Special Article – Surgery Case Reports
Austin J Surg. 2019; 6(22): 1221.
Clinical Characteristics of Capnocytophaga canimorsus: A Case Report and Systematic Literature Review
Aziz Mohammadi M* and Stahl S
Department of Plastic Surgery and Hand and Reconstructive Surgery, Clinical Hospital Luedenscheid, Germany
*Corresponding author: Aziz Mohammadi M, Department of Plastic Surgery, Hand and Reconstructive Surgery, Clinical Hospital Luedenscheid, Germany
Received: September 10, 2019; Accepted: October 23, 2019; Published: October 30, 2019
Abstract
Capnocytophaga canimorsus is a Gram-negative bacterium found in the oral cavity of dogs and cats. Infections with this rod-shaped bacterium can lead to life-threatening septicemia and multiple serious complications. Here, we report the case of a 64-year-old man with no reported risk factors who developed septic shock, multi organ failure, and gangrene of the extremities following a dog bite to the right index finger 3 days prior to the initial symptoms. The patient required intensive treatment, intravenous antibiotic therapy, and multiple surgical procedures including amputation of multiple fingers, toes, and right lower leg. A systematic literature review of 39 cases published in the last 10 years was performed to extrapolate the time to diagnosis and complications by highlighting the risk factors and initial symptoms. Time to diagnosis exceeded 13 days on average. Several cases suggest that smoking may be an underestimated risk factor. Initial symptoms include fever (58%), gastrointestinal manifestations (45%), pain (23%), and malaise (23%). Complication rates may be reduced by early polymerase chain reaction screening and surgical debridement as well as appropriate antibiotic therapy.
Keywords: Dog bite; Capnocytophaga canimorsus; Infection; Amputation; Sepsis; Hand bite
Introduction
Capnocytophaga canimorsus is a Gram-negative bacterium found in the oral cavity of between 25.5% and 74% of dogs and approximately 17% of cats [1,2]. Infections with this rod-shaped bacterium can lead to life-threatening septicemia, with an overall mortality rate of 26%, and is often accompanied with a history of exposure to canines [3]. It has been reported that patients with prior splenectomy, alcoholism, or immune deficiency are more susceptible to infection [3]. As described in several case reports, infection with C. canimorsus can lead to serious complications, including septic shock, organ failure, Disseminated Intravascular Coagulation (DIC), hemolytic-uremic syndrome, thrombotic thrombocytopenic purpura, endocarditis, gangrene of the extremities, and meningitis [3,4]. Here, we report the case of a patient with no related predisposition who developed septic shock, multi organ failure, and gangrene of the extremities following a dog bite to the right index finger. We also searched the Ovid MEDLINE database (January 1, 2007 to July 1, 2017) for “Capnocytophaga canimorsus” and “sepsis.”To minimize the effects of indexing bias, we further included literature from an extensive Internet search and indexed articles. Only English full-text articles were evaluated. Nonsystematic reviews of the scientific literature were classified as expert opinions. Our initial search provided us with 107 articles. We then excluded off-topic publications (e.g., genomic analysis, DNA sequencing, and association with joint arthroplasty) and literature reviews, which resulted in23relevant articles. The reasons for exclusion were documented systematically.
Case Report
A healthy 64-year-old man presented at our clinic with signs of septic shock. Three days before the onset of his symptoms, he had been bitten in the right index finger by his own dog. His medical history revealed transient ischemic attack in 2015 and hypertension. Alcohol abuse was ruled out, but he admitted smoking for 48 pack-years. On arrival, he was dyspnoeic, agitated, and complained of chills. Livedo racemosa was present over his entire body. Examination of his right hand revealed no sign of infection. Laboratory tests showed severe metabolic acidosis, thrombocytopenia, DIC, rhabdomyolysis, and renal failure. Hematologic disease was suspected after peripheral blood smear revealed atypical granulocytes und monocytes. The patient was transferred to our intensive care unit the following day.
Empiric antibiotic treatment with piperacillin/tazobac was initiated after blood samples were obtained for culture. Laboratory data on the second day of admission showed no improvement, so the antibiotic regimen was switched to meropenem with the addition of clindamycin on the following day. Both antibiotics were administered for 6 weeks. Penicillin G and vancomycin were administered intravenously for 3 days until the antibiogram arrived, after which sulbactam/ampicillin was added and continued for 25 days. Continuous venovenous hemodialysis with citrate was initiated 3 days after the first patient contact and continued for 1 month. The patient also received repeated plasmapheresis, packed red blood cell transfusion, and fresh-frozen plasma because of DIC with purpura fulminans and hemolysis.
On the fourth day of admission, surgical debridement of the bite wound was performed. The metacarpophalangeal joint of the index finger was exposed and debrided. No evidence of purulent infection or septic arthritis was found. No bacterial growth was reported on tissue culture obtained during surgery. The wound was covered with polyurethane-silica hybrid foam (Syspur-derm; Hartmann).
The patient developed dry gangrene of multiple digits, toes, and right lower leg. Amputations of the end phalanx of the third digit of both hands, middle phalanx of the fourth digit of the left hand, all toes of the left foot, and second to fifth toes of the right foot were performed on the ninth day of admission (Figure 1). Vacuumassisted closure was applied on the right lower leg. Two days later, debridement was repeated, the left foot was covered with a splitthickness skin graft from the thighs, and the metacarpophalangeal joint of the right index finger was covered with a fasciocutaneous rotational flap. Necrosis of the right lower leg required amputation below the knee. The patient was extubated, vasopressor therapy was reduced, and he was transferred to the medical ward after 30 days of intensive treatment. He recovered well and was released 66 days after admission in good general condition.
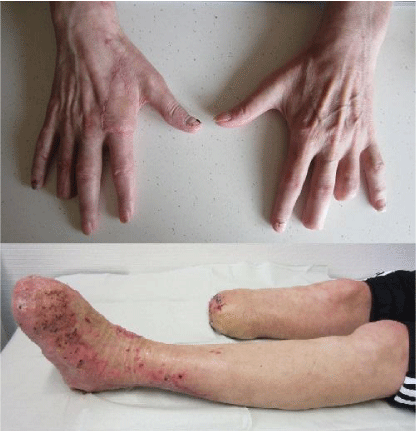
Figure 1: Patient postoperative photographs. Multiple amputations of upper
and lower extremities following a severe Capnocytophaga canimorsus
infection.
Discussion
This case demonstrates the necessity for rapid diagnosis, early surgical debridement, and antibiotic treatment. A literature review was performed to summarize the clinical characteristics of severe C. canimorsus infection in order to reduce the time to diagnosis (Table 1). All patients had been in contact with a canine; with the exception of 1 patient who had been scratched by a cat (Figure 2). Our review revealed that 45% of patients with septic shock involving C. canimorsus had none of the commonly reported risk factors at the time of admission. However, we noticed that little research has been conducted on comorbidities, such as cardiovascular, respiratory, endocrinological, and hematologic diseases, as possible risk factors. Furthermore, in the majority of case reports, smoking was not considered as a risk factor. Nonetheless, tobacco use has been reported to enhance C. Canimorsus growth by increased acquisition of iron, which provides a favorable environment for the bacteria to grow [5]. Only 4 other cases with altered confusional state have been reported (Figure 3) [6-9]. Livedo racemosa and sepsis due to C. canimorsus infection was described in 2 cases [10]. Furthermore, in accordance with previous reports, organ failure followed by acute respiratory distress syndrome was the most common clinical presentation (Figure 3) [3,11,12].
Case
Reference
Type of study
Patients (n)
Age
(years)Sex
Clinical course
Surgical intervention
Time to surgery (days)
1
Submitted
Case report
1
64
M
2, 4, 6a, 10
Yes
4
2
Hawkins J et al., 2017 [5]
Case report
1
68
M
1,4
Yes
6
3
Morandi E et al., 2017 [6]
Case report
1
41
M
2, 3, 4, 8
Yes
Not reported
4
Eefting M et al., 2017 [7]
Case report
1
29
M
3, 4, 8
Unknown
n.a.
5
Hastbacka J et al., 2016 [8]
Cohort study
16
55.5
(50.5–57.5)M (63%), F (37%)
1 (18%), 2 (18%), 3 (56%), 6 (81%), 7 (94%), 8 (56%)
25%
Not reported
6
Dedy N et al., 2016 [9]
Case report
1
68
F
2, 3, 4, 6a, 8
Yes
0
7
Hloch E et al., 2014 [10]
Case report
1
62
F
4, 6ab, 7, 8
Unknown
n.a.
8
Shahani L et al., 2014 [11]
Case report
1
62
M
2, 4, 6a, 7
Yes
14
9
Tan V et al., 2014 [12]
Case report
1
54
M
2, 3, 4, 6a, 8
Yes
24
10
Nishioka H et al., 2014 [13]
Case report
1
80
F
1, 4
None
n.a.
11
Ugai T et al., 2013 [14]
Letter to editor
1
53
F
3
None
n.a.
12
Yamamoto U et al., 2013 [15]
Case report
1
49
M
3, 4, 6ab
None
n.a.
13
Ma A et al., 2013 [16]
Case report
1
56
M
5, 8
None
n.a.
14
Brichacek M et al., 2012 [17]
Case report
1
72
M
1, 5, 8
None
n.a.
15
Matulionyte? R et al., 2012 [18]
Case report
1
46
F
1, 3, 4, 8
Yes
3
16
Christiansen C et al., 2012 [19]
Case report
2
59, 59
M (50%), F (50%)
2, 4, 7, 8
Yes
Not reported
17
Sacks R et al., 2012 [20]
Case report
1
42
F
1, 3
Unknown
n.a.
18
Chary S et al., 2011 [21]
Case report
1
67
M
3, 6a
None
n.a.
19
Band R et al., 2011 [22]
Case report
1
52
M
4, 6a, 7, 8
Unknown
n.a.
20
O'Rourke G et al., 2010 [23]
Case report
1
53
F
2, 4, 6a, 7, 8
Yes
Not reported
21
Stiegler D et al., 2010 [24]
Case report
1
40
M
1
Unknown
n.a.
22
Low S et al., 2008 [25]
Case report
1
48
F
2, 3, 4, 6a, 8
Yes
19
23
Nelson M et al., 2008 [26]
Case report
1
31
M
9
None
None
24
Wald K et al., 2007 [27]
Case report
1
61
F
4, 8
Unknown
n.a.
Descriptive statistics
Σ = 40
μ = 55.1
M (60%), F (40%)
μ = 10
Table 1: Literature review. Clinical course: 1 = death, 2 = amputation, 3 = shock, 4 = disseminated intravascular coagulation, 5 = thrombotic thrombocytopenic purpura, 6 = organ failure ([a] kidney failure, [b] liver failure), 7 = thrombocytopenia, 8 = acute respiratory distress syndrome, 9 = vertebral osteomyelitis, 10 = purpura fulminans. Time to surgery was defined as days until first surgical intervention (day of admission = 0).
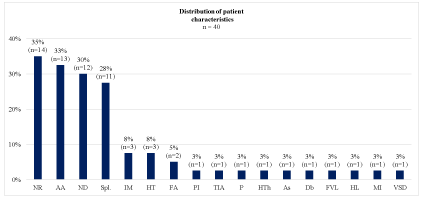
Figure 2: Distribution of patient characteristics.
AA: Alcohol Abuse; As: Asthma; Db: Diabetes; FA: Functional Asplenia
following hematopoietic cell transplant; FVL: FACTOR V LEIDEN; HL:
Hodgkin’s Lymphoma; HT: Hypertension; HTh: Mild Hypothyreosis; IM:
Immunosuppressive Medication; MI: Myocardial Infarction; ND: Nicotine
Dependence; NR: No Reported Predisposition Characteristics; P: Chronic
Pancreatitis; PI: Parasitic Infection; Spl: Splenectomy; TIA: Transient
Ischemic Attack; VSD: Ventricular Septal Defect
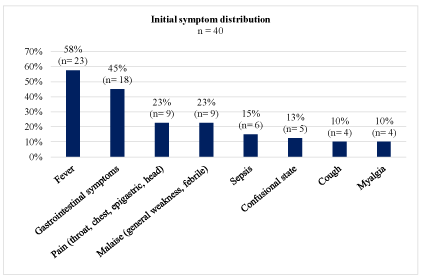
Figure 3: Distribution of initial symptoms.
This article highlights the importance of early recognition of C. canimorsus-involved sepsis in immune competent patients with no obvious risk factors who have been in recent contact with a canine. Although it has been reported that Polymerase Chain Reaction (PCR) is the most accurate diagnostic test for C. canimorsus, we found only 1 case reporting the use of PCR [13,14]. In addition, with a turnaround time of roughly 10 hours, PCR is a quicker diagnostic tool than blood culture [15].
It is well known that C. canimorsus is very difficult to culture [3]. Our review confirmed that diagnostics were confirmed through blood culture an average of 9 days after blood sampling (median, 5.5 days; minimum, 2 days; maximum, 30 days) [6-27]. Initial trauma (ie, dog bite) was reported an average of 4 days before admission (median, 2 days; minimum, 1.5 days; maximum, 28 days) [6-27]. Because none of the reviewed reports mentioned the day of blood sampling relative to the day of admission, uncertainty remains regarding the average time to diagnosis [28-33].
Due to the search terms used, selection bias cannot be ruled out, which may present an overestimation of illness severity. However, the pathogenicity and severity of C. canimorsus infection are well described in the literature. A thorough patient history can contribute to the awareness of potential C. canimorsus infection, which can be confirmed using PCR as a diagnostic tool [15]. Early debridement of animal bites could reduce bacterial load and potentially prevent septicemia.
References
- Blanche P, Bloch E, Sicard D. Capnocytophaga canimorsus in the oral flora of dogs and cats. J Infect. 1998; 36: 134.
- Suzuki M, Kimura M, Imaoka K, Yamada A. Prevalence of Capnocytophaga canimorsus and Capnocytophaga cyno- degmi in dogs and cats determined by using a newly established species-specific PCR. Vet Microbiol. 2010; 144: 172-176.
- Butler T. Capnocytophaga canimorsus: An emerging cause of sepsis, meningitis, and post-splenectomy infection after dog-bites. Eur J Clin Microbiol Infect Dis. 2015; 34: 1271-1280.
- Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: Review of the literature and cases report. Eur J Epidemiol. 1996; 12: 521-533.
- Gaastra W, Lipman LJ. Capnocytophaga canimorsus. Vet Microbiol. 2010; 140: 339-346.
- Hawkins J, Wilson A, McWilliams E. ‘Biting the hand that feeds’: Fever and altered sensorium following a dog bite. BMJ Case Rep. 2011; 28: 1071-1073.
- Morandi E, Pauzenberger R, Trasch C, Rieger U, Pierer G, Djedovic G. A small ‘lick’ will sink a great ship: Fulminant septicaemia after dog saliva wound treatment in an asplenic patient. Int Wound J. 2017; 14: 1025-1028.
- Dedy N, Coghill S, Chandrashekar N, Bindra R. Capnocytophaga canimorsus Sepsis Following a Minor Dog Bite to the Finger: Case Report. J Hand Surg Am. 2016; 41: 81-84.
- Wald K, Martinez A, Moll S. Capnocytophaga canimorsus infection with fulminant sepsis in an asplenic patient: Diagnosis by review of peripheral blood smear. Am J Hematol. 2008; 83: 879-879.
- Sotiriou A, Sventzouri S, Nepka M, Magira EE. Acute generalized livedo racemosa caused by Capnocytophaga canimorsus identified by MALDI-TOF MS. Int J Infect Dis. 2015; 33: 196-198.
- Hastbacka J, Hynninen M, Kolho E. Capnocytophaga canimorsus bacteremia: clinical features and outcomes from a Helsinki ICU cohort.. Acta Anaesthesiologica Scand. 2016; 60: 1437-1443.
- Pers C, Gahrn-Hansen B, Fredriksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: Review of 39 cases. Clin Infect Dis. 1996; 23: 71-75.
- Gottwein J, Zbinden R, Maibach RC, Herren T. Etiologic diagnosis of Capnocytophaga canimorsus meningitis by broad-range PCR. Eur J Clin Microbiol Infect Dis. 2006; 25: 132-134.
- Mally M, Cornelis GR. Genetic tools for studying Capnocytophaga canimorsus. Appl Environ Microbiol. 2008; 74: 6369-6377.
- Beernink TM, Wever PC, Hermans MH, Bartholomeus MG. Capnocytophaga canimorsus meningitis diagnosed by 16S rRNA PCR. Pract Neurol. 2016; 16: 136-138.
- Eefting M, Paardenkooper T. Capnocytophaga canimorsus sepsis. Blood. 2010; 116: 1396.
- Hloch O, Mokra D, Masopust J, Hasa J, Charvat J. Antibiotic treatment following a dog bite in an immunocompromized patient in order to prevent Capnocytophaga canimorsus infection: a case report. BMC Res Notes. 2014; 7: 432.
- Shahani L, Khardori N. Overwhelming Capnocytophaga canimorsus infection in a patient with asplenia. BMJ Case Rep. 2014.
- Tan V, Schwartz J. Renal failure due to Capnocytophaga canimorsus generalized Shwartzman reaction from a dog bite (DF-2 nephropathy). (Proc Bayl Univ Med Cent). 2014; 27: 139-140.
- Nishioka H, Kozuki T, Kamei H. Capnocytophaga canimorsus bacteremia presenting with acute cholecystitis after a dog bite. J Infect Chemother. 2015; 21: 215-217.
- Ugai T, Sugihara H, Nishida Y, Yamakura M, Takeuchi M, Matsue K. Capnocytophaga canimorsus sepsis following BMT in a patient with AML: possible association with functional asplenia. Bone Marrow Transplantation. 2013; 49: 153-154.
- Yamamoto U, Kunita M, Mohri M. Shock following a cat scratch. BMJ Case Rep. 2013.
- Ma A, Goetz M. Capnocytophaga canimorsus Sepsis with associated Thrombotic Thrombocytopenic Purpura. Am J Med Sci. 2013; 345: 78-80.
- Brichacek M, Blake P, Kao R. Capnocytophaga canimorsus infection presenting with complete splenic infarction and thrombotic thrombocytopenic purpura: a case report. BMC Res Notes. 2012; 5: 695.
- Matulionyte R, Lisauskiene I, Kekstas G, Ambrozaitis A. Two Dog-Related Infections Leading to Death: Overwhelming Capnocytophaga canimorsus sepsis in a patient with cystic echinococcosis. Medicina Kaunas. 2012; 48: 112-115.
- Christiansen C, Berg R, Plovsing R, Moller K. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012; 44: 635-639.
- Sacks R, Kerr K. A 42-Year-Old Woman with Septic Shock: An Unexpected Source. J Emerg Med. 2012; 42: 275-278.
- Chary S, Joshi M, Reddy S, Ryan C, Saddi V. Septicemia due to Capnocytophaga canimorsus following dog bite in an elderly male. Indian J Pathol Microbiol. 2011; 54: 368-370.
- Band RA, Gaieski DF, Goyal M, Perrone J. A 52-Year-Old Man with Malaise and a Petechial Rash. The Journal of Emergency Medicine. 2011; 41: 39-42.
- O’Rourke GA, Rothwell R. Capnocytophagacanimorsis a cause of septicaemia following a dog bite: A case review. Aust Crit Care. 2011; 24: 93-99.
- Stiegler D, Gilbert JD, Warner MS, Byard RW. Fatal Dog Bite in the Absence of Significant Trauma: Capnocytophaga canimorsus Infection and Unexpected Death. Am J Forensic Med Pathol. 2010; 31: 198-199.
- Low S, Greenwood JE. Capnocytophaga canimorsus: infection, septicaemia, recovery and reconstruction. J Med Microbiol. 2008; 57: 901-903.
- Nelson MJ, Westfal RE. Case Report: Vertebral Osteomyelitis/Discitis as a Complication of Capnocytophaga canimorsus. Bacteremia. J Emerg Med. 2008; 35: 269-271.