
Case Report
Austin J Surg. 2020; 7(3): 1250.
Clinic Pathological Profile of Male Breast Cancer
Essam Elshiekh1*, Tarek Salah Youssef1, Mohamed Yousif Elsabrout1, Ahmed Alghazaly1, Ahmed Mohamed Ramadan2, Hossam Elashtokhy3, Ahmed I Ebeed4, Mosad Mohamed El-lity2, Mohamed Ibrahim5
1Surgical Oncology Department, Tanta Cancer Center, Egypt
2Pathology Department, Tanta Cancer Center, Egypt
3Medical Oncology Department, Tanta Cancer Center, Egypt
4Radiology Department, Tanta Cancer Center, Egypt
5General Surgery Department, Fayoum Faculty of Medicine, Fayoum University, Egypt
*Corresponding author: Essam Elshiekh, Surgical Oncology Department, Tanta Cancer Center, Egypt
Received: April 18, 2020; Accepted: July 25, 2020; Published: August 01, 2020
Abstract
Background: Male breast cancer is a rare cancer of less than 1% of breast cancer and <1% of all neoplasia, usually presented in advanced stage than female breast cancer.
Patients and Methods: Retrospective study done at Tanta cancer center and General surgery department, Fayoum faculty of medicine, Fayoum University, Egypt. With collection of data for male breast cancer diagnosed to analyze for the biological behavior of the tumor in the period between January 2014 and December 2018.
Results: 25 patients were diagnosed with male breast cancer and underwent surgical interventions after metastatic workup and found that age ranged between 44-72 years with median age of 56.5 years and was found to express estrogen and progesterone receptors in a rate less than female breast and presented with a large size of the tumor and axillary nodes with an advanced stage than the female patients.
Conclusion: Male breast cancer presented with prolonged symptoms more than female with large size of tumor and advanced stage and has survival rate equal to or little better than females. It is important to have early detection program for male as in females to detect breast cancer to obtain diagnosis at early stage.
Keywords: Male Breast Cancer; Clinicopathological Profile
Abbreviations
IDC: invasive duct carcinoma; ILC: Invasive Lobular Carcinoma; MRM: Modified Radical Mastectomy; MBC: Male Breast Cancer; ER: Estrogen Receptor; PR: Progesterone receptor; Her2/neu: Herceptin; OS: Overall Survival; DFS: Disease Free Survival; LVI: Perivascular Invasion; PNI: Perineural Invasion; CT: Computerized Tomography; DM: Diabetes Mellitus; LN: Lymph Node; FBC: Female Breast Cancer; US: Ultrasound; CXR: Chest X-Ray
Introduction
Breast cancer is the most common malignancy in women [1]. Breast cancer in females is diagnosed around the 6th to 7th decade of life, but the diagnosis of breast cancer in a middle-aged adult male is very rare [2]. The age of incidence of breast cancer in males tend to be higher than the females and is attributed to delay reporting in males. Male Breast Cancer (MBC) is relatively uncommon malignancy with less than 1% incidence. MBC presents at a later age with a more advanced presentation as compared to the female breast cancer. Due to the rareness of the number of cases and trials regarding the MBC, we follow the protocol of treatment used in female breast cancer. Mastectomy and hormonal therapy remains the mainstay of treatment. Moreover, the data and trials about prognosis of MBC still limited. Men with breast cancer are a disadvantaged minority. They have been diagnosed with a disease which some considered to be an afflicted. This lack of awareness explains why more than 40% present with advanced or metastatic disease at time of diagnosis [3]. The biology of Male Breast Cancer (MBC) differs significantly from that of Female Breast Cancer (FBC) [4-7]. Despite this, at present, most treatment decisions are based on the Randomized Controlled Trials (RCTs) in FBC. Mastectomy has been the standard surgical protocol for MBC whereas breast conserving therapy is widely used for selected females with the disease and has been shown to be effective in the long-term [8-10]. For men with breast cancer, combined approaches and thoughtful surgery are needed to achieve maximal rate of cure together with a minimum of long-term psychological distress.
Patients and Methods
This is a retrospective study done at Tanta cancer center between January 2014 to end of December 2018 at surgical department of Tanta Cancer Center, Egypt and General surgery department, Fayoum faculty of medicine, Fayoum University, Egypt. When all cases with male breast cancer were collected and their data are registered and analyzed. The inclusion criteria were > 18 years old male patients with localized, locally advanced or metastatic breast cancer confirmed histologically and who have benefited from therapeutic treatment in our structure.
There were 25 cases diagnosed as male breast cancer and all was subjected for diagnosis pathologically either by FNAC or true cut tissue core biopsy and all had their metastatic workup in the form of U/S of abdomen and pelvis, CXR or CT chest when needed and bone scan together with the full laboratory examination including full CBC, urea and creatinine, albumin serum level, liver enzymes, alkaline phosphatase and coagulation profile.
Data regarding general characteristics of patients (age, presenting signs and symptoms, duration of symptoms, and site and location of tumor), histopathology of primary tumor, treatment modalities (surgery, chemotherapy, radiation and hormone therapy), disease-free survival, and overall survival were obtained by reviewing medical records.
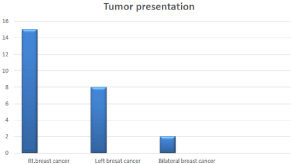
Figure 1: Tumor Presentation.
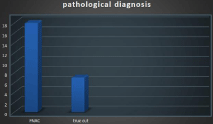
Figure 2: Pathological Diagnosis.
Clinical follow-up included history taking, physical examination, laboratory tests and radiologic imaging tests every 6 - 12 months for detection of relapse.
Results
General Characters of Patients
All patients are male with age between 44-70 years old with median age of 56.5 years, presented with either mass in the breast or retro areolar or presented by ulceration of the nipple or discharge with or without axillary Lymph node enlargement, of our series 15/25 patients (60%) presented on Rt. breast, 8/25(32) on Left breast and 2/25(8%) presented by bilateral synchronous fungating male breast cancer (Figure 1).
The tumor usually presented as mass in 17/25 (68%) cases presented as retro areolar mass with or without nipple invasion while presented as upper outer quadrant mass in 8/25(32%) cases (Table 1).
The size of tumor was determined by the use of US breast or mammography pre operatively and found that 32% of cases 8/25 were < 3cm in diameter while 40% of cases 10/25 presented as 3-4 cm in dimensions and >4cm detected in 7/25 patients (28%), nipple and areola complex infiltration detected in 15/25(60%) cases while was free in 10/25 patients (40%) with no cases presented with skin affection.
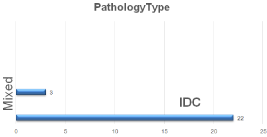
Figure 3: Type of Pathology.
Item
No
Percentage
Age
40-50 years' old
51-60 years' old
61-70 years old44-70 (median 56.5ys)
7
6
12
28%
24%
48%Size of mass
<3cm
3.1-4cm
>4cm
8
10
7
32%
40%
28%Site of tumor
Retro areolar
UOQ
15
10
60%
40%Skin infiltration
Yes
No
15
10
60%
40%Comorbid diseases
*DM
Yes
No
8
17
32%
68%*Hypertension
Yes
No
7
18
28%
72%Cardiac diseases
Yes
No
5
20
20%
80%
Table 1: Characteristics of patients.
8/25 patients had DM (diabetes mellitus) and controlled by its specific treatment before surgery and 7/25 had hypertension under medical treatment while 5/25 had cardiac problems that approved by the use of routine echocardiography for all patients with cardiac consultation before surgical interference.
Pathological and Biological Findings
All patients suspected to have male breast cancer were subjected to be examined clinically and had the pathological diagnosis either by FNAC or true cut core tissue biopsy and then all radiological metastatic workup to improve the presence of distant metastasis or not to have their protocol of treatment then the specimen examined pathologically either intra operatively or postoperatively with the routine markers done for all patients to indicate the biological behavior of the tumor and detect its stage and grade .
Pathological diagnosis was detected by the use of FNAC in 18/25 cases (72%) while another 28% of cases (7/25) diagnosed by tissue biopsy by lumpectomy (Figure 2).
During post-operative examination of specimen, IDC (infiltrating duct carcinoma) diagnosed as the most common pathological presentation in 22/25 cases (88%) while only 3/25 (12%) of cases diagnosed as mixed duct and lobular carcinoma, with tumor grading diagnosed as grade II in 17/25 cases and diagnosed in high grade GIII in 8/25 cases. Multifocality diagnosed in 5/25(20%) cases with no cases diagnosed as multicenteric.
Item
Number of patients
Percentage
Type of pathology
IDC
IDC & ILC
22
3
88%
12%Grades
2
3
17
8
68%
32%LVI
Yes
No
18
7
64%
36%PNI
Yes
No
5
20
20%
80%IDC
Yes
No
0
25
0%
100%Lymph nodes(LNs)
0-2 nodes infiltrated
3-5 nodes infiltrated
5-10 nodes infiltrated
>10 nodes infiltrated
10
5
3
7
40%
20%
12%
28%
ER
+ve
-ve
17
8
68%
32%PR
+ve
-ve
17
8
68%
32%Her2/neu
+ve
-ve
10
15
40%
60%Ki67
+ve
-ve
20
5
80%
20%Triple negative
8
32%
Multicentericity
Yes
No
0
25
0%
100%Multifocality
Yes
No
5
20
20%
80%Perinodal invasion
Yes
No
10
15
40%
60%
Table 2: Pathological characters of tumors.
LVI (lymphovascular invasion) detected in 18 of 25 cases (72%) and 28% was negative for LVI, while PNI (perineural invasion) detected only in 5 out of 25 cases (20%) with the rest of cases 80% has no PNI with No itraductal component detected in all cases during the post-operative examination.
With examination of the axillary LNs (Lymph nodes) harvested during the surgical protocol, 10/25 (40%) has 0-2 axillary lymph node infiltration with 3-5 nodes infiltrated in 5/25 patients (20%) while 6-10 nodes found infiltrated in 3/25 patients (12%) and >10 nodes infiltrated in 7/25 patients (28%) while the largest number dissected of 39 nodes.
Biological test of the tumor was detected in the form of exam for ER (estrogen receptors), PR (progesterone receptors), Her2/neu (Herceptin receptors) and KI67. ER receptors detected as +ve in 17/25 cases and –ve in 8/25 cases of the study and PR detected as +ve in 17/25 and –ve in 8/25 of cases also while Her2/neu was +ve in 10/25 cases (40%) and 60% of cases was –ve (15/25) and KI67 was detected in 16/25 cases as +ve and 9/25 cases as –ve to determine the biological behavior of the tumor.
Radiological exam
Type of metastasis No=25
Lung
Liver
Bone
Brain
Preoperative
Yes
No
1/25 (4%)
24/25(96%)1/25(4%)
1/25(4%)
1/25(4%)
0
Postoperative
Yes
No
7/25(28%)
18/25(72%)
2/25(12%)
23/25(88%)
3/25(20%)
22/25(80%)
2/25 (12%)
23/25(88%)
0
25(100%)
Table 3: Metastatic workup.
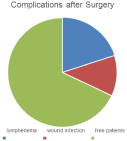
Figure 4: Complications after Surgery.
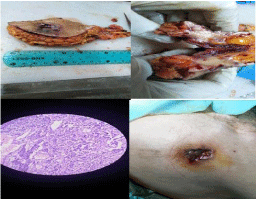
Figure 5: Example to cases, presentation, mass after excision, cut section
and microscopic appearance.
Perinodal invasion detected in 10/25 cases and 15/25 was negative for invasion.
All cases had their metastatic workup in the form of U/S abdomen and pelvis to detect liver metastasis and suspected cases had triphasic CT to confirm the diagnosis with CXR (chest X-ray) for all patients to detect pulmonary metastasis and CT chest for cases suspected together with bone scan done for all patients routinely in the protocol of study to detect bone metastasis except 2 cases presented as bilateral fungating male breast cancer and underwent bilateral toilet mastectomies in the same sitting to guard against the bleeding (Figure 3) (Table 2).
Treatment Protocol
After the metastatic workup and clinicopathological diagnosis, 5/25 (20%) of patients had neoadjuvant chemotherapy by breast protocol of chemotherapy then all patients decided to have MRM (modified radical mastectomy ) and axillary dissection for LNs except in 2 cases of bilateral fungating ulceration that had bilateral toilet mastectomies in the same sitting to prevent complications., with complications detected as wound infection in 3/25 cases and limb lymphedema detected in 5/25 cases postoperatively (Figure 4).
Then all patients subjected to adjuvant chemotherapy and Radiotherapy in the protocol of breast cancer and followed by hormonal therapy as tamoxifen for 5 years as the protocol of treatment.
During follow up, 5/25 cases detected by CT abdomen and pelvis to have Liver focal lesions and had triphasic CT that detect liver metastasis in 3 cases and liver cyst in 2 cases while pulmonary metastasis diagnosed by CT chest in 2cases and bone metastasis in 2/25 cases by the bone scan in suspected cases (Figure 5).
Metastatic workup (Table 2)
Survival: OS (overall survival) was 24/25 patients and only one patients died in 1st year post-operative from metastasis that was multiple (Liver, Pulmonary and Bone) while the Disease Free Survival (DFS) was 19/25 (76%) patients and 6/25 (24%) was live with different metastasis either single or multiple after one year of follow up while after 5 years of follow up, the OS was detected for 20/25 (80%) with 5/25 died with the next 5 years of surgery while the disease free survival was detected in 19/25 (76%) and still one patient alive with liver metastasis after ablation with radiofrequency.
Adjuvant Therapy: All patients undergone adjuvant therapy in the form of chemotherapy, radiotherapy hormonal therapy and only 10/25 patients had targeted therapy in the form of Herceptin due to positive receptors, all done according to the international protocol. Protocol with Anthracycline dependent protocol in the form of Adriamycin and epirubcin and taxanes protocol in metastatic breast cancer with radiotherapy and Herceptin for patients approved to be positive for Her/2neu receptors.
Discussion
In our study we are trying to collect the data of 25 patients diagnosed as male breast cancer. This is a retrospective study done at Tanta cancer center between January 2014 to end of December 2018 at surgical department of Tanta Cancer Center, Egypt and General surgery department, Fayoum faculty of medicine, Fayoum University, Egypt. When all cases with male breast cancer were collected and their data are registered and analyzed. The inclusion criteria were >18 years old male patients with localized, locally advanced or metastatic breast cancer confirmed histologically and who have benefited from therapeutic treatment in our structure with pre-operative investigations and follow up closely post-operative as the protocol of our center.
Male Breast Cancer (MBC) is a rare disease and accounts for less than 1% of all cases of malignancy in men [11]. The incidence of MBC has significantly increased from 0.86 to 1.08 per 100,000 populations over the past 26 years in the United States [12]. MBC in EGYPT is still less than 1% of breast cancers and <1% of all neoplasias at all. Because of its low incidence, MBC has not been studied as extensively as female breast cancer. Most studies related to MBC are retrospective analyses with a small number of patients. Appropriate management guidelines for MBC have not yet been clearly established, and limited information is available regarding the epidemiology, treatment, and prognosis of the disease [13]. Therefore, the treatment guideline has been extrapolated from the data based on female breast cancer.
However, the incidence of MBC has been increasing significantly along with the increasing incidence of female breast cancer and the use recent of recent techniques and facilities in diagnosis together with programs of early detection, although geographic variations in the incidence of MBC was reported [12,14,15]. In Europe, approximately 1% of all BC occurs in males, but the incidence is much higher in other areas such as sub-Saharan Africa with 15% [16,17]. Therefore, the study of MBC will become more important every day. In this study, we investigated clinicopathological characteristics, treatment patterns, and outcomes of MBC in Egypt.
MBC in Western countries was presented mostly in their 60s (range, 63 - 68 years), which is 10 years later than in females [16,18,19]. However, as in female breast cancer, the onset of MBC in Korea is 10 years earlier than in Western countries and appears to peak in their 50s [20,21]. In our study the mean age was 56.5 years old with the peak of cases in their 60s.
Infiltrating ductal carcinoma (IDC) is the most frequent invasive carcinoma in men, accounting for 70 - 95% of MBC, and lobular carcinoma is rare (around 1% of all cases) due to lack of terminal lobules in the male breast [19,22,23,24]. In our study, the majority of cases was IDC 22/25 cases (88%) while 3/25 (12%) was mixed ILC&IDC and no pure lobular carcinoma with no cases with intra ductal component detected in this study.
The expressions of estrogen and progesterone receptor in MBC are higher than in female breast cancer, and they have been reported to be 81 - 90.6% and 74 - 81.2%, respectively [25,26]. In our study, the expressions of estrogen and progesterone receptor in MBC were nearly equal or little lower than in female breast cancer Estrogen and progesterone receptors expressed in 68% of cases while Her/2neu receptors expressed in 40% of cases.
The most common surgical treatment for MBC is modified radical mastectomy, which has displaced radical mastectomy with no significant difference in survival [19,25,27]. However, lumpectomy does not play an important role in the treatment of MBC, because of small volume of male breast tissue and the advanced stage at diagnosis due to diagnosis masked as Gynecomastia [12,25]. For invasive breast cancer, axillary lymph node assessment is usually performed either via sentinel node biopsy or axillary sampling/clearance [24]. Sentinel lymph node biopsy is established as an accurate and low morbid procedure in female breast cancer, and sentinel node biopsy plays an important role in MBC and is being currently advocated as the standard surgical procedure [15]. In our study, 2 patients did not receive axillary node dissection because they had bilateral toilet mastectomy due to ulceration in both breasts with all other patients underwent modified radical mastectomy either after neoadjuvant therapy or from the start. Generally, men with BC had a longer duration of symptoms than women, delay in diagnosis contributes to advanced stage [11,28]. Tumor size and axillary lymph node involvement are the most important prognostic factors for male and female breast cancer [26,29]. When matching age and stage, men and women have similar prognosis [12,26]. In our study, although the sample size was too small, there was no difference in disease-free survival and overall survival between MBC and female ductal carcinoma. Although it was not statistically significant, older age, larger size, axillary lymph mode metastasis, advanced stage, higher grade, and hormone receptor negativity were related to poor overall survival in MBC. It is known that men with BC have poorer overall survival rates than women, but this is probably due to older age at diagnosis, more advanced stage at presentation, and higher rates of death from comorbid disease, but not due to the biology of the disease itself [11,12,30]. With the Overall Survival (OS) in this study was 80% after 5 years of follow up while DFS was 76% after 5years.
Conclusion
The tumor biology of MBC is not significantly different from that of females; however, limited public awareness and absence of adequate screening for MBC result in delayed diagnosis and poor prognosis. With the age of diagnosis was older than female breast cancer but the survival is nearly the same or little better than the female. Therefore, these results suggest that early detection, adequate treatment, and close follow-up would be the mainstay for improving survival of MBC.
References
- Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell biochemistry and biophysics. 2015; 72: 333-338.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019; 69: 7-34.
- Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006; 367: 595-604.
- Shaaban AM, Ball GR, Brannan RA, Gabor Cserni, Anna Di Benedetto, Jo Dent, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012; 133: 949-958.
- Orr N, Cooke R, Jones M, Olivia Fletcher, Frank Dudbridge, Sarah Chilcott-Burns, et al. Genetic variants at chromosomes 2q35, 5p12, 6q25.1, 10q26.13, and 16q12.1 influence the risk of breast cancer in men. PLoS Genet. 2011; 9: e1002290.
- Johansson I, Nilsson C, Berglund P, Carina Strand, Goran Jonsson, Johan Staaf, et al. High-resolution genomic profiling of male breast cancer reveals differences hidden behind the similarities with female breast cancer. Breast Cancer Res Treat. 2011; 129: 747-760.
- Piscuoglio S, Murray M, Ng CKY, Elena Guerini-Rocco, Luciano G Martelotto, Felipe C Geyer, et al. The genomic landscape of male breast cancer. SABCS. 2014; 22: S6-S06.
- Fisher B, Anderson S, Bryant J, Richard G Margolese, Melvin Deutsch, Edwin R Fisher, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347: 1233-1241.
- Veronesi U, Cascinelli N, Mariani L, Marco Greco, Roberto Saccozzi, Alberto Luini, et al. Twenty-year follow-up of a randomized study comparing breast- conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347: 1227-1232.
- Litière S, Werutsky G, Fentiman IS, Emiel Rutgers, Marie-Rose Christiaens, Erik Van Limbergen, et al. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20-year follow-up of the EORTC 10801 phase 3 randomized trial. Lancet Oncol. 2012; 13: 412-419.
- Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002; 137: 678-687.
- Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population- based study. Cancer. 2004; 101: 51-57.
- Malani AK. Male breast cancer: a different disease than female breast cancer? South Med J. 2007; 100: 197.
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56: 106-130.
- Contractor KB, Kaur K, Rodrigues GS, Kulkarni DM, Singhal H. Male breast cancer: is the scenario changing. World J Surg Oncol. 2008; 6: 58.
- Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected etiological factors. Int J Cancer. 1993; 53: 538-549.
- Carlsson G, Hafstrom L, Jönsson PE. Male breast cancer. Clin Oncol. 1981; 7: 149-155.
- Ioka A, Tsukuma H, Ajiki W, Oshima A. Survival of male breast cancer patients: a population-based study in Osaka, Japan. Jpn J Clin Oncol. 2006; 36: 699-703.
- Nahleh Z, Girnius S. Male breast cancer: a gender issue. Nat Clin Pract Oncol. 2006; 3: 428-437.
- The Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2004 using breast cancer registration program [In Korean] J Breast Cancer. 2006; 9: 151-161.
- Son BH, Kwak BS, Kim JK, Kim HJ, Hong SJ, Lee JS, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg. 2006; 141: 155-160.
- Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002; 17: 457-462.
- Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005; 10: 471-479.
- Meguerditchian AN, Falardeau M, Martin G. Male breast carcinoma. Can J Surg. 2002; 45: 296-302.
- Agrawal A, Ayantunde AA, Rampaul R, Robertson JF. Male breast cancer: a review of clinical management. Breast Cancer Res Treat. 2007; 103: 11-21.
- Donegan WL, Redlich PN, Lang PJ, Gall MT. Carcinoma of the breast in males: a multi institutional survey. Cancer. 1998; 83: 498-509.
- Chung HC, Koh EH, Roh JK, Min JS, Lee KS, Suh CO, et al. Male breast cancer-a 20-year review of 16 cases at Yonsei University. Yonsei Med J. 1990; 31: 242-250.
- Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006; 367: 595-604.
- Willsher PC, Leach IH, Ellis IO, Bourke JB, Blamey RW, Robertson JF. A comparison outcome of male breast cancer with female breast cancer. Am J Surg. 1997; 173: 185-188.
- Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005; 10: 471-479.