
Review Article
Austin J Surg. 2020; 7(4): 1253.
Cardiac Myxomas: A Single Center Experience with 33 Cases
Ermal Likaj*, Selman Dumani, Saimir Kuci and Ali Refatllari
Cardiac Surgery Department, University Hospital Center Mother Theresa, Albania
*Corresponding author: Ermal Likaj, Cardiac Surgery Clinic, Rruga e Dibres, Albaniat
Received: September 02, 2020; Accepted: September 24, 2020; Published: October 01, 2020
Abstract
Objectives: In this single-center study we reviewed our experience with a significant number of cardiac myxomas operated in the period January 2004 to January 2016.
Patients and Methods: There were 33 consecutive patients operated for cardiac myxoma. We had a female dominance in the population with 23 (69.7%) females and 11 (30.3%) males. Mean age at operation was 55±12.33 years. A detailed clinical and echocardiographic early and long-term examination of these patients was done during this period.
Results: Most myxomas originated from the left atrium in 31 (94%) patients, and the remainder from the right atrium in 2 (6%) patients. On echocardiography, the myxomas produced a prolapse into the left ventricle in 42.4% of the patients and mitral stenosis in 30.3%. Cardiac signs like dyspnea (57.57%) and syncope (27.27%) appeared in the majority of the patients and led to the diagnosis. Preoperative embolic events had occurred in 12.12%. Coronary angiography was done in 27 patients and two of them had isolated coronary stenosis.
Median sternotomy and cardiopulmonary bypass with separate bicaval cannulation was the routine approach. Bilateral atriotomy or transseptal incisions were the preferred approaches that were used. The atrial septum at the site of insertion was resected in 25 (75.75%) of the patients and it was closed with a pericardial patch in 12 (36.36%) patients. Mean cross-clamp time was 39.6±14.7 min. Three patients received a concomitant surgical procedure: bypass grafting (2 patients) and mitral valve replacement (1 patient).
The early mortality rate was 3.0% (one 80 years old patient died of massive gastrointestinal bleeding). Early complications included atrial fibrillation (3 patients), pericardial effusion (2 patients), revision for bleeding (2 patients) and stroke (1 patient). Postoperatively, 85.23% of the patients remained without cardiac symptoms. Long term follow up was 63.48±43.55 months and the prognosis was excellent. No patient is re-operated up to date for recurring myxoma.
Conclusion: Myxomas were usually detected and operated on in symptomatic patients. A high index of suspicion seems important for early diagnosis. Echocardiography was the key imaging examination for the diagnosis. Immediate surgical treatment was indicated because of the high risk of embolization or of sudden cardiac death.
Keywords: Myxoma; Embolization; Tumor
Introduction
Intracardiac myxoma is the most common tumor of the heart with an estimated incidence of 0.5 per million population per year [1]. In cardiac surgery, regarding the operations with cardio-pulmonary bypass, 0.3% are resections of a cardiac myxoma [2]. Up to 80% of myxomas are localized in the left atrium, of which 75% involve the interatrial septum; 7-20% are found in the right atrium; the rest of up to 10% each are either biatrial, in the right ventricle, or in the left ventricle [2-4]. The symptoms related to the presence of the tumor inside the heart depend on the size and the localization of this tumor. In this single-center study we reviewed our experience with a significant number of cardiac myxomas operated in the period January 2004 to January 2016. A detailed clinical and echocardiographic early and long-term examination of these patients was done during this period.
Patients and Methods
There were 33 consecutive patients operated for cardiac myxoma. We had a female dominance in the population with 23 (69.7%) females and 11 (30.3%) males. Mean age at operation was 55±12.33 years.
Results
Echocardiography
Echocardiography was the gold standard for the detection and the description of the tumor. Most myxomas originated from the left atrium in 31 (94%) patients, and the remainder from the right atrium in 2 (6%) patients. On echocardiography, the myxomas produced a prolapse into the left ventricle in 42.4% of the patients and mitral stenosis in 30.3%. Detailed data obtained on echocardiography are presented in the following Table 1.
Finding in ECHO
Number of patients
Percentage
Left atrium insertion
31
94
Right atrium insertion
2
6
Floating left atrial myxoma
14
42.4
Prolapse into left ventricle
14
42.4
Elevated pulmonary artery pressure
8
24
Reduced left ventricular function
2
6
Mitral stenosis
10
30.3
Mitral insufficiency
4
12
Left atrial dilatation
14
42.4
Right atrium dilatation
8
24
Table 1: Echocardiography data.
Clinical Signs
Cardiac signs like dyspnea (57.57%) and syncope (27.27%) appeared in the majority of the patients and led to the diagnosis. Preoperative embolic events had occurred in 12.12%. Coronary angiography was done in 27 patients and two of them had isolated coronary stenosis (Table 2).
Sign
Number of patients
Percentage
Dyspnea NYHA II
III
IV4
10
512.12
30.3
15.15Syncope
9
27.27
Stroke
4
12.12
Peripheral embolism
1
3
Palpitations
15
45.45
Fatigue
14
42.42
Fever
1
3
Table 2: Clinical signs.
Operative data
Median sternotomy and cardiopulmonary bypass with separate bicaval cannulation was the routine approach. Bilateral atriotomy or trans-septal incisions were the preferred approaches that were used. The atrial septum at the site of insertion was resected in 25 (75.75%) of the patients and it was closed with a pericardial patch in 12 (36.36%) patients. Mean cross-clamp time was 39.6±14.7 min. Three patients received a concomitant surgical procedure: bypass grafting (2 patients) and mitral valve replacement (1 patient) (Table 3).
Data
Number
Percentage
Median sternotomy
33
100
Surgical approach
Bilateral atriotomy
Trans-septal incision
Right atrium
Left atrium
15
12
1
5
45.45
36.36
3
15.15Atrial septum resection
25
75.75
Pericardial patch
12
36.6
Concomitant procedure
CABG
MVR
2
1
6
3Mean cross-clamp time 39.6±14.7 min
Table 3: Operative data.
Complication
Number
Percentage
Atrial fibrillation
3
Pericardial effusion
2
Revision for bleeding
2
Postoperative stroke
1
GI bleeding
Mortality 3.0 %
Follow up 63.48±43.55 months
Reoperations none
Table 4: Postoperative data.
The early mortality rate was 3.0% (one 80 years old patient died of massive gastrointestinal bleeding). Early complications included atrial fibrillation (3 patients), pericardial effusion (2 patients), revision for bleeding (2 patients) and stroke (1 patient). Postoperatively, 85.23% of the patients remained without cardiac symptoms. Long term follow up was 63.48±43.55 months and the prognosis was excellent. No patient is re-operated up to date for recurring myxoma.
Discussion
Primary tumors of the heart are rare and the myxoma is the most frequent benign primary heart tumor as it accounts for 0.3% of open-heart surgery performed worldwide [5,6] (Figure 1). The clinical presentation in the majority of patients consists of important hemodynamic symptoms related to blood flow obstruction and embolic phenomena.
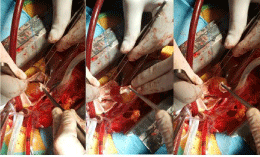
Figure 1: Open-heart surgery.
Furthermore, the myxoma may threaten valve obstruction and, with future tumor expansion, a left ventricular outflow tract obstruction. These patients can have an increased risk for acute cardiogenic shock or sudden cardiac death [5,7].
A higher risk of embolization has also been reported and events occur in 30-43% of the patients [2,3]. Embolization from a mitral valve myxoma might occur more often than from an atrial myxoma due to motion of the valve leaflets. The high pressure within the left ventricle during systole seems to give rise to embolizations more frequently from the left than from the right side of the heart [3]. Embolizations occur more often from polypoid tumors floating in the blood stream than from solid round tumors [9]. Tumorsize may play an additional role in embolization [10]. Asymptomatic cases were rare. This important fact may have an impact on screening methods for such patients.
The surgical access to the myxoma may vary depending on the tumor location. Myxoma excision by way of left atriotomy may be feasible in most cases. This approach facilitates the exposure of the left-ventricular-sided aspect of the mitral valve apparatus. Trans-septal and bi-atrial approach assures a complete revision of atria and total resection of the tumor. In most of the cases presented here, and in most cases presented in the literature, the originally compromised mitral, tricuspid, or aortic valves and the interventricular septum were completely preserved, and the patients were treated by resection of the tumor alone.
Closure of an Atrial Septal Defect (ASD) due to tumor resection was performed in 25 cases. About half of them received a pericardial patch to close the defect. We can emphasize at this point that the resection was wide at our series of patients in order to lower at maximum the chances for tumor recurrence. In those rare instances in which the tumor arises from an Atrioventricular Valve (AV) valve, the valve occasionally requires valvuloplasty or even replacement. Special care must be taken to avoid intraoperative systemic or pulmonary embolization of the myxoma. With systolic prolapse of some tumors into the left ventricular outflow tract, patients are at a higher risk of intraoperative embolization.
The prognosis for patients with solitary myxomas after surgical resection has been excellent. Postoperative complications were comparable to other cardiac operations (Table 4). Usually, the hospital mortality after the removal of an atrial myxoma is about 4% [2]. All current surgical techniques seem to provide low recurrence rates. Late recurrences have been reported to occur in 0.4-5% of surgically treated patients from up to 22 years after operation [2]. Cardiac myxomas seem to recur more often in young males, or in patients with multifocal origins, and in those who have a family history of the tumor [2,7]. Therefore, it is necessary to perform routine echocardiography frequently throughout a patient’s life. Except for patients with multifocal, atypical, or familial myxomas, echocardiography at 5-yearintervals for several years should be adequate.
The courses of our patients indicate that the many risks inherent in this disease may be kept very low with immediate surgical treatment after early diagnosis. As this disease may mimic a huge variety of other cardiac diseases, a possible myxoma should always be considered since relatively low risk cardiac surgical treatment is performable for most patients, and its postoperative prognosis is excellent.
Conclusion
Myxomas were usually detected and operated on in symptomatic patients. A high index of suspicion seems important for early diagnosis. Echocardiography was the key imaging examination for the diagnosis. Immediate surgical treatment was indicated because of the high risk of embolization or of sudden cardiac death.
References
- Mac Gowan SW, Sidhy P, Aherne T, Luke D, Wood AE, Neligan MC, et al. Atrial myxoma: national incidence, diagnosis and surgical management. Ir J Med Sci. 1993; 162: 223-226.
- Castells E, Ferran V, Octavio de Toledo MC, Calbet JM, Benito M, Fontanillas C, et al. Cardiac myxomas: Surgical treatment, long-term results and recurrence. J Cardiovasc Surg Torino. 1993; 34: 49-53.
- Chakfe N, Kretz JG, Valentin P, Geny B, Petit H, Popescu S, et al. Clinical presentation and treatment options formitral valve myxoma. Ann Thorac Surg. 1997; 64: 872-877.
- Bjessmo S, Ivert T. Cardiac myxoma: 40 years’ experience in 63 patients. Ann Thorac Surg. 1997; 63: 697-700.
- Vassiliadis N, Vassiliadis K, Karkavelas G. Sudden death due tocardiac myxoma. Med Sci Law. 1997; 37: 76-78.
- Reynen K, Rober U, Daniel WG, Henge E, Schuler S. Herz operation enwegen Herztumoren in Deutschland - Ergebnisseeiner Umfragefur das Jahr. 1996. Z Kardiol. 1998; 87: 331-335.
- Cilliers AM, van Unen H, Lala S, Vanderdonck KH, Hartman E. Massive biatrial myxomas in a child. Pediatr Cardiol. 1999; 20: 150-151.
- Keeling I, Oberwalder PJ, Schuchlenz H, Anelli-Monti M, Rigler B. Left ventricular outflow tract obstruction due to valve myxoma. Ann Thorac Surg. 2000; 69: 1591-1592.
- Ha JW, Kang WC, Chung N, Chang BC, Rim SJ, Kwon JW, et al. Echocardiographic andmorphologic characteristics of left atrial myxoma and their relationto systemic embolism. Am J Cardiol. 1999; 83: 1579-1582.
- Endo A, Ohtahara A, Kinugawa T, Mori M, Fujimoto Y, Yoshida A, et al. Characteristics of 161patients with cardiac tumors diagnosed during 1993 and 1994 in Japan. Am J Cardiol. 1997; 79: 1708-1711.