
Special Article – Endoscopic Surgery
Austin J Surg. 2019; 6(22): 1220.
Obliteration Operation of Cavity for Symptomatic Sellar Arachnoid Cysts via Endoscopic Endonasal Transsphenoidal Approach
Wang P, Wang JW, Zou DW and Wu N*
Department of Neurosurgery, Southwest Hospital, Army Military Medical University, China
*Corresponding author: Wu Nan, Department of Neurosurgery, Southwest Hospital, Army Military Medical University, Gaotanyan 30#, Shapingba, Chongqing, China
Received: September 05, 2019; Accepted: October 21, 2019; Published: October 28, 2019
Abstract
Background: Various ways are used to treat symptomatic sellar arachnoid cysts (ACs) such as cyst aspiration and wall fenestration; however, many complications exist and it is easy to recur.
Object: This paper described a new way to alleviate symptomatic sellar arachnoid cysts.
Methods: Retrospective analysis for the patients who underwent an endonasal transsphenoidal obliteration of symptomatic sellar ACs with gelatin sponge and biological membrane.
Results: Between January 2015 and December 2018, 8 patients (3 women and 5 men, mean age 40 years) with symptomatic sellar ACs were identified. Clinical presentation included headache (5 patients), impaired vision (5 patients) and vision field defects (4 patients). During intraoperative, we repaired diaphragma sellae using biological membrane; then obliterating cyst cavity with gelatin sponge; and the last repaired sellar floor with two biological membranes. Headache, impaired vision and vision field defects all improved postoperatively. One case occurred with complication of CSF leak; but recovery after two weeks. No endocrine dysfunction, meningitis or neurological deficits occurred. The mean follow-up time were 10.5 months (from 9 to 15 months). Cyst cavity volume eliminated in two patients and other six patients experienced cyst shrinking from MR imaging.
Conclusion: Symptomatic sellar ACs can be effectively cured by cyst obliteration with gelatin sponge and repaired diaphragma sellae and sellar floor with biological membrane via endonasal transsphenoidal for patients. It is with low complications occurring and the recurrence rate is low. It is a good way to alleviate symptoms for sellar ACs.
Keywords: Endoscopic endonasal transsphenoidal approach; Symptomatic sellar arachnoid cysts; Obliteration operation with gelatin sponge; Biological membranes; low recurrence rate
Abbreviations
ACs: Arachnoid cysts; CSF: Cerebrospinal Fluid; MR imaging: Magnetic Resonance Imaging; SAS: Subarachnoid Space
Introduction
Arachnoid cysts (ACs) arises in brain and most of them are located in the middle cranial fossa with roughly 3% growing in the sellar [1-3]. Some sellar ACs are asymptomatic and does not need to be treated. However, some others present symptoms such as headache, endocrine dysfunction or diminution of vision; and the symptoms ACs should be treated [4-6]. Unfortunately, the optimal treatment of symptomatic sellar ACs is always a challenge to neurosurgeons [5,7]. According to the literatures, fenestration of the cyst’s anterior wall through transsphenoidal treatment or craniotomy and excision of the cyst’s membranes maybe a good way to alleviate symptoms [1,8-10]. However, the recurrence of ACs is so high that many neurosurgeons holds a controversial opinion on this method. Besides this idea, some authors prefer to obliteration of the cyst cavity alone and the materials used for cavity obliteration are always fat, fascia and muscles [11- 14]. Unlucky, there also exist many complications such as visual loss [14,15], CSF leak [11,13,14,16], intracranial infection [17] and the most important is that it will cause extra damage for other tissues because the fat, fascia and muscles derive from patients themselves [14]. Actually, another obvious disadvantage is the difficulty to control infection once it (intracranial or sphenoid sinus infection) occurs.
Based on the literature reviews above we described a new way to operate this surgery. In this process, we made a fenestration of the cyst’s anterior wall firstly and used biological membrane to reinforce diaphragma sellae; then obliterating the cyst cavity with gelatin sponge; nest, two biological membranes were used to repair sellar floor; one was located between subdural and gelatin sponge, the other was kept in epidural. In this study, we described 8 patients who experienced this surgery and as a result the volume of ACs got smaller for 6 patients and another two of them even vanished completely. In addition, the most significance is its recurrence is relatively low and it does not destroy CSF flow dynamics for most traditional method that always enlarge the communication between the AC and SAS.
Methods
Patient’s information
8 patients in this study were operation between January 2015 and December 2018, and the age ranged from 32 to 49 years. The symptoms included headache, endocrine dysfunction, diminution of vision or vision field defects (Tables 1 & 2). The symptoms kept the longest was 1.6 years and the least was over 1 month. In these symptoms headache and diminution of vision were the most common and vision field defects kept in the second. All of them were operated via endonasal transsphenoidal approach; repaired diaphragma sellae using biological membranes made with collagen substitute [18], obliterated cavity using gelatin sponge and repaired sellar floor with two biological membranes.
Preoperative evaluation
Firstly, pain evaluation was done by VAS score; optical examination employed uncorrected visual acuity (UCVA) and visual field was detected through 2010 Carl Zeiss Meditec. Standard hormonal assays were detected to represent endocrine function, including levels for adrenocorticotropic hormone (ACTH), cortisol (COR), thyroid-stimulating hormone (TSH) and thyroxine (T3, T4, FT3, FT4), growth hormone (GH), Insulin-like growth factor-1 (IGF-I), insulin-like growth factor binding protein-3 (IGFBP-3), luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone (T), corporin (P), estradiol (E2) and prolactin (PRL). No patients received hormone replacement therapy before surgery. Besides, diabetes insipidus defined as urine volume over 4000ml was assessed for each patient. The last we detected electrolyte (K+, Na+, Cl-) and assessed whether it was in the normal range.
Postoperative evaluation
Pain VAS score also was important and assessed after operation 7d. Then was the vision and visual field examination postoperative day 5-7. Visual function was considered improved if UCVA value increased and visual field defect decreased. Besides, standard hormonal assays including ACTH, COR, TSH, T3, T4, FT3, FT4, GH, IGF-1, IGFBP-3, LH, FSH, T, P, E2 and PRL were demonstrated after surgery 1d, 3d, 7d and 1 months for postoperative patients. Besides, diabetes insipidus was assessed through urine volume and detected every 1h and 24h. Electrolyte (K+, Na+, Cl-) was employed to detect and alleviated if it was imbalance.
MR Imaging
All patients underwent enhanced brain MR imaging (Diffuse+ FLAIR) pre- and postoperative. After surgery, MR imaging was first examined on day 1 and then subsequent detection at 3 to 6- month intervals; and then re-examination every 1 to 2- years.
Surgical techniques
Before surgery, vibrissa was cut off completely and all patients were operated through a direct endonasal trans sphenoidal approach via neuroendoscopy in the whole surgery. The whole steps were as follows; looked for sphenoidal ostium, made mucosal flap from nasal septum, opened sphenoid sinus anterior wall, abraded bone around sphenoid sinus, cut sellar floor and cyst membrane, released liquid from cyst cavity, observed diaphragma sellae, repaired diaphragm sellae, obliterated cyst cavity with gelatin sponge, repaired sellar floor using biological membrane with a location between subdural and gelatin sponge, repaired sellar floor using another biological membrane with a location at epidural, put hemostatic gauze on the second biological membrane, put mucosal flap on hemostatic gauze and the last put tela iodoformum in nose. Here we should take care was abrading sellar floor bone slightly and let sellar bone opening large enough. Dura was cut by bistoury and scissored dura via endoscopic scissor in a U-shaped fashion. The dura didn’t need to open too large but it was large enough to pass through the endoscope and we should pay attention to the opening margin couldn’t extend to lateral sellar edges so that a dural margin remained circumferentially to help to hold the biological membrane and promoted gelatin sponge obliterated cyst cavity. Then opened ACs membrane and acquired some ACs membrane to pathology. Once ACs membrane was opened, clear liquid was pouring forth and then observed vascularity in diaphragm sellae, diaphragm pulse with breath and arachnoid diverticula using the endoscope. According to the size of diaphragm sellae and then tailored biological membrane which must cover the whole diaphragm sellae. The next was obliterating cyst cavity with gelatin sponge and we must insure there was no cavity residual. However, excess gelatin sponge should be avoided because extra optic injury may be produced if there were much more gelatin sponge. Next, cut out two suitable biological membranes, both them must cover the dura opening and extended to dura opening at least 1-2mm. One biological membrane was put into subdural and another was in epidural. All the process must not damage the pituitary gland to avoid worsening pituitary dysfunction. Then covered hemostatic gauze and mucosal flap on outer biological membrane; and lastly put tela iodoformum in nose and pulled it out after surgery 3 to 7d. A brief summary of the surgical procedure described above was listed as follows (Figure 1). For the patients, not all of they did lumbar cistern drainage pre- and postoperative.
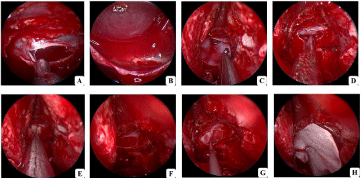
Figure 1: Intraoperative steps for the intrasellar AC operation (Case 1). A Cut sellar floor and cyst membrane; B Released liquid from cyst cavity and observed
diaphragma sellae; C-D Repaired diaphragm sellae using biological membrane; E-F Obliterated cyst cavity with gelatin sponge; G Repaired sellar floor using
biological membrane with a location between subdural and gelatin sponge; H Repaired sellar floor using biological membrane with a location at epidural.
Results
Patients’ information
As shown in Tables 1-3, 3 women and 5 men with a mean age of 40 years (range from 32 to 49 years) were identified. Clinical presentation included headache (5 patients; case 3, 4, 5, 6 and 8), low energy (2 patients; case1 and 5), endocrine dysfunction (none), diminution of vision (5 patients; case 1, 2, 4, 5 and 8), visual field defects (4 patients; case 1, 2, 5 and 8), hyponatremia (none), diabetes insipidus (none) and electrolyte imbalance (none). The most common symptom was headache (5 patients; 62.5%) and diminution of vision (5 patients; 62.5%) followed by visual field defects (4 patients; 50.0%); the third was low energy (2 patients; 25.0%). There was no endocrine dysfunction, hyponatremia, diabetes insipidus and electrolyte imbalance. For headache, the vas score of two patients was 1 (case 3 and 6; 25.0%), and another three patients the vas score was 2 (case 4, 5 and 8; 37.5%). Optical damage mainly caused diminution of vision occurring 5 in 8 patients (case 1, 2, 4, 5 and 8; 62.5%); and the second was visual field defects (4 in 8 patients; case 1, 2, 5 and 8; 50.0%) and 4 patients had both diminution of vision and visual field defects (case 1, 2, 5 and 8; 50.0%). According to MR imagine, the mean diameter was 1.5×1.7×1.7 cm with the largest diameter was 2.5×2.4×3.5 cm (case 1) and the shortest was 0.9×1.0×1.0 cm (case 7).
Endonasal treatment
There were 5 cases requiring a vertical incision (case 2, 4, 5, 6, 7) and the other (case 1, 3 and 8) required horizontal incision to access to the cyst according to the location of pituitary gland (Table 3). We send one cyst membrane (case 1) to pathological examination and the results showed it was arachnoid tissue (Figure 1A). We send the liquid of two cases (case 1 and 5) to test CSF routine and biochemistry and the results showed it was CSF. After the liquid in cyst cavity pouring forth completely, we observed cyst’s cavity and diaphragma sellae using endoscope; we found abundant vessels formation on diaphragma sellae, which pulsed with breath. One case infected after surgery in sphenoid sinus and cyst cavity; we opened sphenoid sinus and cyst cavity again; cleaned out all the gelatin sponge and found there was no CSF in the cavity.
Endocrine, Visual Improved, Pain alleviating, MR Imaging, Urine volume and Electrolyte outcome
According to Tables 1-3, all patients underwent endonasal cyst fenestration and obliteration using gelatin sponge successfully. After surgery for 1-3d, MR imaging detected and all cyst cavities for every patient were obliterated with gelatin sponge successfully. After operation 1d, all the patients felt painful with VAS score from 1 to 3, however all of them alleviated after surgery at 7d. Then long-term followed-up these patients and the mean length of MR imaging was 10.5 months (range from 9 to 15 months). We found the whole of patients recovered very soon. The proportion of cyst cavity obliteration entirely without any CSF re-accumulation was 25% for MR imaging at 9 months (Case 1, Figure 2) and 15 months after surgery (case 4); and 75% of cyst cavity became smaller after postoperative for MR imaging (case 2, 3, 5, 6, 7 and 8). For complications, there was one case with the complication of postoperative CSF leak, who recovered after staying in bed for 2 weeks; vascular injuries and meningitis were not found. For another symptoms after operation, they also improved his/her visual fields and no patients formed new visual defects. Regarding endocrine dysfunction, hormone deficiency was not detected after surgery. 2 patients after surgery with hyponatremia had no further episodes for 2 weeks (Case 5) and 1 months (Case 7) since surgery. For urine volume, the ration of diabetes insipidus was 0% and the mean urine volume was 2000 ml (1500 to 3000 ml).
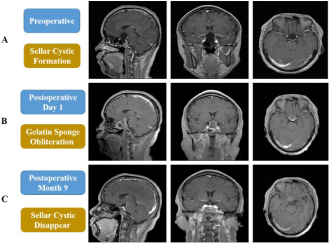
Figure 2: Pre- and postoperative MR imaging for patient (Case 1). A Sellar cystic formation preoperative MR imaging; B Gelatin sponge obliteration successfully
postoperative day 1; C Sellar cystic disappeared postoperative 9 months.
Case Report
39-years-old man (case 1) had a nine-month history of diminution of vision (left 0.12, right 0.20; Table 2) and vision field defect (Table 2). No other symptoms existed. MR imaging showed a saddle area cystic sellar lesion measuring 2.5×2.4×3.5 cm; the pituitary gland and stalk were located at posterior and lateral (Figure 2A). His preoperative endocrine and electrolyte detection showed all were normal. Because of his obvious symptoms and the large size of cyst, an endonasal cyst fenestration and obliteration with gelatin sponge was performed (Figure 1). We used endoscope to observe diaphragma sellae and found a defect of diaphragma sellae; then repairing it with biological membrane; obliterating the cyst cavity with gelatin sponge appropriately; the last repaired sellar floor with two biological membranes, one was between subdural and gelatin sponge and another was in epidural. Postoperative day one MR imaging showed obliteration of the cyst cavity with gelatin sponge successfully (Figure 2B), and there was no CSF leak, hormonal dysfunction, diabetes insipidus, electrolyte imbalance and encephalitis (Table 1). The vision improved at postoperative day 7. His last MRI imaging detection was after surgery 9 months and there was no cyst re-accumulation (Figure 2C).
Preoperative symptoms
Postoperative symptoms
Complications
Recurrence
Case
Age
Sex
Headache(VAS)
Vision Changing
Hormone Changing
Headache
Vision
Hormone Changing
CSF
Infections
Follow-up time
Yes/No
alleviating
Improvement
Leak
39
M
No
Yes
No
/
Improvement
/
No
No
9 M
No
2
38
M
No
Yes
No
/
/
/
No
No
9 M
No
3
32
M
Yes (1)
No
No
alleviating
Improvement
/
Yes
No
11 M
No
4
36
F
Yes (2)
Yes
No
alleviating
Improvement
/
No
No
15 M
No
5
46
F
Yes (2)
Yes
No
alleviating
Improvement
/
No
No
10 M
No
6
38
M
Yes (1)
No
No
alleviating
Improvement
/
No
No
13 M
No
7
43
F
No
No
No
/
/
/
No
No
11 M
No
8
49
M
Yes (2)
Yes
No
alleviating
Improvement
/
No
No
6 M
No
Note: VAS represents - VAS pain grade
Table 1: The information for intrasellar ACs patients pre- and postoperative.
Preoperative Vision Changing
Postoperative Vision Improvement
Case
Age
Sex
Eyesight
Vision field defect
Eyesight
Vision field defect improvement
Left
Right
Yes/No
Left
Right
Yes/No
1
39
M
0.12
0.2
Yes
0.9
0.7
No
2
38
M
0.1
0.5
Yes
0.7
0.6
Yes
3
32
M
1.5
1.5
No
1.5
1.5
/
4
36
F
0.8
0.6
No
0.9
0.9
/
5
46
F
0.7
0.5
Yes
1
0.9
Yes
6
38
M
1.5
1.5
No
1.5
1.5
/
7
43
F
1.5
1.5
No
1.5
1.5
/
8
49
M
0.7
0.2
Yes
1
0.7
Yes
Note: Eyesight detection via standard logarithmic visual acuity chart; Vision field detection via Carl Zeiss Meditec
Table 2: The vision information for intrasellar ACs patients pre- and postoperative.
Case
Age
Sex
Preoperative MRI
Intraoperative
Postoperative MRI
Diameter (cm)
PG
Location
Seeing defect in diaphragma sella
Diameter (cm)
Follow-up
time
1
39
M
2.5×2.4×3.5
posterior, lateral
Yes
0.0×0.0×0.0
9 M
2
38
M
1.6×1.8×1.7
superior, lateral
No
0.3×0.4×0.2
9 M
3
32
M
0.9×1.5×1.1
posterior
Yes
0.3×0.5×0.7
11 M
4
36
F
1.5×2.0×1.8
posterior, lateral
Yes
0.0×0.0×0.0
15 M
46
F
2.2×1.7×1.8
posterior, lateral
No
1.1×1.0×0.8
10 M
6
38
M
0.9×1.2×0.7
posterior, lateral
Yes
0.2×0.5×0.3
13 M
7
43
F
0.9×1.0×1.0
superior, lateral
No
0.3×0.6×0.5
11 M
8
49
M
1.5×1.7×1.9
superior, posterior
Yes
0.3×0.5×0.7
6 M
Note: PG: Pituitary Gland
Table 3: The MR imaging for intrasellar ACs patients pre- and postoperative.
Discussion
The mechanism of intrasellar arachnoid cysts (ACs) formation remains unknown and two opinions in all contribute to the mechanism of ACs formation [12,17,19]. The first opinion attributes to a defective of diaphragma sellae, and basal arachnoid membrane pass the defective diaphragma sellae and herniates; thus leading to a formation of hump, which actually maybe an arachnoid diverticulum. This arachnoid can make a communication between subarachnoid space (SAS) and cyst’s cavity. However, this arachnoid diverticulum may close because of a dynamic reconfiguration of the cyst’s normal structure, an adhesion of the arachnoid caused by meningitis, hemorrhagic and inflammatory. As a result, there was no communication between SAS and cyst’s cavity; thus leading to the formation of ACs [12,17]. The second states that seller ACs locate between the arachnoid layers, which may be originated above the diaphragm and expand along the aperture. Besides, other authors also find basal arachnoid membrane covering the diaphragma sellae enlaregs not only superiorly but also inferiorly along the pituitary stalk. Just because this extending for basal arachnoid membrane, the intrasellar ACs may be formed gradually and then CSF penetrate into the cyst cavity through the weak area of diaphragma sellae [19,20].
Another controversial issue is how cyst cavity enlarges. Traditional studies think it attributes to either a secretory mechanism of the arachnoid cells allowing fluid volume become more and more gradually or a result from a “ball-valve” mechanism allowing fluid into cyst but blocking its outflow [1,20,21]. Besides, subtle osmotic gradients may be another factor to promote the growth of arachnoid cysts [10]. However, in our study we found fluid poured out of the cavity and detected it through CSF routine and biochemistry for two cases and the results proved this fluid was accordance with the feature of CSF, which proved indirectly that seller cyst is formed through “ball-valve” mechanism, since if other mechanism such as a secretory mechanism or subtle osmotic gradients mechanism promotes cyst growth, the character of the fluid is different from CSF. What’s more, we found the defects of diaphragma sellae directly in 5 cases; the same phenomenon was found by Schroeder and Gaab and they observed the arachnoid membrane opened and closed with basilar artery pulsation. Beyond doubt in my opinion the growth of cyst cavity come from the results of “ball-valve” mechanism.
Symptoms ACs need surgery and many ways are used to operate seller cyst. Firstly, building a permanent communication between cyst cavity and the intraventricular or subarachnoid space is used in traditional [22]. Various treatments such as cyst aspiration, wall fenestration, wall excision, cystoventriculostomy and cystoperitoneal are confirmed useful [23,24]. unluckily, many complications exist and it is easy recurrence; thus leading to operation again [25,26]. The postoperative complications through this method include seizures, neurological injury, infection, cerebritis, intracystic haemorrhage, endocrine dysfunction, catheter occlusion, cyst reaccumulation, subdural hygroma, hyponatraemia, electrolyte imbalance and failure to alleviate symptoms that led to a second intervention [13,22]. According to a survey the study showed there were even 60% recurrence with high mortality and morbidity through a cyst fenestration via an open craniotomy. Compared with craniotomy the risk of complications is much lower for cyst fenestration via endoscope; but cyst volume can’t shrink completely and is easily recurrence [28-30]. Recently, Kenichi Oyama et al [16] did a new surgery for this kind of disease. Firstly, opened a small bony window from the upper third of sellar floor to the planum sphenoidale; then I-shaped keyhole dural opening about 1cm was made; next, opened the arachnoid membrane of the prechiasmatic cistern; Subsequently, opened the anterior surface of the cyst wall after an examination of the structure around the cyst wall; thus result of a communication between the cyst cavity and the prechiasmatic cistern; the last, fascia lata was performed as dural plasty and sutured it with monofilament sutures. In this group, 6 patients followed-up visit and there was no CSF leak after surgery. However, two case recurred due to the reformation of the cyst and experienced a surgery again.
In our group, we repaired the diaphragma sellae defects with biological membranes and tamped the cyst cavity with gelatin sponge; then repaired sellar floor cleft with two biological membranes. Actually, the same way has been done by McLaughlin et al [14]. The difference between McLaughlin and our group is the material we used. They used fat, however we used gelatin sponge. In our opinion, we think gelatin sponge is more suitable for the stuffing, because once infection happens after surgery, it is not easy to control as fat is no blood supply. For gelatin sponge it is easy to absorb completely and formed adhesion so that it can consolidate the diaphragma sellae defects and if it is infection after surgery, it is easy to control. Besides it will not cause extra damage for other tissues is another advantage.
Conclusion
Symptomatic intrasellar ACs need to be treated. The best way to alleviate symptoms and avoid recurrence is to repair diaphragma sellae defects, tamp cyst cavity with gelatin sponge, repair sellar floor cleft using artificial dura mates. This way is a good way to cure this disease and the recurrence is so low. The last I want to share is that endoscope is a good instrument in this surgery compared with craniotomy.
Acknowledgement
This work is supported by Science foundation of southwest hospital (SWH2016ZDCX2004).
References
- Chen Y, Fang HJ, Li ZF, Yu SY, Li CZ, Wu ZB, et al. Treatment of Middle Cranial Fossa Arachnoid Cysts: A Systematic Review and Meta-Analysis. World Neurosurg. 2016; 92: 480-490.
- El-Ghandour NM. Endoscopic treatment of middle cranial fossa arachnoid cysts in children. J Neurosurg Pediatr. 2012; 9: 231-238.
- Li Y, Chen X, Xu B. The efficacy of neuroendoscopic treatment for middle cranial fossa arachnoid cysts assessed by MRI 3D segmentation and modeling. Childs Nerv Syst. 2014; 30: 1037-1044.
- Andre A, Zerah M, Roujeau T, Brunelle F, Blauwblomme T, Puget S, et al. Suprasellar Arachnoid Cysts: Toward a New Simple Classification Based on Prognosis and Treatment Modality. Neurosurgery. 2016; 78: 370-379.
- Hall S, Smedley A, Sparrow O, Mathad N, Waters R, Chakraborty A, et al. Natural History of Intracranial Arachnoid Cysts. World Neurosurg. 2019.
- Lee JY, Lee YA, Jung HW, Chong S, Phi JH, Kim SK, et al. Long-term endocrine outcome of suprasellar arachnoid cysts. J Neurosurg Pediatr. 2017; 19: 696-702.
- Charalampaki P, Filippi R, Welschehold S, Conrad J. Endoscopic and endoscope-assisted neurosurgical treatment of suprasellar arachnoidal cysts (Mickey Mouse cysts). Minim Invasive Neurosurg. 2005; 48: 283-288.
- Castle-Kirszbaum MD, Uren B, King J, Wang YY, Goldschlager T. Glimpse into Pathophysiology of Sellar Arachnoid Cysts. World Neurosurg. 2018; 119: 381-383.
- Shim KW, Park EK, Lee YH, Kim SH, Kim DS. Transventricular endoscopic fenestration of intrasellar arachnoid cyst. Neurosurgery. 2013; 72: 520-528.
- Thompson TP, Lunsford LD, Kondziolka D. Successful management of sellar and suprasellar arachnoid cysts with stereotactic intracavitary irradiation: an expanded report of four cases. Neurosurgery. 2000; 46: 1518-1522.
- Cavallo LM, Prevedello D, Esposito F, Laws ER, Jr., Dusick JR, Messina A, et al. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008; 31: 55-64.
- Dubuisson AS, Stevenaert A, Martin DH, Flandroy PP. Intrasellar arachnoid cysts. Neurosurgery. 2017; 61: 505-513.
- Lorente-Munoz A, Lisbona-Alquezar MP, Alberdi-Vinas J, Orduna-Martinez J, Gonzalez-Martinez L, Fernandez-Liesa R. Intrasellar arachnoid cysts. Two case reports and literature review. Neurocirugia (Astur). 2013; 24: 277-282.
- McLaughlin N, Vandergrift A, Ditzel Filho LF, Shahlaie K, Eisenberg AA, Carrau RL, et al. Endonasal management of sellar arachnoid cysts: simple cyst obliteration technique. J Neurosurg. 2012; 116: 728-740.
- Weil RJ. Rapidly progressive visual loss caused by a sellar arachnoid cyst: reversal with transsphenoidal microsurgery. South Med J. 2001; 94: 1118- 1121.
- Oyama K, Fukuhara N, Taguchi M, Takeshita A, Takeuchi Y, Yamada S. Transsphenoidal cyst cisternostomy with a keyhole dural opening for sellar arachnoid cysts: technical note. Neurosurg Rev. 2014; 37: 261-267.
- Hornig GW, Zervas NT. Slit defect of the diaphragma sellae with valve effect: observation of a “slit valve”. Neurosurgery. 1992; 30: 265-267.
- Deng K, Yang Y, Ke Y, Luo C, Liu M, Deng Y, et al. A novel biomimetic composite substitute of PLLA/gelatin nanofiber membrane for dura repairing. Neurol Res. 2017; 39: 819-829.
- Meyer FB, Carpenter SM, Laws ER, Jr. Intrasellar arachnoid cysts. Surg Neurol. 1987; 28: 105-110.
- Yuce O, Doger E, Celik N, Emeksiz HC, Bulduk EB, Camurdan MO, et al. Extensive middle cranial fossa arachnoid cysts and different clinical presentation in two patients. J Clin Res Pediatr Endocrinol. 2014; 6: 174-176.
- Kimiwada T, Hayashi T, Narisawa A, Shirane R, Tominaga T. Shunt placement after cyst fenestration for middle cranial fossa arachnoid cysts in children. J Neurosurg Pediatr. 2015; 16: 533-539.
- Guzel A, Trippel M, Ostertage CB. Suprasellar arachnoid cyst: a 20- year follow-up after stereotactic internal drainage: case report and review of the literature. Turk Neurosurg. 2007; 17: 211-218.
- Murakami M, Okumura H, Kakita K. Recurrent intrasellar arachnoid cyst. Neurol Med Chir (Tokyo). 2003; 43: 312-315.
- Ozek MM, Urgun K. Neuroendoscopic management of suprasellar arachnoid cysts. World Neurosurg. 2013; 79: S19 e13-18.
- Ersahin Y, Kesikci H, Ruksen M, Aydin C, Mutluer S. Endoscopic treatment of suprasellar arachnoid cysts. Childs Nerv Syst. 2008; 24: 1013-1020.
- Ogiwara H, Morota N, Joko M, Hirota K. Endoscopic fenestrations for suprasellar arachnoid cysts. J Neurosurg Pediatr. 2011; 8: 484-488.
- Baykan N, Isbir O, Gercek A, Dagcnar A, Ozek MM. Ten years of experience with pediatric neuroendoscopic third ventriculostomy: features and perioperative complications of 210 cases. J Neurosurg Anesthesiol. 2005; 17: 33-37.
- Golash A, Mitchell G, Mallucci C, May P, Pilling D. Prenatal diagnosis of suprasellar arachnoid cyst and postnatal endoscopic treatment. Childs Nerv Syst. 2001; 17: 739-742.
- Sood S, Schuhmann MU, Cakan N, Ham SD. Endoscopic fenestration and coagulation shrinkage of suprasellar arachnoid cysts. Technical note. J Neurosurg. 2005; 102: 127-133.
- Tirakotai W, Schulte DM, Bauer BL, Bertalanffy H, Hellwig D. Neuroendoscopic surgery of intracranial cysts in adults. Childs Nerv Syst. 2004; 20: 842-851.