
Special Article – Vein Thrombosis Surgery
Austin J Surg. 2020; 7(1): 1242.
Surgical Thrombectomy for Treatment of Acute Iliofemoral Venous Thrombosis
Igor M Ignatyev*
Interregional Clinical and Diagnostic Center, Kazan State Medical University, Russia
*Corresponding author: Igor M. Ignatyev, Department of Vascular Surgery, Interregional Clinical and Diagnostic Center, 12 A, Karbyshev st., 420101, Kazan, Russian Federation, Russia
Received: January 20, 2020; Accepted: February 20, 2020; Published: February 27, 2020
Abstract
Objective: To assess the effectiveness of open surgical thrombectomy in acute iliofemoral venous thrombosis.
Methods: Between January 2012 and October 2018, a total of 65 patients underwent transfemoral venous thrombectomy (VT) in acute iliofemoral venous thrombosis. Ten patients received a venous hybrid operation comprising ballooncatheter thrombectomy and stenting of residual stenosis of iliac vein. The control group consisted of 44 patients who received standard anticoagulant therapy. The results were evaluated by duplex ultrasound (DUS). The assessment of clinical effectiveness was made with Venous Clinical Severity Score (VCSS), Villalta Score and health-related quality of life (HRQoL).
Results: Secondary patency of iliofemoral segment at 6 months of monitoring after thrombectomy was reported in 97% of cases. Meanwhile, the recanalization of iliofemoral segment was registered only in 27% (P< .0001) of patients who had anticoagulant therapy alone. The median preoperative VCSS was 7, which dropped to 2 at 6 months (P=.002). There were five cases of successful re-thrombectomy and stenting (three cases). Cumulative primary and secondary patency rates of iliofemoral veins at 72 months were 88% and 95%, respectively. The data Villalta score in long-term follow-up in patients after surgery was significantly lower than of patients treated with anticoagulation (P<.001). HRQoL of patients after 6 years of VT was improved, its mean score decreased from 45.3 (8.6) to 23.6 (6.1; P< .001).
Conclusion: According to selective indications open surgical thrombectomy in iliofemoral venous thrombosis with using current methods of deep vein restoration patency increases the effectiveness of treatment of this severe pathology and prevents from progression of postthrombotic syndrome.
Keywords: Acute iliofemoral venous thrombosis; Open surgical thrombectomy; Stenting; Recanalization; Duplex ultrasound
Introduction
Deep Venous Thrombosis (DVT) of lower extremities is one of the most widespread vascular diseases, from 160 to 300 cases per 100 000 of general population occur annually [1,2]. Symptomatic pulmonary embolism (PE) accompanies approximately 10% of DVTs and hospital discharge data suggest an incidence of 23 per 100 000 population [3].
The treatment of DVT has two main goals. During an acute period, it is a prevention of complications such as PE and phlegmasia cerulea dolens, in the long-term - minimization of postthrombotic syndrome (PTS), that is 40-60% of patients. One in ten patients has venous leg ulcer that results in disability [4,5]. The most complicated disturbances of venous hemodynamics occur when iliofemoral veins are damaged [6,7].
The traditional method of treatment of acute DVT is anticoagulant therapy that is based on unfractioned and low-molecular-weight heparins, vitamin K antagonist and direct oral anticoagulants. However, these medications have no thrombolytic effect but they prevent from thrombus prolongation, reccurence of thrombosis and they let the risk of PE development decrease [8].
Aggressive methods of DVT treatment have been developed in recent years. These include regional catheter-directed and pharmacomechanical thrombolysis. The advantage of this approach is shown in randomized trial CaVenT (Catheter-directed Venous thrombolysis in acute iliofemoral vein Thrombosis) [9]. However, data from the randomized controlled ATTRACT (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis) trial revealed that the addition of catheter-based intervention to standard-of-care anticoagulation failed to significantly decrease the occurrence of postthrombotic syndrome in patients who received this treatment strategy when compared with its occurrence in patients who received anticoagulation alone [10]. Although ATTRACT has failed to meet its primary endpoints, its results will offer a springboard for ongoing research in this area. If there are any contraindications to thrombolytic therapy, it is recommended to perform open venous thrombectomy [2]. When this active strategy of DVT treatment is used, the risk of residual obstruction and venous valves damage with reflux decreases. As a result, it prevents from severe complications of PTS [11,12].
The aim of this study is to assess the effectiveness of open thrombectomy in acute iliofemoral venous thrombosis.
Materials and Methods
Between January 2012 and October 2018, a total of 65 patients underwent transfemoral venous thrombectomy (VT) in setting of acute iliofemoral venous thrombosis. 51 patients had occlusive thrombosis and 14 patients had floating thrombus on duplex ultrasound. Isolated thrombosis was registered in four cases. Iliofemoral thrombosis with total occlusions of deep veins and phlegmasia cerulea dolens was observed in one patient. Ten patients underwent a venous hybrid operation consisting of balloon-catheter thrombectomy and stenting of residual stenosis.
The patients characteristics are represented in Table 1. In the control group, there were 44 patients with iliofemoral thrombosis (18 men, 26 women, mean age is 54 (range 22-68) that received standard anticoagulant therapy alone for 6 months.
TABLECREATED
Table 1: Preoperative patient characteristics (n=65).
Laboratory tests included the D-dimer levels and thrombophilia work-up, including protein C, protein S, and antithrombin III deficiency, factor V Leiden mutation, prothrombin G20210A gene mutation, antiphosphatidylserine antibody, and homocysteine levels.
The diagnostic imaging included duplex ultrasound (DUS, LOGIQ E9, GE Medical Systems, Wauwatosa, Wisconsin), magnetic resonance (GE, Signa, HDxt 1.5 T, Milwaukee, Wisconsin) and multi-slice computed tomographic venography (AQUILION 64, Toshiba, Japan), contrast venography (INNOVA 3100, GE, Cedex, France), perfusion lung scintigraphy (single-photon emission tomograph Millenium MPR, GE, Milwaukee, Wisconsin). In those patients suspected of having PE, CT angiogram of chest, abdominal and pelvic was performed.
Main indications for open surgical thrombectomy included: symptomatic iliofemoral venous thrombosis, when thrombolysis had failed or was contraindicated; total thrombosis of deep veins in the setting of phlegmasia cerulea dolens; no more than 10 days from the onset of the presenting symptoms; the absence of severe accompanying pathology.
General characteristics of surgical venous thrombectomy types and endovascular procedures are presented in Table 2.
TABLECREATED
Table 2: Types of surgical thrombectomy and endovascular procedures.
Surgery technique
The operations were carried out in standardized fashion under general anesthesia. The proximal common iliac vein (CIV) was approached through a short incision above the inguinal ligament (Figure 1A). The common femoral vein (CFV), distal part of external iliac vein (EIV), deep femoral vein (DFV) and femoral vein (FV) were exposed, using a standard longitudinal inguinal incision. After administration of unfractioned heparin intravenously injection (100 IU/kg) the CFV and CIV, at the iliocaval confluence, were controlled with vessel loops. A longitudinal venotomy was performed on the anterior surface of the CFV and the iliac vein were then cleared of thrombus using Fogarty venous catheter (8-9F) (Figure 1B). Great attention was paid to clearing DFV and GSV of thrombus (Figure 1C). At the time of venous thrombectomy, the positive end-expiratory pressure was increased by 15 mm Hg to prevent PE. There were no inferior vena cava (IVC) filters implanted. The venotomy is closed using running sutures (7-0 Prolene). To prevent recurrent thrombosis, an arteriovenous fistula (AVF) was created in all patients in order to increase venous blood flow in the treated segment over the critical three-month post-operative period. The same limb saphenous vein was used to create an AVF between the femoral vein and artery (8-0 Prolene), and pulsatile fistula flow was confirmed using continuous-wave Doppler. The AVF was wrapped with a polytetrafluorethylene (PTFE) cuff, which was fixed with 2-0 Prolene suture. The ends of suture were excluded through perforations of sterile button, which was placed either on skin surface or in subcutaneous tissue with marking the place near the wound. The femoral vein was ligated by atraumatic absorbable suture 3-0 Vicryl distal to the profunda branches or plicated with 2-3 longitudinal sutures (6-0 Prolene) (Figure 1D). The patency of iliac vein was verified by intraoperative ascending venography. In cases of residual thrombi or residual stenosis, for example, caused by an iliac spur (May-Thurner Syndrome), the iliac vein underwent angioplasty and stenting (Wallstent-Uni Endoprosthesis; Boston Scientific, Natick, Massachusetts; Figures 2A,B). When thrombus encroached into IVC, the retroperitoneal incision was extended and open thrombectomy was performed directly from IVC.
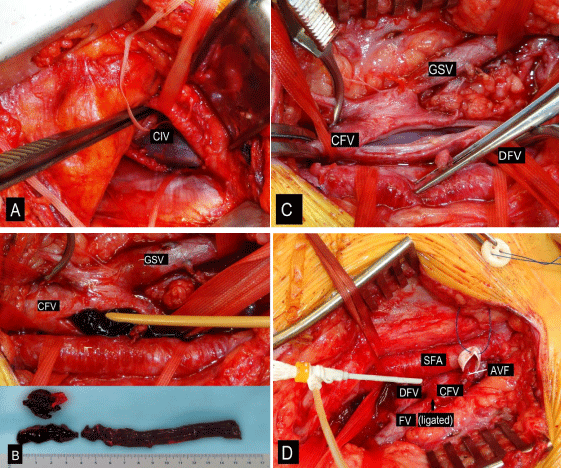
Figure 1: A, Exposure of proximal CIV from retroperitoneal approach. B,
Longitudinal venotomy of the CFV, CFV and the iliac vein were then cleared
of thrombus with the help of Fogarty catheter; bottom of the figure – fragments
of excluded thrombi. C, CFV lumen after thrombectomy. D, AVF between
CFV and SFA wrapped in PTFE cuff, that is fixed with 2-0 Prolene suture.
The ends of suture were excluded through perforations of sterile button,
which was placed on skin surface. The femoral vein is ligated by atraumatic
absorbable suture 3-0 Vicryl distal to the profunda branches (arrow). The
sheath was introduced between the sutures of CFV.
AVF: Arteriovenous Fistula; CFV: Common Femoral Vein; GSV: Great
Saphenous Vein; DFV: Deep Femoral Vein; SFA: Superficial Femoral Artery;
PTFE: Polytetrafluorethylene
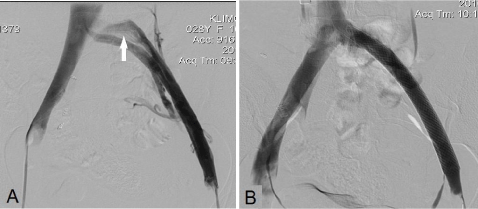
Figure 2: A, Intraoperative venogram of residual compression stenosis of
left CIV after thrombectomy. “Bull’s – Eye” sign, typical for CIV compression
(arrow). B, Balloon dilatation and stenting of residual stenosis of CIV by
Wallstent® 16×80 mm with complete restoration of its patency.
CIV: Common Iliac Vein
Postoperatively, all patients received full anticoagulation, first low-molecular-weight heparin (enoxaparin, nadroparin), and then with rivaroxaban 20 mg for six months. Life-long anticoagulant treatment was recommended for patients with thrombophilia. D-dimer was tested during the first and second in-hospital stay within one week after surgery.
Compression therapy was started immediately after admission and continued after surgery with custom-made compression stockings with a pressure range of 23 to 32mmHg. Compression therapy was suggested for six months and more.
Patients were followed up after 3 months, 6 months and yearly thereafter. The follow-up workup consisted of a clinical examination, as well as DUS investigation of the pelvic and leg veins. The assessment of the clinical effectiveness of surgery was made with Venous Clinical Severity Score (VCSS). We used Villalta Score [13] for determine PTS. The Chronic Venous Insufficiency Questionnaire (CIVIQ) is a tool designed to assess health-related quality of life (HRQoL) [14]. Of the original 65 patients, we managed to obtain long-term data for 36 patients, who came back for a detail follow-up examination. The mean follow-up time at examination was 48.5% months. In the control group in the long-term period 24 patients were observed (mean time – 42.9% months).
Statistical analysis
Statistical analysis was performed using Statistica 10 (StatSoft, Tulsa, OK, USA). The clinical characteristics of patients are presented by methods of descriptive statistics. Continuous data are presented as the means ± SD values. Wilcoxon signed-rank test was used for statistical analysis of VCSS.
Continuous variables were analyzed using Student’s t-test or Mann-Whitney U-test for nonparametric independent variables when appropriate. Comparisons of categorical variables were performed with the χ2 test. The assessment of cumulative patency was performed with Kaplan-Meier curve. A value of P<0.05 was accepted as representing a significant difference.
Results
The immediate procedural success rate of open surgical VT was 100%. Technical success of stenting of compressive stenosis of left CIV (May-Thurner syndrome) amounts to 100%.
Thrombosis of the operated segment in early follow-up term was observed in three cases. In two of three cases the reason of thrombosis was non-diagnosed compressive stenosis of left CIV. Wound complications (transient lymphorrhea and hematoma) were registered in three cases. No perioperative death or pulmonary embolism occurred.
In mid-term follow-up we observed four recurrent thrombosis after surgery. Of them one case was with functioning AVF. Thrombotic occlusion of the stent CIV occurred in one patient. Instent restenosis > 50% in long-term follow-up was seen in two cases (it is no symptomatic). The diagnosis was confirmed using DUS in all patients.
The AVFs were closed after three months under the control of duplex ultrasound. It consists in a distant elimination of fistula under ultrasound control by tightening polypropylene suture till Doppler signal from fistula disappears. This method is simple and reliable, and it does not require traumatic redo surgery [15]. In 8 cases AVF has occluded spontaneously.
There were four successful re-thrombectomy. Of these in two patients it combined with residual stenosis stenting of CIV. Restenting occlusion of the stented iliac vein with patency restore was performed in one patient.
There was a secondary patency of iliofemoral segment verified by duplex ultrasound at 6 months after the VT in 97% of cases (Figure 3). Meanwhile, the patients who received a standard anticoagulant therapy demonstrated the recanalization of iliofemoral veins in 27% of cases. Patency rate of iliofemoral veins after surgery was significantly higher, than its frequency of recanalization after anticoagulant treatment (97% vs 27%, P< .0001). The recanalization of femoropopliteal veins at 6 months after the VT was registered in 65% (39/60) of patients.
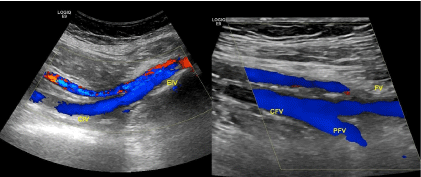
Figure 3: Echograms of iliofemoral veins at 6 months after thrombectomy.
CIV, EIV, CFV, DFV and FV are patent. Recanalization of FV.
CIV: Common Iliac Vein; EIV: External Iliac Vein; CFV: Common Femoral
Vein; DFV: Deep Femoral Vein; FV: Femoral Vein
The primary one-year patency rate was 88% (and after six years) and secondary one- year (and six-year) patency rate was 95% (Figure 4).
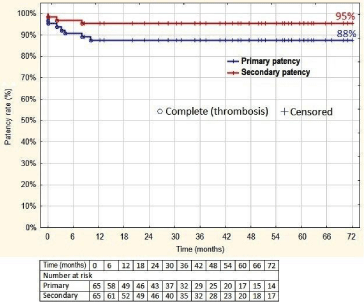
Figure 4: Cumulative primary and secondary patency rate of iliofemoral veins
after thrombectomy (%). The number of patients at risk at each interval is
given below the graph. All standard errors of the mean < 10%.
The median preoperative VCSS was 7, which dropped to 2 at six months (P=.002).
The data Villalta score in long-term follow-up in the patients’ group after surgery (1st group) and patients who received anticoagulant therapy (2nd group) are shown in Table 3. This means, that according to the Villalta score, symptoms of PTS were absent or mild in 88.9% (32/36) patients. In the 2nd group of patients this data was 41.7% (10/24) and significantly lower than in the 1st group (88.9% vs 41.7%, P< .001).
TABLECREATED
Table 3: Villalta score.
HRQoL of patients after 6 years of VT was improved, its mean score decreased 45.3(8.6) to 23.6(6.1; P< .001).
Discussion
According to recommendations of medical societies and forums, the quality of evidence of surgical thrombectomy remains low [16,17]. However, when compared to systemic anticoagulation, which was performed in 10 trials and one of them was randomized, the metaanalysis of the open thrombectomy results in iliofemoral venous thrombosis demonstrated statistically a significant reduction in the risk of PTS development (RR, 0.68; 95% CI, 0.46–0.99) [11].
In Swedish randomized research, published in 1984, it was shown that at 6 months after thrombectomy, the symptoms of PTS were completely absent in 42% of cases, while after the standard anticoagulant therapy, it was only in 7%. Iliac veins patency in the surgical group amounted to 76%, in the group after conservative therapy – to 35%. Valves competence of femoropopliteal segment as compared to above-mentioned groups, was 52% and 35%, correspondingly. During the control research after 5 and 10 years, the correlation between vein patency and valves competence remained identical [18]. There were similar results in other researches [19-21]. Symptoms of PTS were absent or mild in 95% of patients [2]. Our results also correlated with the literature data. Cumulative primary and secondary patency rates of the venous segments at 72 months were 88% and 95%, respectively.
In 2012, the clinical recommendations of the Society for Vascular Surgery and American Venous Forum were published. In this literature, the majority of experts suggest the strategy of early thrombus removal in selected patients meeting the following criteria: the first episode of acute iliofemoral deep venous thrombosis; symptoms <14 days in duration; low risk of bleeding, and ambulatory with good capacity and an acceptable life expectancy (Grade 2C) [16]. The major preference is given to the percutaneous methods of treatment: catheter-directed and pharmacomechanical thrombolysis. In contraindications to thrombolysis, it is recommended to perform open venous thrombectomy. However, in some studies, terms for thrombectomy vary from 5-7 to 10 days [22,23]. It confirms the necessity of individual approach in the treatment of DVT.
At the present time, the open thrombectomy from deep veins of lower extremity is performed on selective indications. They are total deep vein thrombosis with the development of phlegmasia cerulea dolens and contraindications to thrombolytic therapy associated with a high risk of bleeding in various pathologies (active internal bleeding, recent cerebrovascular accidents, malignant tumor, major trauma or surgery within 10 days, the age over 75, coagulopathy, thrombocytopenia or absolute endocarditis, intracardiac thrombosis, severe uncontrolled hypertension, pregnancy, septic thrombosis, allergy to thrombotic agents) [24,25]. According to the randomized trial CaVenT data, about 55% of patients had contraindications for thrombolytic therapy and 30% of them were candidates for surgical thrombectomy [9,24]. In our study, about one third of the patients had contraindications for thrombolysis.
PTS is a long-term complication of DVT. Various studies have shown efficacy of open surgical venous thrombectomy and a positive impact on PTS according to Villalta Score and HRQoL [11,12,26]. Our study has similar results showing improvement in PTS and quality of life at 6 years followup. In addition, we found statistically significant improvement of morphological and clinical results after surgery when compared to patients treated with anticoagulation alone.
We agree with others who postulate that further randomized trials will show that strategies of thrombus removal will be acknowledged as first-line therapy for patients with extensive venous thrombosis [27].
While choosing a surgical strategy we follow definite points. It is known that the initial development of thrombosis in calf deep veins is widespread and its clinical features are minimal. Manifestation of thrombosis develops in ascending thrombosis propagating to popliteal and femoral vein with maximal clinical indications during the prolongation of thrombus on common femoral and iliac veins. Consequently, sometimes it is difficult to identify the thrombus age localized in the femoro-popliteal-crural segment. That is why we use total open thrombectomy only in case of phlegmasia cerulea dolens.
Moreover, open thrombectomy can be performed when anticoagulant therapy with progressive symptoms of disease is not efficient and when there is a lack of necessary technical conditions for regional thrombolysis during in-patient monitoring.
Conclusion
According to selective indications open surgical thrombectomy in iliofemoral venous thrombosis with using current methods of deep vein restoration patency increases the effectiveness of treatment of this severe pathology and prevents from progression of PTS.
Ethical Approval
Local ethical board of Interregional Clinical and Diagnostic Centre (Kazan, Russia) approved study of Igor Ignatyev on surgical venous thrombectomy and it also approved the patient consent that was written in the form a document, the title of which is “Patient consent to medical examination and surgery” (Protocol No 26 of 11.05.2011).
References
- Coiteux I, Mazzolai L. Deep venous thrombosis: epidemiology, risk factors and natu-ral history. Schweiz Rundsch Med Prax. 2006; 95: 455-459.
- Hölper P, Kotelis D, Attigah N, Hyhlik-Dürr A, Bockler D. Long-term results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010; 39: 349- 355.
- Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 1991; 151: 933-938.
- Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999; 2: 39-49.
- Colledge-Smith PD. The aethiology and pathophysiology of chronic venous insufficiency and leg ulcers. In: Johnson CD, Taylor I, editors. Recent advances in surgery. Edinburg: Churchill Livingstone. 2000: 125-140.
- Johnson BF, Manzo RA, Bergelin RO, Strandness DE. Relationship between changes in the deep venous system and the development of the post thrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one-to-six year follow-up. J Vasc Surg. 1995; 21: 307-312.
- Akesson H, Brudin L, Dahlström JA, Eklöf B, Ohlin P, Plate G. Venous function assessed during 5-year period ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Endovasc Surg. 1990; 4: 43-48.
- Schwarzbach MH, Schumacher H, Böckler D, Fürstenberger S, Thomas F, Seelos R, et al. Surgical thrombectomy followed by intraoperative endovascular reconstruction for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2005; 29: 56-66.
- Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomized controlled trial. Lancet Haematol. 2016; 3: 64-71.
- Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharma-comechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017; 377: 2240-2252.
- Meissner MH. Rationale and indications for aggressive early thrombus removal. Phlebology. 2012; 27: 78-84.
- Casey ET, Murad MH, Zumaeta-Garcia M, Elamin MB, Shi Q, Erwin PJ, et al. Treatment of acute iliofemoral deep vein thrombosis. J Vasc Surg. 2012; 55: 1463-1473.
- Kahn SR. Measurement properties of the Villalta scale to define and classify the se-verity of the post-thrombotic syndrome. J Thromb Haemost. 2009; 7: 884-888.
- Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996; 5: 539-554.
- Pokrovsky A, Ignatyev I, Gradusov E. First experience of performing hybrid operations in chronic venous obstructions of iliofemoral segments in patients with postthrombotic syndrome. Vasc Endovasc Surg. 2017; 5: 447-452.
- Meissner MH, Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, et al. Early thrombus removal strategies for acute deep venous thrombosis: Clinical Practice Guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Sur. 2012; 55: 1449-1462.
- Nicolaides AN, Fareed J, Kakkar AK, Comerota AJ, Goldhaber SZ, Hull R, et al. Prevention and treatment of venous thromboembolism. International Consensus Statement. Int Angiol. 2013; 32: 225.
- Plate G, Einarsson E, Ohlin P, Jensen R, Qvarfordt P, Eklöf B, et al. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg. 1984; 1: 867-876.
- Neglén P, al-Hassan HK, Endrys J, Nazzal MM, Christenson JT, Eklof B, et al. Iliofemoral venous thrombectomy followed by percutaneous closure of the temporary arteriovenous fistula. Surgery. 1991; 110: 493-499.
- Hartung O, Benmiloud F, Barthelemy P, Dubuc M, Boufi M, Alimi YS. Late results of surgical venous thrombectomy with iliocaval stenting. J Vasc Surg. 2008; 47: 381-387.
- Juhan CM, Alimi YS, Barthelemy PJ, Fabre DF, Riviere CS. Late results of iliofemoral venous thrombectomy. J Vasc Surg. 1997; 25: 417-422.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrom-botic therapy for venous thromboembolic disease: ACCP evidencebased clinical practice guidelines. (8th ed). Chest. 2008; 133: 454S-545S.
- Lindow C, Mumme A, Asciutto G, Strohmann B, Hummel T, Geier B. Longterm results after transfemoral venous thrombectomy for iliofemoral deep venous thrombosis. Eur J Vasc Endovasc Surg. 2010; 40: 134-138.
- Masuda EM. Open venous thrombectomy: a technique that is still necessary in some patients. VEITH symposium On-Demand. 2016.
- Vedantham S, Thorpe PE, Cardella JF, Grassi CJ, Patel NH, Ferral H, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2006; 17: 435-437.
- Wagenhäuser MU, Sadat H, Dueppers P, Meyer-Janiszewski YK, Spin JM, Schelzig H, et al. Open surgery for iliofemoral deep vein thrombosis with temporary arteriovenous fis-tula remains valuable. Phlebology. 2018; 33: 600-609.
- Comerota AJ. The future of deep venous thrombosis and post-thrombotic syndrome in 2020. Phlebology. 2012; 27: 95-102.