
Research Article
Austin J Surg. 2020; 7(4): 1256.
MSCT Virtual Modeling of the Robotic-Assisted Operations on the Spleen
Stepanova Yu A*, Shirokov VS, Ionkin DA, Alimurzaeva MZ and Berelavichus SV
A.V. Vishnevsky National Medical Research Center of Surgery, Moscow, Russia
*Corresponding author: Yulia A Stepanova, A.V. Vishnevsky National Medical Research Center of Surgery, Russia
Received: November 16, 2020; Accepted: November 27, 2020; Published: December 04, 2020
Abstract
Purpose: To develop the method of preoperative MSCT assessment of the possibility a robot-assisted surgical intervention performing in patients with spleen focal lesions.
Materials and Methods: At A.V. Vishnevsky National Medical Center of Surgery 20 surgical interventions using the robotic surgical complex Da Vinci in patients with spleen focal lesions were performed (women predominated - 17 (85.0%), mean age - 34.15 years) in the period from 2009 to 2020.
The technique that makes it possible to simulate the optimal the location of the laparoscopic and manipulation ports on the abdominal wall in relation to the area of surgical actions at surgery was developed, based on our own experience of previously performed robot-assisted surgical interventions on the abdominal and retroperitoneal organs with the aim of more adequate placement of ports for trocars on the anterior abdominal wall and effective implementation of robotic-assisted surgical intervention on the spleen.
Results: Preoperative morphological verification of spleen focal lesions was confirmed histologically in all cases: lymphangioma - 8; true cyst - 7; cyst - 3; pseudocyst - 1; hamartoma - 1.
The localization of the lesions was presented as follows: upper pole - 11: upper pole and middle third - 4; middle third - 1; lower pole - 4.
An important aspect of the preoperative examination was the 3D-reconstruction of the MSCT-images, which made it possible to assess in more detail both the lesion itself and its localization in the organ parenchyma and the relationship with the surrounding intraorgan arteries and veins.
Based on the analysis of the data of the preoperative examination in the presented 20 cases, it was decided on the possibility of a robot-assisted surgery on the spleen. The volume of surgical interventions performed on the spleen was as follows: robot-assisted partial splenectomy (or spleen resections) - in 15 patients; cyst fenestration - in 2 patients; splenectomy - in 3 patients.
In 2 cases with combined lesions, combined surgical interventions were performed: for pancreatic tail adenocarcinoma (up to 2.0 cm) and lymphangioma of the spleen - robot-assisted resection of the pancreatic tail and partial splenectomy; for gastrointestinal tumors of the small intestine and spleen cyst - robotic-assisted resection of the small intestine (up to 43.0 mm) and partial splenectomy.
Conclusion: The use of the presented technique in patients with spleen focal lesions made it possible to simulate the main stages of a robotic-assisted intervention before surgery, optimize the operation of robot manipulators, and reduce the risk of damage to organs and tissues in the area of surgical treatment and in its immediate vicinity. The clear layout of the ports, determined at the preoperative stage, allowed avoiding additional trauma to the anterior abdominal wall associated with the need to reinstall the trocars.
Keywords: Spleen lesions; MSCT virtual modeling; Robotic-assisted operations; Partial splenectomy
Introduction
Although robotic surgery is still not widely used, it is currently one of the cutting edge advances in surgery with broad application prospects. With increased precision and maneuverability, this technology allows surgeons to perform operations that have traditionally defied minimal access. As a result of this advantage, minimal access surgery can be applied to a wider range of surgical conditions.
Spleen resection is a method of preserving the organ parenchyma, which is used for trauma, focal lesions, or hematological diseases. Despite the improvement of laparoscopic techniques over the past few decades, minimally invasive partial splenectomy (or spleen resections) (laparoscopic or robot-assisted) is currently not widely used. This conclusion was made by A. Balaphas et al., who searched the MEDLINE database (PubMed) and included in their study all articles that dealt with truly minimally invasive partial splenectomy (that is, segmental or lobar devascularization of the spleen with transection of the parenchyma). Of the 195 publications found, 33 were included, which were mainly clinical observations and case series, in which a total of 187 patients were represented [1]. The authors separately emphasized that a robotic approach can be an interesting technical option. Basically, this cautious approach is due to the small number of cases.
In recent years, robot-assisted partial splenectomy has been described in the literature mainly as single observations [2-5] or as a small series [6]. Robotic-assisted splenectomy is used more often and to a greater extent [7-9]. It should be noted that all authors emphasize that the 3D operating vision and mechanical arm of the Da Vinci robotic surgical system overcome the limitation of the flexibility of vision and operating instruments, which can significantly improve the controllability, stability and accuracy of surgery.
Thus, today the feasibility of performing robot-assisted interventions in small spaces is beyond doubt. However, against this background, progress in the implementation of robotic systems in abdominal surgery is still quite modest. In the medical literature, there are few works on the use of these systems in the treatment of diseases of the abdominal cavity and retroperitoneal space. The limited use of robot-assisted operations on the abdominal and retroperitoneal organs is associated with the need for most of these interventions to manipulate in various parts of the abdomen, moving the bowel loops, which is extremely difficult to perform with the manipulators of the robotic complex. At the same time, in abdominal surgery, there is a large number of operations that require high accuracy of movements in the absence of physiological tremor, which can be achieved with the help of robotic assistance. However, difficulties with the correct placement of trocars, which entail a decrease in the area of surgical impact of robotic instruments, collision of manipulators (arms) of the robot, great difficulties in the work of a surgeon and an assistant, are one of the factors limiting the use of robotic-assisted operations in various diseases of the abdominal cavity and retroperitoneal space. Therefore, it is advisable to search for solutions that provide the possibility of optimal use of robotic technology for performing operations [10].
Purpose
To develop the method of preoperative MSCT assessment of the possibility a robot-assisted surgical intervention performing in patients with spleen focal lesions.
Materials and Methods
At A.V. Vishnevsky National Medical Center of Surgery 20 surgical interventions using the robotic surgical complex Da Vinci in patients with spleen focal lesions were performed (women predominated - 17 (85.0%), mean age - 34.15 years) in the period from 2009 to 2020.
The technique that makes it possible to simulate the optimal the location of the laparoscopic and manipulation ports on the abdominal wall in relation to the area of surgical actions at surgery was developed, based on our own experience of previously performed robot-assisted surgical interventions on the abdominal and retroperitoneal organs [10,11] with the aim of more adequate placement of ports for trocars on the anterior abdominal wall and effective implementation of robotic-assisted surgical intervention on the spleen.
MSCT virtual modeling of the robotic-assisted operations on the sleen.
The study was performed on a multispiral computed tomography (Philips Brilliance iCT). Scanning was performed from the level of the right dome of the diaphragm to the pelvic floor. Intravenous bolus contrast enhancement was used in all studies. Ioversol was injected into the cubital vein (at a concentration of 350 mg of iodine per ml) at a rate of 4 ml / s. Following the contrast agent bolus, 50 ml of physiologic saline solution was administered. Scanning was performed using the bolus tracking technique. A dual head Opti Vantage was used to inject the contrast agent.
Scanning was started at a contrast medium density in the aortic of 150 HU. Scanning into the arterial phase was started 10 seconds after the aorta threshold was reached. After 35 sec from the start of scanning, images were acquired in the portal phase. The delayed phase was performed after 6-7 minutes (this phase is needed to clarify the nature of the lesion). The next step was to assess multiplanar reconstructions (to determine the type of the lesion, its localization in the spleen, the relationship to nearby organs when the lesion spreads beyond the spleen, arteries, veins, tightness of adherence, compression, deviations from the physiological course of the vessel). After the final verification of the diagnosis, a decision was made on the possibility of performing a robot-assisted intervention. Virtual simulation of a robot-assisted operation was carried out on a workstation of a Brilliance iCT tomograph with a «virtual radio frequency ablation» application. Using the data of the portal phase of the study in a semi-automatic mode with the possibility of manual adjustment, the lesion was examined simultaneously in all projections, including three-dimensional. The simulation started with Port 1, which was simulated by a robotic videol aparoscope. Simultaneous visualization of all projections of the patient’s body was carried out, “Port 1” for the laparoscope was virtually positioned, taking into account the peculiarities of the anatomy of the abdominal cavity, anterior abdominal wall and bone structures (costal arch, ilium). The inside of the “Port 1” was directed directly to the surgical site. Typically, the “Port 1”.
installation point was the navel area. After setting other “ports”, “Port 1” was moved to a more convenient position. The next two working robotic ports (“Port 2,3”) were set taking into account all anatomical features so that the distance between each of them and “Port 1” was not less than 12 cm. The inner part of the ports was oriented to the periphery of the surgical area. The insertion point of the auxiliary trocar was determined after the placement of robotic trocars 1-3. The accessory port was located on the opposite side of the surgical site in the largest gap between the robotic ports. After that, the image was saved with virtually installed instruments of the robot relative to the operation area and the patient’s body surface. For more accurate transfer of virtually installed trocar points to the anterior abdominal wall of the patient, a self-constructed navigation grid was used. The cells of this grid are 5 × 5 cm in size. X-ray positive markers are fixed at the corners of each square. Before MSCT, the mesh was placed on the anterior abdominal wall of the patient in such a way that the central mark was located in the navel. After an adequate virtual location of the “ports” against the background of the navigation grid, a clear “binding” of the installation points of the robotic and accessory ports to the X-ray positive marks was obtained. Immediately before the operation, the navigation mesh was placed on the anterior abdominal wall of the patient in the manner described. Having clear information about the location of trocar points relative to the marks, the insertion sites of the ports on the anterior abdominal wall were marked.
Results
Preoperative MSCT examination made it possible to make a preliminary morphological diagnosis and localize of the spleen lesion.
Preoperative morphological verification of spleen focal lesions was confirmed histologically in all cases:
• lymphangioma - 8;
• true cyst - 7;
• cyst - 3;
• pseudocyst - 1;
• hamartoma - 1.
Such morphological forms as “true cyst” and “cyst” are singled out separately, since the generally accepted differential diagnosis of “true” and “false” cysts based on the histological detection of the epithelial lining is not always possible, since with their prolonged existence, the cell lining of the cyst can atrophy under pressure of the contents or when the inflammatory process is attached, exfoliate during the operation or during puncture and evacuation of the contents [12].
A single lesion was identified in 16 cases, two lesions - in 1 and multiple - in 3.
The sizes and localization of the spleen lesions are presented in Table 1.
No.
Nature of the lesion
Sizes
Localization
Additional Characteristics
1
cyst
7x6,3 cm
lower pole small cysts up to 3-4 mm along the edge of the upper lobe
-
2
cyst
from 3 mm to 5,2 cm
upper pole and middle third
predominatly plurale
3
cystic lesion
11 cm 2,5 cm
upper pole and middle third
two cysts
4
suppurative cyst
up to 10 cm
middle third
-
5
cystic lesion
up to 7x6 cm
upper pole
plurale
6
cystic lesion
7 cm
lower pole
-
7
cyst
12 cm
upper pole
-
8
cystic lesion
up to 7 cm
upper pole and middle third
Pancreatic tail tumor
9
pseudocyst
up to 10,8 cm
upper pole
10
cyst
6,5 cm
upper pole
gastrointestinal stromal tumor of the small intestine
11
cystic lesion
10 cm
upper pole (to the gate)
-
12
cyst
up to 10 cm
upper pole and middle third
-
13
cyst
9 cm
upper pole
-
14
cystic lesion
10 cm
upper pole
-
15
cyst
10 cm
lower pole
-
16
cyst
8,5 cm
upper pole
-
17
cyst
8 cm
upper pole
-
18
cystic lesion
8 cm
upper pole
-
19
solid lesion (hamartoma)
8x9 cm
lower pole
-
20
cyst
5x6 cm
upper pole
-
Table 1: Size and localization of the spleen lesions according to preoperative MSCT.
Thus, the localization of the lesions was mainly in the projection of the upper pole of the spleen:
• upper pole - 11:
• upper pole and middle third - 4;
• middle third - 1;
• lower pole - 4.
An important aspect of the preoperative examination was the 3D-reconstruction of the MSCT-images, which made it possible to assess in more detail both the lesion itself and its localization in the organ parenchyma and the relationship with the surrounding intraorgan arteries and veins (Image). Image: 3D-reconstruction of the spleen hamartomas MSCT-images (the hypervascular lesion of the spleen lower pole is determined).
Based on the analysis of the data of the preoperative examination in the presented 20 cases, it was decided on the possibility of a robotassisted surgery on the spleen. The volume of surgical interventions performed on the spleen was as follows: robot-assisted partial splenectomy (or spleen resections) - in 15 patients; cyst fenestration - in 2 patients; splenectomy - in 3 patients.
It should be noted that, in 2 cases with a combined lesions, combined surgical interventions were performed: for pancreatic tail adenocarcinoma (up to 2.0 cm) and lymphangioma of the spleen - robot-assisted resection of the pancreatic tail and partial splenectomy; for gastrointestinal tumors of the small intestine and spleen cyst - robotic-assisted resection of the small intestine (up to 43.0 mm) and partial splenectomy.
Also, in one case, in a patient with hamartoma, a robotic-assisted resection of the lower pole of the spleen for better hemostasis was supplemented with RFA along the spleen section using our own technique (Cool-Type + FORCE Triada “Wallylab”).
It should be noted that, in some cases, it is necessary to install an additional (assistant) trocar for the introduction of a vacuum aspirator tip or clamp. In our study, this was done in 7 cases.
Analysis of the localization and sizes of spleen focal lesions, placement of trocar ports and the results of robot-assisted surgical interventions made it possible to generalize about the most effective location of trocar ports in the three most typical localizations of spleen focal lesions (Table 2, Figure 1).
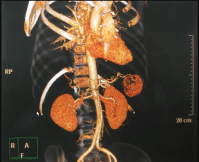
Figure 1: Schematic presentation of port locations in a patient with a large
cyst of the spleen upper pole (P1 and P2 are working ports; AP - accessory
port). The most effective position of the patient will be on the side at an angle
of 45°.
Part of the spleen
Robotic ports
Localization
Upper pole
robotic video laparoscope "Port 1"
above the navel
working port 1 "Port 2"
in the epigastrium along the midline 1-2 cm Below the xiphoid process of the sternum
working port 2 "Port 3"
in the left hypochondrium along the mid-clavicular line
accessory port
in the epigastrium along the midline 6 cm below the xiphoid process of the sternum
Lower pole
robotic video laparoscope "Port 1"
in the paraumbilical zone below the navel
working port 1 "Port 2"
1-2 cm to the right of the midline of the abdomen in the epigastrium
working port 2 "Port 3"
3-4 cm below the costal arch along the anterior axillary line on the left
accessory port
left hypochondrium at navel level Middle third
similar to the arrangement of ports in the upper pole localization
lesion localization
similar to the arrangement of ports in the lower pole lesion
MSCT modeling
According to the data of individual marking during preoperative
Table 2: The most effective position trocar ports with three most typical localizations of focal defeats elezenka 2.
The most effective position trocar ports with three most typical localizations of focal defeats elezenka 2.
MSCT modeling (Figure 1).
There were no complications during robotic-assisted surgery and in the early postoperative period. All interventions were performed with minimal blood loss (no more than 100 ml). The average duration of an operation with 3D computer modeling was reduced to 90 minutes, compared to 130 minutes for operations without preparation according to this technique. A conflict between robot manipulators (collision) did not arise in any case.
Partial splenectomy (resection of the spleen) is based on the topographic anatomy of its segmental blood supply and requires precise dissection of the parenchyma and effective isolation of individual arteries and veins of a sufficiently small caliber. A robotic surgical system allows you to effectively perform these actions due to high resolution and stereoscopic vision (there is no limitation of the flexibility of vision and operating instruments), as well as reduced tremor and excellent maneuverability [11,13,14].
Conclusion
The use of the presented technique in patients with spleen focal lesions made it possible to simulate the main stages of a roboticassisted intervention before surgery, optimize the operation of robot manipulators, and reduce the risk of damage to organs and tissues in the area of surgical treatment and in its immediate vicinity. The clear layout of the ports, determined at the preoperative stage, allowed avoiding additional trauma to the anterior abdominal wall associated with the need to reinstall the trocars.
References
- Balaphas A, Buchs NC, Meyer J, Hagen ME, Morel P. Partial splenectomy in the era of minimally invasive surgery: the current laparoscopic and robotic experiences. Surgical Endoscopy. 2015; 29: 3618-3627.
- Vasilescu C, Tudor S, Popa M, Tiron A, Lupescu I. Robotic partial splenectomy for hydatid cyst of the spleen. Lan-genbeck’s Arch Surg. 2010; 395: 1169-1174.
- Berelavichus SV, Smirnov AV, Ionkin DA, Kriger AG, Dugarova RS. Robotassisted and laparoscopic partial splenectomy for nonparasitic cysts. Khirurgiia (Mosk). 2015; 7: 41-48.
- Kirih MA, Liang X, Jinwei C, Junhao Z. Robot-Assisted Partial Splenectomy for Splenic Cysts: A Case Report. Journal of Cancer Science and Clinical Therapeutics. 2019; 3: 166-170.
- Kirih MA, Liang X, Xie Y, Cai J, Zheng J, Xu F, et al. Robot-Assisted Partial Splenectomy for Splenic Epidermoid Cyst. Case Rep Surg. 2020.
- Giulianotti PC, Buchs NC, Addeo P, Ayloo S, Bianco FM. Robot-assisted partial and total splenectomy. Int J Med Robot. 2011; 7: 482-488.
- Patriti A, Casciola L. Robot-Assisted Splenectomy: Technique and Indications. Patriti and Casciola, Adv Robot Autom. 2012; S1: 002.
- Micu B, Micu C, Pop TR, Constantea N. Robotic Splenectomy using the DaVinci Platform. In: Vlad S, Roman N. (editors) International Conference on Advancements of Medicine and Health Care through Technology; 12th - 15th October 2016, Cluj-Napoca, Romania. IFMBE Proceedings. 2017; 59.
- Kashi PK, Rojas C, Casablanca Y, Garg R. A seven-step surgical strategy for robotic splenectomy. Int J Gynecol Cancer. 2020; 1-2.
- Berelavichus SV, Karmazanovsky GG, Kriger AG, Fedorov AV, Shirokov VS, Kondratyev EV, et al. Virtual simulation of robotic-assisted abdominal surgery. Surgery. Journal them. NI. Pirogov. 2011; 6: 18-21.
- Berelavichus CB, Fedorov AB, Krieger AG, Gorin DS. Robotic surgery. Surgery. Journal them. N.I. Pirogov. 2008; 12: 68-73.
- Stepanova Yu A, Alimurzaeva MZ, Ionkin DA. Ultrasonic differential diagnostics of cyst and cystic tumors of the spleen. Medical Visualization. 2020; 24: 63-75.
- Gutt CN, Oniu T, Mehrabi A, Kashfi A, Schemmer P, Buchler MW. Robotassisted abdominal surgery. Br J Surg. 2004; 91: 1390-1397.
- Velarde D. Benefits of robotic surgery has popularity skyrocketing.