
Special Article – Surgery Case Reports
Austin J Surg. 2019; 6(25): 1229.
Two Cases of Gastric Cancer Treated with Stereotactic Body Radiotherapy Using Cyberknife®
Satani K1*, Onishi H2, Suzuki T1, Morita S3, Nonaka H4, Saito R3, Komiyama T3 and Takahashi H1
1Kasugai CyberKnife Rehabilitation Hospital, Japan
2Department of Radiology, University of Yamanashi, Japan
3Department of Gastroenterology, Shinsyu Ueda Medical Center, Japan
4Department of Radiology, Fujiyoshida Municipal Medical Center, Japan
*Corresponding author: Satani K, Kasugai CyberKnife Rehabilitation Hospital, 436 Kokufu, Kasugai-cho, Fuefuki-city, Yamanashi, 406-0014, Japan
Received: September 26, 2019; Accepted: November 05, 2019; Published: November 12, 2019
Abstract
We report two cases of gastric cancer treated with stereotactic body radiotherapy (SBRT) using CyberKnife®. Case 1 was advanced gastric cancer (T3N0M0 stage IIA) in an 80-year-old man. Endoscopy revealed a bulky type 3 tumor on the lesser curvature of the gastric upper body. Case 2 was recurrent gastric cancer in an 82-year-old man who had undergone endoscopic submucosal dissection for a previous tumor. Endoscopy revealed a bulky recurrent tumor on the gastric cardia. Both the patients received SBRT using CyberKnife® for symptom improvement and local control of the tumor. After treatment, both the patients showed early reduction of the tumor and improvement of the symptoms caused by the tumor, without serious complications or tumor progression for 8 and 4 months, respectively. SBRT was useful as a local treatment with low invasiveness and rapid action for locally advanced gastric cancer. Although some issues still require clarification, such as the optimal prescription dose and fractionation of irradiation, we expect SBRT to be a suitable treatment option for locally advanced gastric cancer in cases where other treatments are difficult to perform, such as elderly patients and patients with complications.
Keywords: Stereotactic body radiotherapy; CyberKnife®; Gastric cancer
Abbreviations
SBRT: Stereotactic Body Radiotherapy; CT: Computed Tomography; ESD: Endoscopic Submucosal Dissection; BED: Biological Effective Dose; OAR: Organs at Risk; GTV: Gross Tumor Volume; PTV: Planning Target Volume; DVH: Dose-Volume Histogram; TLS: Target Locating System; CRT: Conventional Radiotherapy
Introduction
Radiotherapy is often used as pre-operative or post-operative adjuvant therapy or as palliative treatment for advanced gastric cancer to improve hemorrhage or passage disorders [1-9]. The use of Stereotactic Body Radiotherapy (SBRT) has made it possible to concentrate the dose on the lesion and reduce the dose to the surrounding organs, thereby resulting in higher local control and fewer adverse events [10]. Moreover, SBRT can be completed in a short period, reducing the patient’s physical burden. Accordingly, SBRT is expected to be one of the treatment options used in the multidisciplinary treatment for advanced gastric cancer. Herein, we report our experience of performing SBRT for advanced gastric cancer using CyberKnife®.
Case Presentation
Case 1
An 80-year-old man presented with hematemesis, upper abdominal discomfort, and appetite loss, and was diagnosed with advanced gastric cancer. Endoscopy revealed a bulky type 3 tumor on the lesser curvature of the gastric upper body(Figure 1A). Abdominal Computed Tomography (CT) revealed a bulky tumor on the lesser curvature of the gastric body with a diagnosis of cT3N0M0, stage IIA carcinoma (Figure 2A). Biopsy revealed a well-differentiated tubular or papillary adenocarcinoma. The patient did not undergo surgery because of his advanced age, but instead received a daily dose of 80mg S-1. S-1 was administered for 4 weeks, followed by 2 weeks of rest. Tumor growth was controlled for 5 months, after which it regrew. The patient was referred to our hospital for tumor control and symptom improvement.
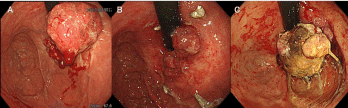
Figure 1: Temporal changes observed on gastroscopy for case 1. (A):
Gastroscopy findings before treatment: endoscopy revealed a bulky type 3
tumor on the lesser curvature of the gastric upper body. (B): Gastroscopy
findings 1 months after treatment: the tumor was obviously reduced with a
remarkable response. (C): Gastroscopy performed 8 months after treatment
showed re-growth of the tumor.
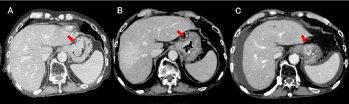
Figure 2: Temporal changes on abdominal computed tomography (CT)
for case 1. (A): CT scan before treatment: CT revealed a bulky tumor on
the lesser curvature of the gastric upper body. (B): CT scan 4 months after
treatment: the bulky tumor was flat and obviously reduced. (C): CT scan 8
months after treatment: Re-growth of the tumor was observed, with ascites
and liver metastasis.
* The red arrows point to the tumor.
Case 2
An 82-year-old man who underwent surgery for right renal cancer was diagnosed with gastric cardia cancer (adenocarcinoma, cT1bN0Mx) during follow-up after multidisciplinary treatment for multiple metastases. Although he underwent Endoscopic Submucosal Dissection (ESD), relapse was observed after 6 months. Endoscopy revealed a bulky recurrent tumor on the gastric cardia (Figure 3A). Abdominal CT revealed a bulky gastric cardia tumor with liver metastasis (Figure 4A). The patient presented with hematemesis, upper abdominal discomfort, and passage obstacle. Biopsy showed a well to moderately differentiated tubular adenocarcinoma. The patient wanted to receive CyberKnife® treatment, for which he was referred to our hospital.
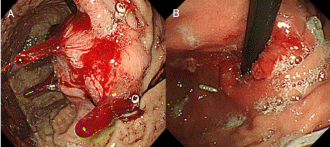
Figure 3: Temporal changes observed on gastroscopy for case 2. (A):
Gastroscopy findings before treatment: endoscopy revealed a bulky recurrent
tumor on the gastric cardia. (B): Gastroscopy findings 1 month after treatment:
the tumor was obviously reduced with a remarkable response.
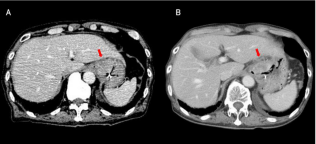
Figure 4: Temporal changes on abdominal computed tomography (CT) for
case 1. (A): CT findings before treatment: CT revealed a bulky recurrent
tumor on the gastric cardia. (B): CT findings 1 month after treatment: the
bulky tumor was flat and reduced.
* The red arrows point to the tumor.
Treatment
Stereotactic body radiotherapy: SBRT precisely administers high target doses in a few fractions and minimizes the dose to adjacent normal tissue structures. Case 1 received 42 Gy (10 fractions of 4.2 Gy per fraction) using CyberKnife® G4 (Accuray, Sunnyvale, CA, USA), corresponding to a Biological Effective Dose (BED) of 59.6 Gy (assuming a/β = 10). Case 2 received 40 Gy (8 fractions of 5 Gy per fraction) using CyberKnife® G4, corresponding to a BED of 60 Gy (assuming a/β = 10). Although the BEDs prescribed in these cases were lower than those for other cancers were [11-13], we ensured that the dose to the Organs at Risk (OAR) does not exceed the tolerated dose. Table 1 shows the OAR dose constraints (Table 1). Gross Tumor Volume (GTV) was determined by considering endoscopic findings and the visible tumor on CT. The Planning Target Volume (PTV) margin was a 3mm increase in the anterior-posterior rightleft direction and a 5mm increase in the superior-inferior direction for GTV. The dose was prescribed using the 95% PTV isodose. The plans were recalculated using the Monte Carlo algorithm. The dose distribution and Dose-Volume Histogram (DVH) of case 1 are shown in Figure 5. Treatment with the CyberKnife® system was performed using fiducial tracking. Metal clips were placed endoscopically around the tumor. Fiducial tracking irradiation was performed using two of five clips in case 1 and two of three clips in case 2. To improve the accuracy further, we frequently acquired images using the Target Locating System (TLS). Patients were irradiated on an empty stomach to ensure the same conditions every time.
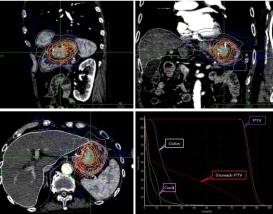
Figure 5: Dosimetric evaluation of computed tomography (CT) images show
the dose distribution in the sagittal, coronal, and axial slices, with a D95
isodose involving the tumor. We planned to concentrate the high dose on the
target. The lower right figure is a dose-volume histogram (DVH).
Organs/tissue at risk
Dose-limiting
Liver
V20 <20%
Stomach
36 Gy =10 cc
30 Gy =100 cc
Intestine
36 Gy =10 cc
30 Gy =100 cc
Spinal cord
Dmax <25 Gy
Table 1: Dose constraints of organs at risk.
CyberKnife®: The CyberKnife® system has a linear accelerator mounted on a robotic arm, providing more flexibility and freedom of motion while delivering the radiation beams. The CyberKnife® system has integrated X-ray imagers that provide continual image guidance and real-time tracking capabilities, ensuring high (i.e., submillimeter) accuracy and precision [14].
Treatment response: Although two of the five placed clips fell off in case 1 and one of the three fell off in case 2 during each treatment period, treatment in both the patients was completed without any complications and without reducing treatment accuracy. The symptoms of hematemesis and upper abdominal discomfort improved from in both cases early after treatment. After 1 month, tumor reduction was confirmed via endoscopic evaluations in both cases. In case 1, the tumors were controlled for 8 months after treatment. However, after 8 months, the tumor rapidly increased in size with ascites retention, and the patient died of disease progression 11 months after treatment (Figures 1B, C, and Figures 2B, C). In case 2, the tumors were controlled for 4 months after treatment. However, the patient died 4 months after treatment owing to deterioration in his general condition (Figures 3B and 4B).
Clinical response
One month after treatment, both cases showed a partial response considering the radiological and endoscopic findings (Figures 1B and 3B).
Adverse event
According to CTCAE scale, v. 4.0., grade 2 gastritis was identified early after treatment in both cases.
Discussion/Conclusion
Although surgery is the standard radical treatment for gastric cancer [15], chemotherapy and palliative treatment are treatment options for inoperable cases owing to advanced stage, advanced age, and complications. Many patients with advanced gastric cancer experience symptoms such as bleeding, obstruction, and perforation of the stomach. These symptoms need to be addressed with topical therapy, as they reduce not only survival but also quality of life. Radiotherapy as palliative treatment is effective for these symptoms [3-5], and higher local tumor control after radiotherapy may benefit patients. The local tumor control rate after radiotherapy is dose dependent. When Conventional Radiotherapy (CRT) was used for palliative treatment of gastric cancer, there was no difference in the palliative effect between high and low BED regimens [16]. In contrast, there was a significant difference in local control when prescription doses were above the BED (assuming a/β = 10) of 41 Gy [17], and higher BED regimens provided better local control. However, the true irradiated dose was unknown because of inter- and intrafractional motion of the stomach, and the optimal prescription dose and fractionation for irradiation have not yet been determined. Furthermore, owing to the adverse effects to the surrounding organs due to the tolerable dose and movement of the surrounding organs, prescription doses cannot be easily increased.
Therefore, treatment requires dose concentration and accuracy. We expected that SBRT could be used to resolve these issues, and CyberKnife® was a suitable option. SBRT using CyberKnife® made it possible to concentrate the dose on the lesion and reduce the dose to surrounding organs. The prescription BED (assuming a/β = 10) was 59.6 Gy in case 1 and 60.0 Gy in case 2. Because of the accuracy obtained via image-guided radiotherapy, it was possible to set a small margin and to quantify more precisely irradiated dose, thereby making it possible to increase the prescription dose. Although these doses exceeded the BED value of irradiation for gastric cancer using CRT considering palliation and local control, the margins are different for CRT and SBRT; hence, it is unclear if the use of increased doses can be generalized for all situations. When SBRT was used to treat cancer at other sites including lung cancer, a BED of ≥100 Gy was effective [18-21], which was less than that. Moreover, the local control period was 8 months in case 1; however, as the tumor size increased rapidly thereafter, the dose seemed insufficient. Furthermore, the optimal prescription dose and fractionation schedule need to be standardized in future studies. However, both the patients that we treated could undergo complete treatment effectively and safely in a short period. In both cases, the tumor reduced rapidly and symptoms of bleeding and obstruction improved. Moreover, tumor progression was controlled for 8 and 4 months, respectively. Adverse events were also mild during the observation period. In addition, as short-term treatment is desirable for elderly patients and patients with poor conditions, SBRT was effective because it involved the use of hypofractionated irradiation. Although there are many issues that yet need to be discussed, we expect SBRT to be useful for locally advanced gastric cancer in elderly patients and patients with complications, both of who are difficult to treat.
In conclusion, although treatment planning and dose prescription are difficult because the tolerable dose of normal gastric mucosa and surrounding organs is low, SBRT was highly precise irradiation method and useful as a low-invasive and speedy local treatment for locally advanced gastric cancer. Moreover, SBRT can be useful for elderly patients and patients who have difficulty in undergoing other treatments owing to complications.
References
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N ENG J Med. 2001; 345: 725-730.
- Saikawa Y, Kubota T, Kumagai K, Nakamura R, Kumai K, Shigematsu N, et al. Phase II study of chemoradiotherapy with S-1 and low-dose cisplatin for inoperable advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2008; 71: 173-179.
- Yoshikawa T, Tsuburaya A, Hirabayashi N, Yoshida K, Nagata N, Kodera Y, et al. A phase I study of palliative chemoradiation therapy with paclitaxel and cisplatin for local symptoms due to an unresectable primary advanced or locally recurrent gastric adenocarcinoma. Cancer Chemother Pharmacol. 2009; 64: 1071-1077.
- Hashimoto K, Mayahara H, Takashima A, Nakajima TE, Kato K, Hamaguchi T, et al. Palliative radiation therapy for hemorrhage of unresectable gastric cancer: a single institute experience. J Cancer Res Clin Oncol. 2009; 135: 1117-1123.
- Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, et al. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011; 137: 125-130.
- Suzuki A, Xiao L, Hayashi Y, Macapinlac HA, Welsh J, Lin SH, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer. 2011; 117: 4823-4833.
- Takahashi T, Saikawa Y, Fukuda K, et al. Chemo radiotherapy for advanced gastric cancer. Jpn cancer chemother. 2012; 39: 2464-2468.
- Ishigami S, Arigami T, Uenosono Y, Okumura H, Uchikado Y, Owaki T, et al. Outcome of advanced gastric cancer patients treated with chemoradiation therapy. Gan To Kagaku Ryoho. 2013; 40: 727-731.
- Hu JB, Sun XN, Gu BX, Wang Q, Hu WX. Effect of intensity modulated radiotherapy combined with s-1-based chemotherapy in locally advanced gastric cancer patients. Oncol Res Treat. 2014; 37: 11-16.
- Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007; 25: 947-952.
- Baumann R, Chan MKH, Pyschny F, Stera S, Malzkuhn B, Wurster S, et al. Clinical Results of Mean GTV Dose Optimized Robotic-Guided Stereotactic Body Radiation Therapy for Lung Tumors. Front Oncol. 2018; 8: 171.
- Andratschke N, Alheid H, Allgäuer M, G. Becker, O. Blanck, J. Boda- Heggemann, et al. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligo metastases in 474 patients with 623 metastases. BMC Cancer. 2018; 18: 283.
- Mazzola R, Fersino S, Aiello D, Gregucci F, Tebano U, Corradini S, et al. Linac-based stereotactic body radiation therapy for unresectable locally advanced pancreatic cancer: risk-adapted dose prescription and imageguided delivery. Strahlenther Onkol. 2018; 194: 835-842.
- About Cyberknife. The CyberKnife. Accuray Inc (Sunnyvale, CA, USA).
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guideline 2014 (ver.4). Gastric Cancer. 2017; 20: 1-19.
- Tey J, Choo BA, Leong CN, Loy EY, Wong LC, Lim K, et al. Clinical outcome of palliative radiotherapy for locally advanced symptomatic gastric cancer in the modern era. Medicine (Baltimore). 2014; 93: e118.
- Kim MM, Rana V, Janjan NA, Das P, Phan AT, Delclos ME, et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008; 47: 421-427.
- Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004; 101: 1623-1631.
- Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypo fractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007; 2: 94-100.
- Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, et al. Prospective Trial of Stereotactic Body Radiation Therapy for both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. 2015; 93: 989-996.
- Onishi H, Shirato H, Araki T, Hiraoka M, Fujino M, Gomi K, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011; 81: 1352-1358.