
Special Article – Vascular Surgery
Austin J Surg. 2019; 6(27): 1236.
Biointegral Xenograft in Management of Aortofemoral Vascular Graft Infections
Rojas M, Stehno O, Štádler P and Vitásek P*
Department of Vascular Surgery, Na Homolce Hospital, Czech Republic
*Corresponding author: Vitasek P, Department of Vascular Surgery, Na Homolce Hospital, Roentgenova 2, Prague 150 30, Czech Republic
Received: November 04, 2019; Accepted: December 06, 2019; Published: December 13, 2019
Abstract
Aortic graft infections represent a major problem in modern vascular surgery. The issue of which specific material should be chosen for a new reconstruction in the infected area remains unsolved. We used a bioprosthesis made from bovine pericardium - Biointegral for 9 patients with intracavitary vascular graft infections between 2017 and 2019. The median age of the patients was 65 years, the average length of hospital stays was 28 days. The early 30 day mortality rate was 22%. Late graft occlusion occurred in two patients. In two cases, we had to treat infections of the biological prosthesis. Despite numerous complications, the use of the Biointegral xenograft may be a valuable option in certain cases of vascular graft infections. To compare the results of the treatment with other types of grafts, further studies would be necessary.
Keywords: Vascular surgery; Infection; Bioprosthesis
Introduction
Infections involving vascular graft prostheses are severe complications in modern vascular surgery. Incidence has been reported between 1% and 6% [1]. The treatment of aortic graft infections is associated with a 30 day mortality rate ranging between 18% [2] and 48% [3]. The risk of synthetic graft infection is also subject to endovascular surgery, mainly stent graft implantation for treatment of aortic aneurysm. In these cases, the estimated incidence is 0,2%-5% [4].
Pt no
Sex/age (y)
Co- morbidities
Primary intervention
Reinterventions: Type/number
Time interval to graft explantation (y)
Symptoms of infection
Bacterial culture
1
F/65
CAD, peptic ulcer disease
Aortofemoral
bypass
Thrombectomy/1
7
Septic fever, duodenal fistula, positive hemoculture, PET CT +
Streptococcus, Lactobacillus, Klebsiella, Candida
2
M/57
Ischemic stroke, obstructive pulmonary disease
ABF (laparoscopic)
Abscess drainage in the groin, prosthetic replacement of the distal part of the graft with rifampin bonded prosthesis / 2
7
Purulent fistula in the groin, PET CT+
Staphylococcus aureus
3
M/66
Ischemic stroke, renal disease
ABF
Abscesses drainage in the groins, prosthetic replacement of the distal part of the graft with rifampin bonded prosthesis / 2
2
Purulent fistula in the groin, PET CT+
Staphylococcus epidermidis, Corynebacterium species
4
M/71
Hypertension, renal disease
ABF
Central and peripheral anastomosesalse aneurysms prosthetic substitution /3
15
Purulent fistula in the groin, sigmoid fistula
Enterococcus, Candida
5
F/58
Hypertension, psoriasis, nicotine abuse, left lower-extremity amputation
ABF
Femorofemoral crossover bypass for one branch occlusion of the graft /1
2
Purulent fistula in the groin, PET CT+, retroperitoneal abscess
Peptostreptococcus, Anaerococcus
M/63
None
ABF
Reconstructions of the anastomosis in the groin for occlusion and repeated infection/ 6
2
Purulent fistulas in the groins
Staphylococcus aureus MRSA
7
F/73
Ischemic stroke, hypertension, colon resection
Ilicofemoral bypass + infrainguinal reconstruction
None
3
Occlusion of the infrainquinal reconstruction, periprosthetic fluid collection, leukocytosis, PET CT +
None
8
M/60
CAD, hypertension, nicotine abuse
ABF
Right branch thrombectomy /1
2
Duodenal fistula, anaemia
Peptostreptococcus, Escherichia coli, bacteroides species
9
F/72
Small bowel resection, cholecystectomy, nephrectomy
ABF
Stent-graft implantation to the central anastomosis, partial replacement of graft branches, femorofemoral crossover bypass / 10
26
Periprosthetic fluid collection, PET CT+
Staphylococcus epidermidis
CAD: Coronary Arterial Disease; ABF: Aortobifemoral Bypass; PET CT +: Positron Emission Tomography scan positive findings
Table 1: Patients characteristics and history before xenograft implantation.
Pt no
Type of surgery, type of xenograft, concomitant procedure
Estimated
blood loss (mL)
Intra/perioperative complications
Antibiotic therapy
Hospital stay (d)
Follow-up (mo)
Graft patency
Complications during follow-up
1
Aortofemoral bypass, tubular graft 7 mm, duodenal resection
5000
Early postoperative revision surgery for bleeding from inferior caval vein
Death 1 day after surgery from hemorrhagic and septic shock
Vancomycin, ampicillin
1
-
Patent
-
2
ABF, bifurcated graft 16/8
1500
None
Oxacillin
14
32
Patent
None
3
ABF, bifurcated graft 16/8
2200
Pleural effusion, complicated healing in the groin
Vancomycin,
rifampicin, penicillin
29
21
Patent
Xenograft reinfection 14 months after the surgery, autologous femoral vein substitution
4
ABF, bifurcated graft 18/9, end-to-end central anastomosis
3000
Persistent fistula in the groin, bowel resection for perforation, end-sigmoid colostomy
Meropenem,
vancomycin
66
8
Successive obliteration of graft branches 5 and 7 months after the surgery
3 reinterventions for false aneurysms of the anastomosis, bilateral extra-anatomic reconstruction.
Death 8 months afterthe surgery
5
Aortofemoral bypass, tubular graft 7 mm, omentoplasty
900
Left leg stump ischemia, medicamentously treated
Penicillin
14
12
Patent
None
6
ABF, bifurcated graft 16/8, autovenous above-knee bypass
1000
Postoperative adhesion-related intestine obstruction without bowel ischemia the 3rd day after the surgery, followed by multiorgan failure
Death on the 5 th postoperative day
Vancomycin,
rifampicin
5
-
Patent
-
7
Ilicofemoral bypass, tubular graft 7 mm, end-to-end central anastomosis, retroperitoneal approach
700
None
Cefuroxim
14
10
Patent
None
8
ABF, bifurcated graft 16/8, duodenal resection
3000
Lower extremity peripheral embolization, below-knee amputation,
Gastroenteroanastomosis
Cefotaxim,
Ampicillin,
metronidazol
53
10
One branch obliteration 10 months after the surgery
None
9
ABF, bifurcated graft 16/8, stent-graft explantation
3200
Renal failure, temporary hemodialysis, pneumonia
Vankomycin,
Meropenem,
amikacin
60
3
Patent
None
Table 2: Perioperative and long term results after xenograft implantation.
Patients with an infection of infrainguinal arterial reconstruction have a 16% risk of limb amputation and 18% risk of death [5].
Patients with vascular graft infections are in major risk of developing sepsis caused by a systemic spread of pathogens. Local complications of infection include pseudoaneurysm development and subsequently life-threatening bleeding. Those conditions require immediate treatment and are associated with poorer prognosis. Higher age and numerous underlying comorbidities significantly impair the prognosis of patients [3].
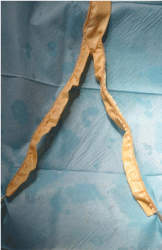
Figure 1: Bioprosthesis Biointegral, bifurcated graft.
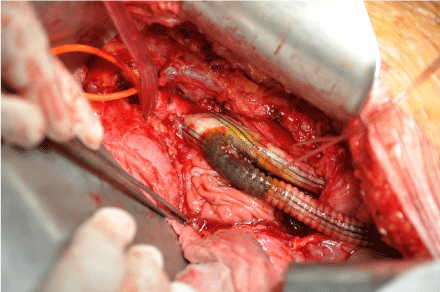
Figure 2: Infected aorto bifemoral graft with prothesis- duodenal fistula
before explantation.
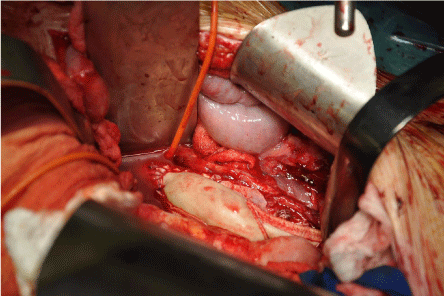
Figure 3: Central anastomosis of the new aortobifemoral bypass Biointegral
bifurcated graft.
Patients with enteric or bronchial fistula have a higher mortality rate. The treatment involves surgical management of the affected intestine or bronchus and lung tissue, which increases surgical load and frequency of complications [6].
The diagnosis of vascular graft infections is highly complex. There are various methods to confirm the diagnosis and determine the extent of the disease. Patients with synthetic vascular grafts manifest clinical symptoms and laboratory signs of sepsis. In case of gastrointestinal bleeding, it is essential to eliminate the possibility of communication with the vascular graft in aortic position by a CT angiography or endoscopic examination of the duodenum. In case of wound dehiscence, development of fluid collection or inflammatory infiltrate in proximity to vascular graft, it is crucial to recognize a direct graft infection and identify infectious agents based on microbiological tests. Samples are collected through local excision, targeted puncture or blood cultures. A microbial genome sequencing could help to identify infectious agents in case of negative results of microbiological examinations [7]. Imaging methods such as sonography, CT angiography, PET CT and magnetic resonance are used to confirm the diagnosis and determine the extent of the infection. The treatment procedure of choice is the surgical removal of the infected graft and debridement of the surrounding tissue [8]. This requires a complete healing of the arterial wall defect at the site of primary anastomosis and providing perfusion to the revascularized tissues. There are several surgical approaches but, unfortunately, none of them is universal and ideal.
Closure of the arterial wall defect at the site of anastomoses using an autologous patch and simultaneously endovascular recanalization of native circulation, though technically possible only in few cases [9].
Closure of the arterial wall defect by autologous patch or arterial suture followed by extra-anatomic prosthetic bypass revascularization. This procedure is associated with a significant risk of infection in the new graft especially at the groin, as well as aortic stump disruption. In addition, the long-term patency of extra-anatomic reconstruction has been reported to be lower [10].
Graft excision followed by autologous in situ bypass. The great saphenous vein or femoral veins are most commonly used for these reconstructions [11]. The material might not always be available in sufficient size and length. Moreover, vein harvesting is associated with further surgical load for the patient.
In situ reconstruction by arterial or venous allograft - cryopreserved or fresh [12,13]. However, grafts might not be immediately available in emergency situations. There is a considerable risk of rejection or reinfection of the transplanted tissue. Another disadvantage of their use is the need for permanent immunosuppression.
Rifampin-bonded [14] and silver-coated synthetic vascular grafts are associated with a higher reinfection rate [15] and therefore should be attempted in areas with potential infection risk rather than primary infectious areas.
Lately, the use of modified materials manufactured from xenogenous tissues for in situ replacement [16] of infected vascular graft have become more common. Those include bovine pericardium [17,18] and vascular grafts containing ovine collagen (Omniflow) [19]. Extracellular matrices from porcine small intestine submucosa [20] (CorMatrix) are used experimentally.
Methods
In our single-center study we retrospectively analyzed clinical data of patients who were surgically treated for vascular graft infections in aortoiliac position for a period of 2 years, from 2017, through 2019. We included patients who underwent in situ reconstruction with xenograft, specifically bioprosthesis Biointegral Surgical No- React® (Biointegral Surgical Inc, Mississauga, ON, Canada) made from bovine pericardium. To confirm the diagnosis and determine the extent of vascular graft infection, we evaluated clinical symptoms and laboratory signs of infection, CT scan and PET CT. In case of positive microbiological test, immediate antimicrobial therapy was initiated. We chose midline laparotomy as a surgical approach of choice for most patients, and a retroperitoneal approach with explantation of primary prosthetic material otherwise. We inserted ureteral stents preoperatively. We performed debridement of the surrounding tissue and in situ implantation of bifurcated or tubular graft Biointegral Surgical No-React® with end-to-end or end-to-side central anastomosis. Postoperatively, we continued the antimicrobial therapy adjusted according to results of microbiological tests for 5 weeks. Patients received low molecular weight heparin as anticoagulant therapy for a duration of 5 weeks as well as antiplatelet therapy. Patients who had uncomplicated postoperative course got a regular medical check-up in 1,3,6,12 and 24 months.
Results
In our study we identified 4 female and 5 male patients, the median age of the patients was 65 years. The primary surgical intervention included aortobifemoral bypass surgery for atherosclerotic occlusive disease in seven cases and right side aortofemoral bypass in one case. One patient had a one side ilico-femoral bypass. Only that one patient did not have any other surgical intervention performed prior to the explantation of the primary reconstruction. Other patients had 1 to 10 surgical interventions performed on the primary reconstruction.
Three patients developed an enteroprosthetic fistula (1 sigmoid and 2 duodenal). In 6 cases, patients presented a draining sinus tract in the groin. In 8 cases, a microbiological examination identified infectious agents preoperatively and a targeted antimicrobial therapy was initiated. In 6 patients, PET was used to determine the extent of the infection with a positive result. The replacement of infected prosthesis with xenograft took place 2 to 26 years following the primary surgery.
In two patients the surgical approach was primary retroperitoneal, in one case a conversion to transperitoneal approach was necessary to perform duodenal resection. Generally the duodenal resection was performed in two patients. In one case, an extraction of the stent graft covering pseudoaneurysm of the central anastomosis was performed. One patient underwent peripheral venous reconstruction simultaneously. In two cases the suture of central anastomosis of the xenograft was sewn end-to-end, in other cases end-to-side.
Two patients died in the early post-operative period. One patient died of hemorrhagic shock that occured as a result of perioperative vena cava inferior injury for which she had undergone a surgical revision. The other patient developed postoperative adhesion-related intestine obstruction the 3rd day after the primary surgery. The condition was surgically treated but the patient died on the 5th day due to multiple organ failure.
The average length of hospital stays for surviving patients was 36 (14-66) days.
In one case, a perioperative peripheral embolization to crural arteries occured. After failure of mechanical thrombectomy, we performed below-knee amputation. The same patient required construction of gastroenteroanastomosis following a duodenal resection. In one case, a persistent enterocutaneous fistula in the groin led to a colon resection and end-sigmoid colostomy construction. One patient required temporary hemodialysis during the postoperative course. Other complications included a pleural effusion managed by chest tube insertion, in two cases localised wound infections in the groin were treated conservatively using vacuum-assisted closure device.
The median follow-up period of patients was 14 (4-32) months.
During this period, we noted two cases of graft reinfection. One of these two patients had a relapsing pseudoaneurysm of the anastomosis in the groin 5 months after the surgery was carried out. He was treated initially with an autogenous vein graft replacement of the distal part of the right branch of the graft, subsequently with an extra-anatomic axillo-femoral bypass.
7 months after the xenograft was implanted, the left branch got occluded and resulted in a similar extra-anatomic reconstruction. The patient died 8 months after the surgery from sepsis, the autopsy did not reveal any signs of the infection of the remaining central part of the xenograft.
The other patient with graft reinfection that occured 14 months after the surgery underwent a successful graft excision and in situ replacement using deep femoral veins.
In one case, one branch of the aortobifemoral graft got occluded. The patient did not develop critical limb ischemia, thus did not require any surgical intervention.
Discussion
We used biological grafts Biointegral in a group of our patients. The main reasons to favor this material were immediate availability and proclaimed infection resistance even for use in infected areas. The use of manufactured bifurcated graft shortens the operative time in comparison with grafts prepared perioperatively by suturing pericardial patches. Compared to deep femoral vein harvesting, the surgical load for a patient is reasonable. Clinical experience with bovine pericardium in cardiovascular surgery shows it may be a valuable option in treatment of vascular graft infections. No react surface treatment of the graft provides biocompatibility and infection resistance. It provides good long-term patency even for low-pressure circulation. Our investigation and experience imply high rates of infection resistance of the Biointegral xenograft even though we detected cases of reinfection and development of pseudoaneurysm of the anastomosis in the groin.
Managing patients with aortofemoral vascular graft infections requires a multidisciplinary team approach. If duodenal resection is necessary, a specialist in abdominal surgery should be present. The anesthesiologist plays a major role in the perioperative and postoperative course, management of sepsis, systemic inflammatory response and multiple organ failure. The strategy of antimicrobial therapy should be established in cooperation with a microbiologist.
Regarding the relatively low frequency of aortofemoral vascular graft infections, differences in disease manifestation, extent of the infection, presence of enteric fistulas, comorbidities, type of infectious agent, studied groups of patients tend to be heterogeneous and small. To confirm the effectiveness of Biointegral xenograft in treatment of vascular graft infections, the comparison of a bigger group with a group of patients treated with other methods like transplantation of allogeneic vascular grafts or autologous vascular grafts in randomized trial would be necessary.
Acknowledgement
Supported by Ministry of Health, Czech Republic-conceptual development of research organization (NNH, 00023884) IG 140101.
References
- Legout L, Sarraz-Bournet B, D’Elia PV, Devos P, Pasquet A, Caillaux M, et al. Characteristics and prognosis in patients with prosthetic vascular graft infection: A prospective observational cohort study. Clin Microbiol Infect [Internet]. 2012; 18: 352-358.
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg. 2008; 47: 264-269.
- Garot M, Delannoy PY, Meybeck A, Sarraz-Bournet B, d’Elia P, d’Escrivan T, et al. Intra-abdominal aortic graft infection: prognostic factors associated with in-hospital mortality. BMC Infect Dis [Internet]. 2014; 14: 215.
- Smeds MR, Duncan AA, Harlander-Locke MP, Lawrence PF, Lyden S, Fatima J, et al. Treatment and outcomes of aortic endograft infection. In: Journal of Vascular Surgery. 2016.
- Wilson WR, Bower TC, Creager MA, Amin-Hanjani S, O’Gara PT, Lockhart PB, et al. Vascular Graft Infections, Mycotic Aneurysms, and Endovascular Infections: A Scientific Statement from the American Heart Association. Circulation. 2016; 134: e412-460.
- Czerny M, Von Allmen R, Opfermann P, Sodeck G, Dick F, Stellmes A, et al. Self-made pericardial tube graft: A new surgical concept for treatment of graft infections after thoracic and abdominal aortic procedures. Ann Thorac Surg. 2011; 92: 1657-1662.
- Ajdler-Schaeffler E, Scherrer AU, Keller PM, Anagnostopoulos A, Hofmann M, Rancic Z, et al. Increased pathogen identification in vascular graft infections by the combined use of tissue cultures and 16s rRNA gene polymerase chain reaction. Front Med. 2018; 5: 169.
- FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: Confusion and inconsistency in the absence of evidence or consensus. Journal of Antimicrobial Chemotherapy. 2005; 56: 996-999.
- Erzurum VZ, Clair D. Endovascular native vessel recanalization to maintain limb perfusion after infected prosthetic vascular graft excision. J Vasc Surg [Internet]. 2005; 41: 332-336.
- Dulbecco E, Camporrotondo M, Blanco G, Haberman D. In situ reconstruction with bovine pericardial tubular graft for aortic graft infection. Rev Bras Cir Cardiovasc. 2010; 25: 249-252.
- Daenens K, Fourneau I, Nevelsteen A. Ten-year experience in autogenous reconstruction with the femoral vein in the treatment of aortofemoral prosthetic infection. Eur J Vasc Endovasc Surg. 2003; 25: 240-245.
- Bisdas T, Bredt M, Pichlmaier M, Aper T, Wilhelmi M, Bisdas S, et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010; 52: 323- 330.
- Kieffer E, Gomes D, Chiche L, Fléron MH, Koskas F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: Early and late results in 179 patients. J Vasc Surg. 2004; 39: 1009-1017.
- Hayes PD, Nasim A, London NJ, Sayers RD, Barrie WW, Bell PR, et al. In situ replacement of infected aortic grafts with rifampicin-bonded prostheses: the Leicester experience (1992 to 1998). J Vasc Surg [Internet]. 1999; 30: 92-98.
- Batt M, Jean-Baptiste E, O’Connor S, Bouillanne PJ, Haudebourg P, Hassen- Khodja R, et al. In-situ Revascularisation for Patients with Aortic Graft Infection: A Single Centre Experience with Silver Coated Polyester Grafts. Eur J Vasc Endovasc Surg. 2008; 36: 182-188.
- Oderich GS, Bower TC, Cherry KJ, Panneton JM, Sullivan TM, Noel AA, et al. Evolution from axillofemoral to in situ prosthetic reconstruction for the treatment of aortic graft infections at a single center. J Vasc Surg. 2006; 43: 1166-1174.
- Kohler C, Attigah N, Demirel S, Zientara A, Weber M, Schwegler I. A technique for a self-made bifurcated graft with bovine pericardial patch in infectious vascular reconstruction. J Vasc Surg Cases Innov Tech. 2016; 2: 158-160.
- Lutz B, Reeps C, Biro G, Knappich C, Zimmermann A, Eckstein HH. Bovine Pericardium as New Technical Option for In Situ Reconstruction of Aortic Graft Infection. Ann Vasc Surg. 2017; 41: 118-126.
- Betz T, Neuwerth D, Steinbauer M, Uhl C, Pfister K, Töpel I. Biosynthetic Vascular Graft: A Valuable Alternative to Traditional Replacement Materials for Treatment of Prosthetic Aortic Graft Infection? Scand J Surg. 2019; 108: 291-296.
- Fallon A, Goodchild T, Wang R, Matheny RG. Remodeling of extracellular matrix patch used for carotid artery repair. J Surg Res. 2012; 175: e25-34.