly washed with octenidine dihydrochloride with 2-phenoxyethanol (Octenisept, Schülke & Mayr GmbH, Germany), with extensive debridement and excision of the necrotic tissue up to the left iliac wing. The wound was left open to heal. The following lab tests were performed: WBC- 13500/mm3; CRP-365 mg/l; ASO-114.8 U/L; procalcitonin-3.83 ng/ml. Intravenous antimicrobial therapy was initiated as per recommendations of the consultant microbiologist with daily dosages: amoxicillin/clavulanic acid 3×1.2 g; clindamycin 3×600 mg and gentamycin 3×80 mg until the infection is cured.
The following day (06.12.2012) during wound care, more necrotic tissue was excised and a sanious discharge from under the rectus abdominis muscle was noticed. The space was widened and thoroughly washed with Octanisept. Once again procalcitonin level was verified and it was 3 ng/ml.
The following day (07.12.2012) during wound inspection, we observed abscess formation in the left lumbar region. After surgical consultation, the abscess was incised and tunnelized to drain towards the pole of the wound – a seton soaked in Octanisept was left in the incision. Procalcitonin level was 1.7 ng/ml. Based on the culture and with drug sensitivity results available, gentamycin was discontinued and tigecycline added at an IV dose of 50 mg every 12h and the patient was qualified for hyperbaric chamber therapy at the Clinic of Hyperbaric Medicine and Sea Rescue in Gdynia, Poland. The patient was transferred to the clinic where she had hyperbaric chamber sessions twice daily for a week. Upon the patient’s return to the Department of Gynaecology in Starogard Gdanski, the wound was almost in granulation stage with negative cultures. A decision was made to close the wound with a secondary suture in operating theatre settings. The wound was closed along 2/3 of its length – the rest was left open to heal by granulation due to loss of a large fragment of skin and subcutaneous tissue. The sutured site was covered with alginate and hydrogel dressings. Stiches were removed after 10 days and the open wound healed by granulation (Figures 3 & 4).
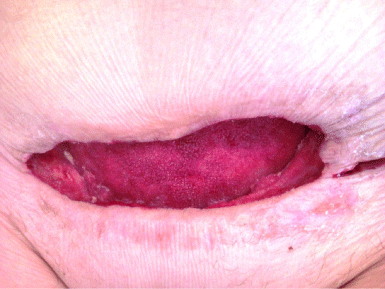
Figure 3: Example of the wound beginning to heal.
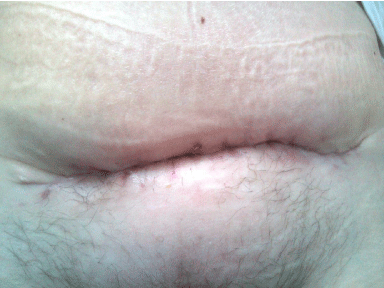
Figure 4: Healed SSI.
The identification of GBS was firstly performed by latex agglutination assay and API STREP kit (bioMerieux, France) and then molecular identification based on PCR with Sag59 and Sag190 species-specific primers (Genomed, Poland) for S. agalactiae were applied [10,11]. The genes encoding capsular polysaccharides Ia, Ib, II-VIII were investigated in GBS isolates using multiplex PCR method with specific primers (Genomed, Poland) according to Poyart et al. [5].
To detect the surface protein genes alp2, alp3, alp4, bca, epsilon and rib multiplex PCR was used with specific primers (Genomed, Poland), according to the procedure proposed by Creti et al. [7] and Gherardi et al. [3].
MICs of penicillin, ampicilin, erythromycin, clindamycin and vancomycin were evaluated using E-test (Oxoid, UK) as recommended by EUCAST (in 2012) [12]. Macrolide resistance phenotype was determined by the double-disc test with erythromycin (15 μg) and clindamycin (2 g) (Oxoid, UK). The erm(A), erm(B), erm(C) and mef(A/E) resistance determinants were detected by multiplex PCR with adequate four pairs of primers (Genomed, Poland) according to Sutcliffe et al. [11].
MLST analysis was performed as described by Jones et al. [13] with the use of oligonucleotide primer pairs (Genomed, Poland) specific for the seven housekeeping loci selected for the GBS MLST. The online database https://pubmlst.org/sagalactiae was used for assigning alleles for seven loci and GBS isolate was defined by the sequence type (ST). The eBURST program was used to group isolates into clonal complex (CC) whose members share at least six of the seven MLST loci [14].
The GBS isolate was characterized as: II serotype, rib gene, cMLSB phenotype with ermB gene and sequence type ST-28 (CC19).
Discussion
GBS strains are subdivided according to their type-specific capsular polysaccharides (CPS) into ten unique serotypes (Ia, Ib, II - IX). This capsule has been recognized as one of the major virulence factors with antiphagocytic function [3,5]. GBS pathogenicity varies between and within serotypes, with considerable variation in genetic content between strains. Particularly, the serotypes most often causing human infections are Ia, II, III and V [16].
The best-characterized GBS protein antigens, which are significant virulence factors, belong to the alpha-like protein (Alp) family. They are called Alpha-C protein, Rib, Alp2, Alp3, Alp4 and Epsilon (Alp1) and are encoded by bca, rib, alp2, alp3, alp4 and epsilon/alp1 genes respectively. The examination of the protein gene profile increases the potential for GBS subtyping [7]. The surface proteins, e.g. Rib protein, play an important role in the pathogenesis of GBS infection. The Rib protein was identified as a unique high-molecular-weight protein in cell wall extracts of type III strains. The available evidence indicates that Rib is expressed by the large majority of serotype III strains, by many type II strains, and by a few type V strains [15,17].
Multilocus sequence typing (MLST) has been previously described in order to identify the emergence and spread of GBS clones and to study their genetic population structure worldwide [8]. MLST has become the conventional method for determining the population structure of GBS and has been applied to the molecular epidemiology of S. agalactiae infections by several investigators. Currently, five main clonal complexes CC1, CC10, CC23, CC19 and CC17 have been identified emphasizing the diversity of S. agalactiae in humans [13].
For many years, group B streptococcus remained susceptible to penicillin, the recommended drug used in the treatment and prophylaxis of infections caused by GBS. However, for patients allergic to penicillin, the alternative drugs according to CDC recommendation from 2002, were macrolides and lincosamides [18]. In relation to high levels of erythromycin resistance, CDC did not recommend the erythromycin anymore in 2010 [4].
In the USA, 29% of GBS isolates were erythromycin resistant [19]. In Europe, erythromycin resistance amounted to 35% [20]. Two principal resistance mechanisms are described for GBS. Genes erm (erythromycin ribosome methylase) such as ermA and ermB (subclass ermTR) codes methylase 23S rRNA, which methylates of erythromycin and clindamycin receptor sites in ribosomes. Expression of the erm gens is described as MLSB phenotype (cMLSB – constitutive; iMLSB - inductive) and points to cross resistance to macrolides, lincosamides and streptogramins B. Genes mefA and mefE (macrolide resistance M phenotype) coded the highly conservative pump which expels antibiotics from the bacterial cell. Expression of these genes is described as M phenotype [11].
To our knowledge, this is the first report of necrotizing fasciitis in a previously healthy patient caused by GBS in Poland. This patient did not suffer from any underlying chronic medical conditions and was probably previously colonized by GBS. Interestingly, in May 2011, she underwent cervical conisation for CIN III and then suffered from a pelvic abscess which was incised and drained, with follow up appendectomy in July 2011. Unfortunately we have no culture results available for the 2011 infectious episode. We may speculate that the infection back then was also of GBS etiology, since microbiological lab results from 05.12.2012 showed that GBS was not only present in the samples (swabs and aspirate) collected from the SSI, but also colonized her vagina, rectum and ear.
To conclude, we would like to open a discussion whether GBS screening before gynaecological procedures should be done routinely, since invasive GBS infections play a role not only in Europe but also in USA, especially among the elderly or non-pregnant adult patients suffering from chronic underlying conditions.
Acknowledgement
The study was supported by a grant from the Polish Ministry of Research and Higher Education no N N401 042 337. The study was approved by Jagiellonian University Bioethical Committee decision no. KBET/143/B/2007.
References
- Trent JT, Kirsner RS. Necrotizing fasciitis. Wounds. 2002; 14: 284-292.
- Sanford Guide to Antimicrobial Therapy. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT, Black D, Freedman DO, Kim K, Schwartz BS (eds.) Antimicrobial Therapy Inc. Sperryville. 2018; 58.
- Gherardi G, Imperi M, Baldassarri L, Pataracchia M, Alfarone G, Recchia S, et al. Molecular epidemiology and distribution of serotypes, surface proteins, and antibiotic resistance among group B streptococci in Italy. J Clin Microbiol. 2007; 45: 2909-2916.
- Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of Perinatal Group B Streptococcal Disease. Revised Guidelines from CDC. MMWR. 2010; 59: 1-32.
- Poyart C, Tazi A, Réglier-Poupet H, Billoët A, Tavares N, Raymond J, et al. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. Journal of Clinical Microbiology. 2007; 45: 1985-1988.
- Lindahl G, Stalhammar-Carlemalm M, Areschoug T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clinical Microbiology Reviews. 2005; 18: 102-127.
- Creti R, Fabretti F, Orefici G, von Hunolstein C. Multiplex PCR assay for direct identification of group B streptococcal alpha-protein-like protein genes. Journal of Clinical Microbiology. 2004; 42:1326-1329.
- Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan MS, Kunst F, et al. Multilocus sequence typing system for group B streptococcus. Journal of Clinical Microbiology. 2003; 41: 2530-2536.
- Ke D, Ménard C, Picard FJ, Boissinot M, Ouellette M, Roy PH, et al. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clinical Chemistry. 2000; 46: 324-331.
- Ke D, Ménard C, Picard FJ, Boissinot M, Ouellette M, Roy PH, Bergeron MG. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clinical Chemistry. 2000; 46: 324-331.
- Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L. Detection of erythromycinresistant determinants by PCR. Antimicrobial Agents and Chemotherapy. 1996; 40: 2562-2566.
- EUCAST – European Committee on Antimicrobial Susceptibility Testing. Version 2.0. Access valid from. 2012; 01-01.
- Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan MS, Kunst F et al. Multilocus sequence typing system for group B streptococcus. Journal of Clinical Microbiology. 2003; 41: 2530-2536.
- Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004; 186:18-30.
- Stålhammar-Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. Journal of Experimental Medicine 1993; 177: 1593-1603.
- Brimil N, Barthell E, Heindrichs U, Kuhn M, Lütticken R, Spellerberg B. Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol. 2006; 296: 39-44.
- Lindahl G, Stalhammar-Carlemalm M, Areschoug T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clinical Microbiology Reviews. 2005; 18: 102-127.
- Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR. 2002; 51: 1-22.
- Castor ML, Whitney CG, Como-Sabetti K, Facklam RR, Ferrieri P, Bartkus JMet al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol. 2008; 2008: 727505.
- Lopes E, Fernandes T, Machado MP, Carriço JA, Melo-Cristino J, Ramirez M, et al. The Portuguese Group For The Study Of Streptococcal Infections. Increasing macrolide resistance among Streptococcus agalactiae causing invasive disease in non-pregnant adults was driven by a single capsulartransformed lineage, Portugal, 2009 to 2015. Euro Surveill. 2018; 23.