
Research Article
Austin J Surg. 2019; 6(19): 1212.
Galectin-3 Expression in Thyroid Papillary Cancer
Ozcinar B1*, Bireller ES2, Ertugrul B3, Ergen HA3 and Cakmakoglu B3
¹Department of General Surgery, Istanbul University Istanbul Faculty of Medicine, Turkey
²Department of Pharmaceutical Microbiology, Istanbul Yeni Yuzyil University, Turkey
³Department of Molecular Medicine, Istanbul University, Aziz Sancar DETAE, Turkey
*Corresponding author: Beyza Ozcinar, Istanbul University Istanbul Faculty of Medicine, Department of General Surgery, Capa 34093, Istanbul, Turkey
Received: August 08, 2019; Accepted: September 16, 2019; Published: September 23, 2019
Abstract
Thyroid cancers are responsible from less than 1% of all cancers, over than 90% of endocrinologic cancers, and 0.4% of cancer-related deaths. Every year, the number of patients diagnosed as having thyroid cancer is increasing. Most frequently used method in differentiation of benign- malignant thyroid nodules is the fine needle aspiration biopsy. However, the biopsy result is benign, the tumor may be identified as malignant in 1% of patients after surgery. Therefore, molecular identifiers are required for the preoperative differentiation of benign malignant thyroid nodules. The aim of the present study is to identify the Galectin-3 expression levels in thyroid cancers, and to identify the association between tumor characteristics and expression.
A total of 25 patients who were diagnosed as having thyroid cancer in the General Surgery Department of Istanbul University Istanbul Faculty of Medicine were included in the study. Accordingly, thyroid tumors and normal tumors, which were excised during the surgery, were stored at -80°C. Then, cDNA was synthesized after the concentrations of RNAs that were isolated from the samples, were equalized. The real time PCR of Galectin-3 and GAPDH Housekeeping gene expressions were calculated. The obtained results were evaluated using 2-ddCt method, and the gene fold change rates of patients compared with control group were calculated.
Evaluation of Galectin-3 gene expression in tumor tissue and normal tissue revealed that Galectin-3 expression decreased 0.24 folds in tumor tissue compared with healthy tissue. However, no statistical significance was detected (p= 0.257). Evaluation of Galectin-3 gene fold change levels in accordance with the surgical border demonstrated no statistical significance, and fold changes were ranged as negative‹focal positive‹positive (p›0.05).
The results showed that more comprehensive studies including the series that also have other differential cancers of thyroid other than papillary cancer are required for the routine use of Galectin-3 gene expression in differentiation of benign-malignant tumors, and in identification of the subtypes of papillary cancer.
Keywords: Papillary thyroid cancer; Galectin-3; Gene expression
Introduction
Thyroid cancers are responsible from less than 1% of all cancers, over than 90% of endocrinologic cancers, and 0.4% of cancer-related deaths [1]. The most frequently used methods in preoperative diagnosis of thyroid cancers are thyroid ultrasonography (USG) and Fine Needle Aspiration Biopsy (FNAB). Thyroid ultrasonography may also be used as a guide for FNAB in addition to its use in evaluation of nodule size, characteristics (border features, blood flow features etc.), differentiation of solid-cystic, and in evaluation of neighboring lymph nodes. Although, FNAB is frequently used in preoperative benign-malignant tumor differentiation, it has some limitations in establishing a final diagnosis. It is known that patients who are diagnosed as having a benign cytology with FNAB, could be detected as malignant in 1% during the surgery [1]. In addition, FNAB lacks sufficient evidence in differentiation of follicular adenoma and follicular cancer. Therefore, other guiding methods are required in addition to FNAB in preoperative evaluation of nodules as benign or malignant.
There are studies in the literature investigating various molecules for using in differentiation of benign-malignant, follicular adenomafollicular cancer, papillary- other cancer types [2-8]. A study series conducted on human tissues; revealed that Galectin- 3 expression increased in multi organ- rooted malignancies such as colon, stomach, central nervous system, breast and thyroid, and it was suggested that it could be used in differentiation of benign-malignant in thyroid nodule [9]. In addition, some studies showed that Galectin-3 was effective in invasion and metastasis in malignant lesions [10-13].
Galectin-3 has different effects in different cell types. Breast cancer studies showed that Galectin-3 prevented apoptosis, and Galectin-3 expression decreased as the tumor grade increased [13- 17]. It is known that the adherence characteristics of cancer cells causes apoptosis in thyroid cancer.
There are different opinions associated with Galectin-3 levels in decrease of differentiation, progression of tumor such as local invasion and metastasis in thyroid papillary cancers. In addition, however Galectin-3 expression was detected in thyroid cancers, Galectin-3 was not detected in benign tumors and normal tissues [18].
Considering all these characteristics, in the present study we aimed to identify Galectin-3 expression levels in thyroid papillary cancers and to identify the association between the tumor characteristics and expression.
Material and Method
A total of 25 patients who were diagnosed as having thyroid cancer in the General Surgery Department of Istanbul University Istanbul Faculty of Medicine were included in the study. The group consisted of 18 women, and 7 men. The histopathologic evaluation revealed that 5 patients were diagnosed with classic type papillary cancer (20%), 17 patients had follicular variant papillary cancer (68%), and the other patients were diagnosed with the other rare papillary cancer subtypes (Tall cell variant, oncocytic variant, and diffuse sclerosing type papillary cancer) (Table 1). Although, no tumor necrosis was detected, lymphovascular invasion in one patient (4%), calcification in 4 patients (16%) and perineural invasion was detected in 2 patients (8%). Although, micro cancer was detected in 8 patients (32%), tumors were multifocal in 16 patients (64%).
Number
%
Histopathologic diagnosis
Classic type papillary cancer
5
20
Follicular variant papillary cancer
17
68
Tall cell variant papillary cancer
1
4
Oncocytic variant papillary cancer
1
4
Diffuse sclerosing type papillary cancer
1
4
FNAB- Bethesda classification
AUS/FLUS
9
36
Follicular neoplasia
1
4
Suspicious for Malignancy
9
36
Malignant
6
24
Table 1: The histopathological characteristics and FNAB results of patients.
Thyroid tumors and normal tissues were homogenized with a hand homogenizer by addition of 1ml RNAzol® RT≈100 mg tissue after providing approximately 100 mg RNAzol® RT solution from MRCgene company. After homogenization, the RNA isolation procedure was completed appropriate to MRCgene protocol. The concentration and purity of obtained RNAs were calculated using a nanodrop device, and RNA samples were stored at -80oC. The purity was determined by calculations at 260nm and 280nm wavelength beam absorption (Optic Density) spectrophotometer, and by the calculation of O.D rate at 260nm/ O.D rate at 280nm. cDNA synthesis stage was initiated after the concentrations of isolated total RNAs were equalized. SCRIPT cDNA Synthesis Kit was manufactured from Jena Bioscience company, and protocols indicated on the kit were used.
cDNA sysnthesis was performed by placing all solutions into 0.2mL PCR tube for 10 minutes at 42°C, and by keeping 1 hour in Thermal Cycler (Biorad T100). After PCR reaction, cDNA samples were stored at -20°C until used in Real-Time PCR. The manufactured qPCR Green Master UNG/lowROX kit and Agilent Technologies Stratagene Mx3005p device were used for calculations of Galectin-3 and GAPDH Housekeeping gene expressions in Real-Time PCR. The obtained results were evaluated using 2-ddCt method, and the gene fold change rates of patients compared with control group were calculated.
The data analyses were performed using a computer aided statistics program. The mean scores, standard deviations, and frequencies were calculated. Group differences for categorical variables were evaluated using Chi-square test, and; Mann-Whitney U test was used for continuous variables. Spearmen’s correlation was performed in comparison of gene analysis of tumor tissue and normal tissue. The differences between more than two groups were identified by one-way ANOVA. The results were evaluated in 95% confidence interval, and significance value was accepted as p‹0.05.
Results
The relative gene expression changes of individuals in patient group are shown in (Figure 1).
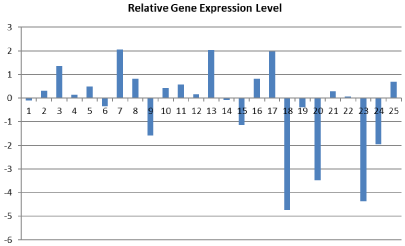
Figure 1: Relative gene expression levels in each patients.
The evaluation of Galectin-3 expressions of tumor and normal tissue revealed that Galectin-3 expression decreased 0.24 fold in tumor tissue compared with normal tissue. However, no statistical significance was detected (p=0.257) (Table 2).
Cancer tissue
CT level (Galectin)
Cancer tissue
CT level (GAPDH)
Healthy tissue CT level (Galectin)
Healthy tissue CT level (GAPDH)
Cancer tissue ΔCT level
Healthy tissue ΔCT level
ΔΔCT level
2-ΔΔCT
Fold change
25,61±2,85
23,02±2,33
24,74±1,47
22,46±2,10
3,38±2,85
3,04±1,47
0,34(-2,96-6,84)
1,92 (0,01-7,78)
-0,24
Table 2: Galectin-3 and GAPDH gene expression levels.
The evaluation of fold change values in accordance with clinical, demographic, and histopathologic parameters revealed that Galectin-3 gene expression significantly increased in patients who were included in FNAB Bethesda 5-6 classification (p=0.046, 95% Cl=0.94-4.99). In addition, statistically significant increase in Galectin-3 gene expression was detected in patients with calcification (p=0.038, 95% Cl=0.79-5.38) (Table 3).
Clinical, Demographical and Histopathological Parameters
Fold change (mean, min-max)
p value
Sex
Female (n=18)
2,21 (0,01-7,78)
Male (n=7)
1,17 (0,32-1,99)
0,904
Multifocality
Presence (n=16)
1,45 (0,01-7,26)
Absence (n=9)
2,74 (0,01-7,78)
0,365
Tumour capsule
Presence (n=8)
1,76 (0,01-7,78)
Absence (n=17)
1,99 (0,01-7,67)
0,620
FNAB category
3-4 (n=10)
0,52 (0,14-1,06)
5-6 (n=15)
2,54 (0,01-7,78)
0,046
Calcification
Presence (n=4)
4,52 (1,36-7,67)
Absence (n=21)
1,42 (0,01-7,78)
0,038
Autoimmune thyroiditis
Presence (n=12)
2,05 (0,01-7,78)
Absence (n=13)
1,79 (0,01-7,26)
0,892
Micro cancer
Presence (n=8)
1,55 (0,01-3,89)
Absence (n=17)
2,09 (0,01-7,78)
0,838
Table 3: Relation of clinical, demographical and histopathological parameters and fold change.
Six (24%) tumors were localized in the right lobe, 4 (16%) tumors were localized in the left lobe, and 15 (60%) were localized both in the right and left lobes. Underlying autoimmune thyroiditis was detected in 12 patients (48%). In evaluation of surgical borders; surgical border was positive in 2 patients (8%), focal positivity was detected in 4 patients (16%), and surgical borders were negative in 19 patients (76%) (Table 4). In evaluation of clinical stages, metastasis was detected in the neck in 2 patients, and distant metastasis was detected in 2 patients (8%). The evaluation of Galectin-3 gene fold change in accordance with surgical border demonstrated no statistical significance however, the fold changes were organized as negative‹ focal positive‹ positive (p›0.05) (Figure 2). The subgroup classification of tumors as classic type papillary cancer (n=5) and follicular variant papillary cancer (n=17) demonstrated that the mean age was smaller in follicular variant papillary cancer compared with classic type papillary cancer (45.4/52), the median tumor size was smaller (16.6/37.5), and Galectin-3 gene expression levels were higher (26/23) compared with the classic type. In addition, Galectin-3 expression levels were similar in normal tissues (25/25) however, the differences were not statistically significant. Researchers suggested that this could be due to small number of patients in subgroup analyses.
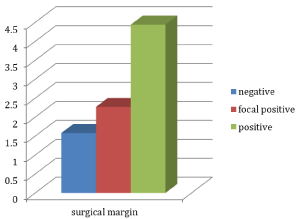
Figure 2: Graphic of Galectin-3 fold change levels according to surgical
margins.
Fold change (mean, min-max)
p
Surgical margin
Negative (n=19)
1,58 (0,01-7,78)
Focal Pozitive (n=4)
2,27 (0,20-7,67)
Pozitive (n=2)
4,44 (1,62-7,26)
0,150 (positive vs negative)
0,626 (focal positive vs negative)
0,355 (focal positive vs positive)
Tumor localization
Right (n=6)
2,44 (0,03-7,78)
Left (n=4)
2,71(1,32-7,67)
Bilateral (n=15)
1,50 (0,01-7,26)
0,697 (right vs bilateral)
0,548 (left vs bilateral)
0,670 (right vs left)
Table 4: The relation of Galectin-3 with surgical margin and tumor localization.
Multifocality was reported to be higher in follicular variant papillary cancers (14/1) (p=0.040). In addition, calcification detection rate (2/1) and perineural invasion detection rate was higher (1/0) in classic type papillary cancers (p=0.019 and p=0.030).
The tumor size of patients with neck metastasis was found to be larger (p=0.001). No significant association was detected between the presence of micro cancer in the tumor, neck metastasis and distant metastasis, and Galectin-3 expression (p›0.005).
Discussion
Galectins are from the protein family that bound high affinity carbonhydrate to B galactozide. Galectin-3; takes part in cell growth, differentiation, cell adhesion, angiogenesis, tumor progression, apoptosis and metastasis, and is expressed in various tumor types such as thyroid, colon and breast.
Many distinctive results were obtained in Galectin-3 expression in benign and malignant lesions. The detailed investigation of material and methods of studies in literature, shows that too different methods have been used for identification of Galectin-3 expession. In addition, no criteria are available for detection of the presence of Galectin-3 expression, and the level which is accepted positive. We evaluated Galectin-3 gene expression using the RT-PCR method in the present study. First, cDNA was synthesized from RNAs, and then gene expression levels were evaluated using qRT-PCR method.
The advantage of this study is that it was performed on fresh tissues, both tumor and normal thyroid tissues of patients were seperately stored and analysed so that comparison could be made accordingly. The most important disadvantage was that the study sample size was small.
The most frequent thyroid cancers are papillary thyroid cancers and Galectin-3 is strongly expressed in malignant transformation of thyroid cells particularly in classic type papillary thyroid cancer [19-23]. First, Xu et al. emphasized that Galectin-3 was expressed in thyroid cancers and this marker could be used for differentiation of benign and malignant thyroid nodules [24]. Bartolazzi et al. found in their study which they conducted with 1009 thyroid tissue specimens and 226 fresh thyroid cytologies, that Galectin-3 sensitivity was 99%, and specificity was 98% in differentiation of benign and malignant thyroid lesions [22]. Sharaky et al. investigated Glypican-3 and Galectin-3 expressions in pathology blocks involving 17 follicular adenoma, 16 classic type papillary cancer, 6 follicular variant papillary cancer, 3 follicular cancers, and 5 medullary cancers, and found Galectin-3 expression sensitivity as 96.8% and specificity as 70.6% in differentiation of benign-malignant thyroid nodules [6]. These results show that Galectin-3 sensitivity was fairly higher, and specificity was a bit lower in differentiation of benign-malignant thyroid tumor.
Because there were controversial results of Galectin-3 in many studies in the literature, this led the researchers to studies conducted using multiple markers. Papotti et al. reported in their study that Galectin-3 and HBME-1 joint expressions were also detected in follicle structures of well-differentiated thyroid cancers, and these two markers may be used in diagnosis of papillary thyroid cancer [25]. Saleh et al investigated Galectin-3, CK19, HBME-1 and RET oncoprotein expressions in thyroid fine needle aspirates, and demonstrated that Galectin-3 had the highest sensitivity and specificity in differentiation of malignant thyroid nodules from benign nodules [26]. Abd-El Raouf et al. found that Galectin-3 expression had a higher sensitivity and specificity in thyroid cancer compared with HBME-1 however, joint evaluation of HBME-1 and Galectin-3 increased the specificity [7].
There are various opinions on the association of Galectin-3 levels and indications of tumor progression such as the decrease in differentiation, local invasivity, lymph node metastasis in thyroid papillary cancers. Some studies reported that Galectin-3 decreased the protein expression in metastatic thyroid cancer [27,28]. Salajegheh et al. detected in their study [29] the increased Galectin-3 expression in mRNA level in 58% of thyroid papillary cancers and in 64% of metastatic lymph nodes. Expression levels were found to be higher in metastatic papillary thyroid cancers compared with non-metastatic papillary cancers. In addition, researchers found that metastatic lymph nodes expressed higher rates of Galectin-3 compared with the primary focus [29]. However, no correlation was detected between the expression levels and clinicopathologic characteristics [29].
Investigation of the association of Galectin-3 gene expression levels and tumor positivity, tumor focal positivity and negativity in surgical resection borders showed that it was the smallest with surgical border negativity, slightly higher with focal positivity, and was the highest in patients with positive surgical border. However, this was not statistically significant, Galectin-3 level may be increasing as the local aggressively increased. We believe that future studies with increased number of patients will provide the significance.
In conclusion, we found that Galectin- 3 gene expression level may be used in differentiation of normal thyroid tissue and thyroid papillary cancer, however, studies with larger series are required. Although, no statistical significance was detected in subtypes of thyroid papillary cancer, Galectin-3 expression in subtypes of follicular variant papillary cancer was higher compared with classic type papillary cancer.
The results showed that more comprehensive studies including the series that also have other differential cancers of thyroid other than papillary cancer are required for the routine use of Galectin-3 gene expression in differentiation of benign-malignant tumors, and in identification of the subtypes of papillary cancer.
Acknowledgements
This work was funded by Istanbul University Scientific Research Project number 37243. Thanks to Cem Horozoglu for contribution at preparation of study.
References
- Gürsoy A, Erdoğan MF. A’dan Z’ye Klinik Tiroidoloji. Tiroid kanserleri ve kanserli hasta takibi. 1. Baski, Ömür Matbaacilik. 2012.
- Yilmaz E, Karsidağ T, Tatar, C, Tüzün S. Serum Galectin-3: Diagnostic value for papillary thyroid cancer. Ulus Cerrahi Derg. 2015; 31: 192-196.
- Sumana BS, Shashidhar S, Shivarudrappa AS. Galectin-3 immunohistochemical expression in thryoid neoplasms. Journal of Clinical and Diagnostic Research. 2015; 11: 7-11.
- Seçkin S, Karagece U. Expression of CK-19, cErb B2, galectin-3 and p53 in papillary thyroid carcinomas. Turk J Med Sci. 2010; 40: 207-212.
- Harazono Y, Kho DH, Balan V, Nakajima K, Zhang T, Hogan V, et al. Galectin-3 leads to attenuation of apoptosis through Bax heterodimerization in human thyroid carcinoma cells. Oncotarget. 2014; 30: 9992-10001.
- Al-Sharaky DR, Younes SF. Sensitivity ans specificity og galectin-3 and glypican-3 in follicular patterned and other thyroid neoplasms. Journal of Clinical and Diagnostic Research. 2016; 10: 6-10.
- Abd El, Raouf SM, Ibrahim TR. Immunohistochemical expression of HBME-1 and galectin-3 in the differential diagnosis of follicular derived thyroid nodules. Pathology research and Practice. 2014; 210: 971-978.
- Dunderovic D, Lipkovski JM, Boricic I, Soldatovic I, Bozic V, Cvejic D and Tatic S. Defining the value of CD56, CK19, Galectin 3 and HBME 1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literatüre. Diagnostic Pathology. 2015; 10: 196-213.
- Krzeslak A, Lipinska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004; 9: 305-328.
- Kawachi K, Matsushita Y, Yanezawa S, Nakano S, Shirao K, Natsugoe S, et al. Galectin-3 expression in various thyroid neoplasms and its possible role in metastasis formation. Hum Pathol. 2000; 31: 428-433.
- Herrman ME, LiVolsi VA, Pahsa TL, Roberts SA, Wojcik EM, Baloch ZW, et al. Immunohistochemical expression of Galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med. 2002; 126: 710-713.
- Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004; 19: 543-549.
- Akinoto Y, Hirabayashi J, Kasai K, Hirano H. Expression of the endogenous 14- kDa beta- galactoside- binding lectin Galektin-3 in normal human skin. Cell Tissue Res. 1995; 280: 1-10.
- Chirariotti L, Berlingieri MT, battaglia C, Benvenuto G, Martelli ML, Salvatore P, et al. Expression of Galectin-1 in mormal human thyroid gland and in differentiated and poorly differentiated thyroid tumors. Int J Cancer. 1995; 64: 171-175.
- Castronovo V, Van Den Brule FA, Jackers P, Clausse N, Liu FT, Gillet C, et al. Decreased expression of Galectin-3 is associated with progression of human breast cancer. J Pathology. 1996; 179: 43-48.
- Idikio H. Galectin-3 expression in human breast carcinoma: Correlation with cancer histologic grade. Int J Oncol. 1998; 12: 1287-1290.
- Saggiorato E, Cappia S, de Giuli P, Mussa A, Pancani G, Caraci P, et al. Galectin-3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular thyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism. 2001; 86: 5152-5158.
- Orlandi F, Saggiorato E, Pivano G, Puligheddu B, Termine A, Cappia S, et al. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Research. 1998; 58: 3015-3020.
- Fischer S, Asa SL. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008; 132: 359-372.
- Zhu X, Sun T, Lu H, Zhou X, Lu Y, Cai X, Zhu X. Diagnostic significance of CK19, RET, galectin-3 and HBME-1 expression for papillary thyroid carcinoma. J Clin Pathol. 2010; 63: 786-789.
- Mehrotra P, Okpokam A, Bouhaidar R, Johnson SJ, Wilson JA, Davies BR, et al. 2004. Galectin-3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology. 2004; 45: 493-500.
- Bartolazzi A, Gasbarri M, Papotti G, Bussolati T, Lucante A, Khan H, et al. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. 2001; 357: 1644-1650.
- Lee YM, Lee JB. Prognostic value of epidermal growth factor receptor, p53 and galectin-3 expression in papillary thyroid carcinoma. Journal of International Medical Research. 2013; 41: 825-834.
- Xu XC, el-Naggar AK, Lotan R. Differential expression of Galektin-1 and Galektin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995; 147: 815-822.
- Papotti M, Rodriguez J, Pompa R, Bartolazzi A, Rosai J. Galectin-3 and HBME-1 expression in well-differentiated thyroid tumors with follicular architecture of uncertain malignant potential. Mod Pathol. 2005; 18: 541-546.
- Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular derived thyroid nodules. Diagn Pathol. 2010; 5: 9-11.
- Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, Amino N, et al. Ubiquitous expression of galectin-3 mRNA in benign and malignant thyroid tumors. Cancer Lett. 2003; 199: 69-73.
- Türkoz HK, Oksüz H, Yurdakul Z, Ozcan D. Galectin-3 expression in tumor progression and metastasis of papillary thyroid carcinoma. Endocr Pathol. 2008; 19: 92-96.
- Salajegheh A, Dolan-Evans E, Sullivan E, Irani S, Rahman A, Vosgha H, et al. The expression profiles of galectin gene family in primary and metastatic papillary thyroid carcinoma with particular emphasis on galectin-1 and galectin-3 expression. Experimental and Molecular Pathology. 2014; 96: 212- 218.