
Original Article
Austin J Surg. 2021; 8(1): 1262.
Is Sub-Commissural Annuloplasty a Safe Adjunct to Sutureless Perceval-S Aortic Valve Implantation?
Roumy A¹*, Verdugo M¹, Gunga MZ¹, Monney P², Rancati V³ and Kirsch M¹
¹Department of Cardiac Surgery, Lausanne University Hospital, Switzerland
²Department of Cardiology, Lausanne University Hospital, Switzerland
³Department of Anesthesia, Lausanne University Hospital, Switzerland
*Corresponding author: Aurelien Roumy, Department of Cardiovascular Surgery, CHUV BH 10- 984, rue du Bugnon 46, 1011 Lausanne, Switzerland
Received: December 17, 2020; Accepted: February 04, 2021; Published: February 11, 2021
Abstract
Background: Sutureless bioprosthesis aortic valves simplify surgery for aortic valve replacement (AVR) but some unexpected anatomical features of the recipients aortic annulus might preclude anchoring and lead to a paravalvular leak. Sub-Commissural Annuloplasty (SCAP) has been sporadically proposed to secure implantation under these circumstances. This study evaluated whether SCAP affects early postoperative outcomes and follow-up after sutureless Perceval-S implantation.
Methods: We included all elective patients who underwent AVR (isolated or combined with coronary bypass) with the Perceval-S valve from March 2016 to August 2019. SCAP was performed each time the surgeon deemed it useful to improve anchoring.
Results: One hundred and three patients were included. The mean age was 73.9±7.2 years and 36 (35%) were women. SCAP was performed in 34 (33%) patients, significantly more frequently in patients with large aortic annulus or bicuspid aortic valve. Perceval-S implantation was successful in 100 (97%) patients. Thirty-day mortality was 2% (n=2), of which one was related to the procedure. There was no significant difference in the incidence of postoperative conduction disorders between patients with and without SCAP (respectively, 3 [9%] vs 7 [10%], p=1.0). At one-year follow-up, no more than trivial paravalvular leak was noted in both groups, and peak and mean gradients were similar in patients with SCAP than in those without (19.1±8.3 vs 17.9±7.1 mmHg, p=0.53 and 10.7±5.0 vs 10.0±3.9 mmHg, p=0.59, respectively).
Conclusions: SCAP is a safe, simple and reproducible technique that might facilitate Perceval-S aortic valve implantation in specific situations.
Keywords: Valve replacement; Sutureless valve; Aortic valve; Annulus plasty
Introduction
To date, Aortic Valve Replacement (AVR) is the only curative therapy to treat aortic valve stenosis. Despite the emergence of the Transcatheter Aortic Valve Implantation (TAVI) procedure, surgical approach remains the gold standard, especially because it allows for the removal of the diseased valve and decalcification of the annulus, optimizing the annulus size and limiting paravalvular leak occurrence. Over the past decade, sutureless and rapid deployment aortic valves have emerged, offering an alternative to conventional AVR.
The Perceval-S aortic valve (LivaNova, London, UK) is a bioprosthesis based on bovine pericardial leaflets mounted into a flexible, self-expandable nitinol stent. This is the only sutureless valve available on the market and it presents several advantages. First, its implantation is rapid, simple and reproducible, which reduces aortic cross-clamping time [1-4]. Moreover, its collapsible design favors minimally invasive surgical approaches [5] and facilitates implantation in challenging situations such as redo operations or calcified aortic root [3,6,7]. It also provides lower transvalvular gradients than conventional stented bioprothesis [1,4,8]. Recent studies have shown that Perceval-S’ rate of adverse events (notably renal insufficiency and blood transfusion) are similar or lower to that of conventional bioprothesis [1-3], while mortality and paravalvular leak rates are lower than in TAVI [3,9-12].
Even if the Perceval-S seems to be attractive and has a broad spectrum of use and advantages, its design based on two anchoring sites, (the first at the annulus level and the second at the Sino-Tubular Junction (STJ) level) has some pitfalls. Consequently, Perceval-S is contraindicated in case of a ratio between the STJ and the aortic annulus greater than 1.3, aneurysmal dilation or dissection of the ascending aortic wall [13]. The shape of the annulus is also crucial. In the Bicuspid Aortic Valve (BAV), for example, an ovoid or scalloped annulus (with unequal sub-commissural height) may preclude the valve anchoring and lead to paravalvular leak or valve migration. In this Situation, Sub-Commissural Annuloplasty (SCAP) has been sporadically performed in order to circularize the annulus before implanting a Perceval-S [14,15]. In our surgical experience, we regularly challenge non-circular annulus and the aim of this study is to evaluate if SCAP can safely address this issue.
Patients and Methods
This is a monocentric retrospective study approved by the ethical committee (CER-VD) under the number 2017-00340. From March 2016 to August 2019, all the patients who benefited from Perceval-S valve implantation in our institution were considered. The choice to use a Perceval-S valve was left to the operating surgeon during surgery, depending on personal preference and the anatomical characteristics cited above. Exclusion criteria were patients <65 years old, anatomical features outside the manufacturer’s recommendations [13], Sievers type 0 BAV, emergencies and combined surgery other than AVR plus coronary artery bypass graft (CABG). Redo operation was not an exclusion criterion.
Perceval-S implantation technique
Procedures were mainly performed through median sternotomy or, alternatively, via an upper J ministernotomy. After starting Cardiopulmonary Bypass (CPB), a transverse aortotomy was made around 1.5 cm above the STJ. The aortic valve cups were excised and the annulus conscientiously decalcified to be sufficiently flexible while avoiding annulus lesion. The size of the Perceval-S valve was chosen according to the dedicated sizer and the manufacturer’s recommendations [13]. The Perceval-S valve was collapsed into the delivery system and positioned using three guiding sutures placed 1mm below the nadir of the aortic valve cusps. These guiding sutures were retrieved after valve expansion. The correct position of the valve inflow ring on the aortic annulus was visually checked. Dilatation with the dedicated balloon was then performed for 30 seconds at 4 atm and 37°C. The aortotomy was closed using a double Blalock running suture, and after deairing the heart, the aortic clamp was removed and the CPB weaned. Intraoperative Transesophagal Echocardiography (TEE) confirmed the good position and functioning of the valve, as well as the absence of paravalvular leak prior to CPB removal. If a more than trivial paravalvular leak was noticed, the surgeon could (i) reposition the same valve after re-collapsing (off-label maneuver), possibly completed by SCAP, or (ii) use a different size of Perceval-S prosthesis, or (iii) switch to a sutured bioprosthesis (considered as implantation failure).
Sub-Commissural Annuloplasty (SCAP)
SCAP consisted of one or two sub-commissural triangle plications (Figure 1), performed by X-stitches using braided Ethibon 2/0: the first one was usually placed under the commissure between the Left Coronary (LC) and Non-Coronary (NC) cusps, eventually completed by additional sutures under the commissure between the NC and the Right Coronary (RC) cusps or the LC and RC cusps. Thus, SCAP permits to reduce slightly the size of the aortic annulus, to circularize an elliptic annulus and/or to reshape the annulus in the horizontal plane by reducing discrepancies between sub-commissural triangle heights.
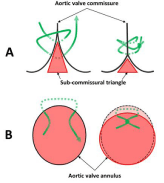
Figure 1: Aortic annulus remodeling by SCAP with a mattress suture 2-0
(green line).
A: Reduction of the height of the sub-commissural triangle.
B: circularization of an elliptic annulus. SCAP, sub-commissural annuloplasty.
End-points
Primary end-points were implantation success and 30-day mortality [16].
Secondary end-points were postoperative stroke, AV-block requiring a permanent pacemaker and paravalvular leak, according to recommendation from Akins et al., [16].
Perceval-S valve hemodynamics (mean and peak gradients) were evaluated via Transthoracic Echocardiography (TTE) at follow-up (14.6±11.2 months) by the cardiologists of the patients. We excluded hemodynamics of the patients in whom Perceval-S implantation failed.
Statistical analysis
Statistical analysis was performed using SPSS BASE 17.0 statistical software (SPSS Inc. Chicago, IL, USA). Categorical variables were expressed as percentages and compared using the Chi-square or Fisher’s exact test, as appropriate (n ≤5 per group). Continuous variables were expressed as mean±1 standard deviation and were compared using the Student’s t test. A two-tailed p-value less than 0.05 was taken to indicate statistical significance.
Results
Patients and operative data
During the observational period of the study, 115 consecutive patients underwent Perceval-S valve implantation in our institution and 103 were included in the present study. The 12 excluded patients (10%) were emergency situations (n=5, including four patients with endocarditis) and combined surgery other than AVR plus CABG (n=7, including three AVR plus mitral, two AVR plus tricuspid and three AVR plus mitral and tricuspid procedures). Preoperative data are reported in (Table 1). Fifty-seven patients (55%) underwent isolated AVR, of which 17 (30%) were performed via ministernotomy. The other 46 patients (45%) underwent combined surgery with CABG (mean anastomoses 1.9). Mean CPB times were 59.3±45.4 min and 89.2±43.5 msin, and aortic cross-clamp times were 39.4±24.2 min and 69.0±35.6 min in isolated AVR and combined procedures, respectively.
All
No-SCAP
SCAP
p value
N=103
N=69
N=34
Gender (M/F)
67/36 (35%)
40/29 (42%)
27/7 (21%)
0.047
Age
73.9±7.2
74.03±0.88
73.51±1.26
0.74
BMI
28.2±5.7
28.4±5.4
27.9±6.4
0.71
BSA
1.88±0.02
1.87±0.22
1.91±0.23
0.35
NYHA class
2.3±0.82
2.4±0.82
2.3±0.83
0.63
I-II
53 (52%)
34 (49%)
19 (56%)
0.54
III-IV
50 (49%)
35 (51%)
15 (44%)
0.54
Hypertension
82 (80%)
55 (80%)
27 (79%)
1
Diabetes
28 (27%)
20 (29%)
8 (24%)
0.64
Smoker
12 (12%)
9 (13%)
3 (9%)
0.75
COPD
16 (16%)
8 (12%)
8 (24%)
0.15
GFR <30ml/min.
5 (5%)
1 (1%)
4 (13%)
0.03
Logistic EuroSCORE II (%)
3.0 ± 2.67
2.9±2.8
3.1±2.5
0.6
<4
81 (79%)
56 (81%)
25 (74%)
0.45
4-8
16 (16%)
10 (15%)
6 (18%)
0.77
>8
6 (6%)
3 (4%)
3 (9%)
0.39
Redo AVR
5 (5%)
5 (7%)
0 (0%)
0.17
LVEF (%)
59.6±10.4
60.8±9.1
57.0±12.4
0.08
>50
83 (81%)
59 (86%)
24 (71%)
0.11
30-50
19 (18%)
10 (15%)
9 (27%)
0.18
<30
1 (1%)
0 (0%)
1 (2.9%)
0.33
Peak gradient (mmHg)
65.3±26.9
67.02±28.0
32.1±25.0
0.41
Mean gradient (mmHg)
39.0±17.0
39.6±17.4
37.8±16.2
0.61
EOA (cm2)
0.78±0.26
0.78±0.27
0.84±0.23
0.24
Biscupid aortic valve
16 (16%)
6 (9%)
10 (29%)
0.01
AVR: Aortic Valve Replacement; BMI: Body Mass Index; BSA: Body Surface Area; COPD: Chronic Obstructive Pulmonary Disease; EOA: Effective Orifice Area; GFR: Glomerular Filtration Rate; LVEF: Left Ventricle Ejection Fraction; NYHA: New-York Heart Association; SCAP: Sub-Commissural Annuloplasty.
Table 1: Patient preoperative demographics.
SCAP technique
In total, 34 patients (33%) required SCAP (Group SCAP); the other 69 patients (67%) benefited from a classical implantation technique (Group No-SCAP). In Group SCAP, a single SCAP was performed in 23 patients (68%) and a double SCAP in 11 patients (32%). Perceval-S sizes and SCAP techniques are reported in (Table 2).
All
No-SCAP
SCAP
p value
N=103
N=69
N=34
Perceval Size
Small
5 (5%)
4 (6%)
1 (3%)
1
Medium
19 (18%)
16 (23%)
3 (9%)
0.11
Large
31 (30%)
26 (38%)
5 (15%)
0.02
X-Large
48 (47%)
23 (33%)
25 (74%)
0.0001
SCAP technique
NC/LC
21 (21%)
-
21 (62%)
-
LC/RC
2 (2%)
-
2 (6%)
-
NC/LC+NC/RC
11 (11%)
-
11 (32%)
-
Native aortic valve
-
-
Tricuspid
87 (84%)
63(91%)
24 (71%)
0.01
Bicsupid
16 (16%)
6 (9%)
10 (29%)
0.01
AV: Aortic Valve; LC: Left Coronary; NC: Non Coronary; RC: Right Coronary; SCAP: Sub-Commissural Annuloplasty.
Table 2: Perceval-S valve size and SCAP technique.
Moreover, the rate of SCAP’s use differed according to the anatomy of the aortic valve, as SCAP was necessary in 24 patients (28%) with a tricuspid aortic valve, versus in 10 patients (63%) among those who had BAV (Figure 2).
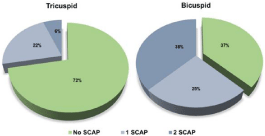
Figure 2: Proportion of the number of required SCAP according to the valve
type. SCAP, sub-commissural annuloplasty.
For patients who underwent in isolated AVR procedure, mean CPB times were 57.0±38.3 min and 64.6±59.9 min, and aortic crossclamp times were 39.7±26.4 min and 38.7±18.7 min in groups No- SCAP and SCAP, respectively (p=0.57 and p=0.90). This shows that SCAP did not significantly increase valve implantation or overall procedure times.
Implantation failure
One hundred patients (97%) benefited of a Perceval-S implantation, which is similar to the reported rate in the current literature17-19. In the three remaining cases, the Perceval-S valve had to be replaced by a sutured bioprosthesis (Trifecta St Jude Medical, MI, USA) in one, while a total aortic root replacement had to be performed using a Freestyle aortic root bioprosthesis (Medtronic, Dublin, Ireland) in the two other.
Clinical outcomes and post-operative hemodynamics
The 30-day mortality rate was 2% (n=2) for a EuroScore II predicted mortality of 2.95% (range 0.5-16.5 %). Both of deaths were in Group No-SCAP (2.9% of 69 patients) but only one was directly related to the procedure; the other one was related to the discovery of a multimetastatic lung cancer leading to an irreversible respiratory insufficiency and multi-organ failure.
There was no significant difference between the two groups regarding the different secondary end-points (Table 3). Only one patient (0.9% of overall) in Group SCAP (2.9% of 34 patients) experienced a stroke with a persistent neurological deficit (p=0.40). Ten patients (10%) presented a new onset of AV-block that required permanent pacemaker implantation, seven (10%) and three (9%) in Goup No-SCAP and Group SCAP, respectively (p=1.00). No residual paravalvular leak more than trivial was found at follow-up.
All
No-SCAP
SCAP
p value
N=103
N=69
N=34
30-days mortality
2 (2%)
2 (3%)
0 (0%)
New AF
20 (19%)
13 (19%)
7 (21%)
1
AV-block with PM
10 (10%)
7 (10%)
3 (9%)
1
Stroke
1 (0.9%)
0 (0%)
1 (6%)
0.4
AF: Atrial Fibrillation; AV-block: Atrio-Ventricular Block; SCAP: Sub-Commissural Annuloplasty.
Table 3: Postoperative clinical outcomes.
Hemodynamics of the Perceval-S at follow-up are shown in (Table 4). There was no significant difference between the two groups, but we noticed a trend towards lower peak and mean gradients in Group SCAP, which is consistent with a larger overall size of implanted valves.
All
No-SCAP
SCAP
p value
N=100
N=67
N=33
Paravalvular leak>trivial
0 (0%)
0 (0%)
0 (0%)
1
Peak Gradient (mmHg)
18.7±7.9
19.1±8.3
17.9±7.1
0.53
Mean Gradient (mmHg)
10.5±4.7
10.7±5.0
10.0±3.9
0.59
LVEF (%)
58.9±10.4
59.4±11.0
57.9±9.1
0.53
LVEF: Left Ventricle Ejection Fraction; SCAP: Sub-Commissural Annuloplasty.
Table 4: Perceval-S valve hemodynamics at follow-up (14.6±11.2 months).
Discussion
For a decade, sutureless and rapid deployment aortic valves have provided a compromise between standard AVR and TAVI by favoring minimal surgical approach, reducing time procedure and limiting paravalvular leak. The sutureless Perceval-S aortic valve was designed to be surgeon-friendly and to make its implantation safe and reproducible. Our results corroborate its safety as early mortality was low and rate of implantation success, stroke, pacemaker requirement and post-operative hemodynamics were similar to those reported in the current literature [18,20].
However, the sutureless feature of the Perceval-S is based on the specific design of an expandable nitinol stent which needs two anchoring sites: (i) the first one, at the annulus level (inflow ring), (ii) and the second one at the STJ level (outflow ring). These characteristics imply some procedural caution to avoid implantation pitfalls. In order to prevent valve migration, the ratio between the aortic annulus and STJ diameters must be <1.3, and the size of the aortic annulus must be <27mm (according that the Perceval-S sizes are: S = 19-21mm, M=21-23 mm, L=23-25 mm, XL=25-27 mm). Moreover, in order to minimize paravalvular leak occurrence, the aortic annulus must be decalcified enough (but avoiding annulus lesion) to be flexible enough to be conformed to the valve by the radial force of the Perceval-S stent. It is also important that annulus has a circular shape and that the heights of the sub-commissural triangles are neither too high nor too unequal. Furthermore, the height of the nitinol struts obligates to slightly modify the surgical technique by performing a horizontal and higher aortotomy than with sutured prosthetic valves, which could reduce the accessibility and the exposition of the diseased aortic valve.
Pre-operative imaging (cardiac-CT or echocardiography) is helpful to assess the shape and size of the annulus as well as to choose the best surgical approach [21]. That allows to estimate the likelihood of Perceval-S implantation success, but despite of this, some anatomic features of the aortic annulus (shape, flexibility, sub-commissural triangle height, calcification, etc…) are unpredictable before surgery and could hamper Perceval-S implantation. In such situations, the surgeon should typically substitute a sutured valve, which is more time consuming and may be challenging, especially through a minimally invasive approach [1-4].
In order to address unexpected anatomical features of the annulus, some techniques of annuloplasty have been described sporadically to decrease the risk of implantation failure. In a short series, Ferrari et al., described how they sneaked a purse string suture of 3-0 polypropylene all around the aortic annulus before implanting a rapid-deployment aortic valve (Intuity, Edwards Lifesciences, Irvine, CAL, USA). Before closing the aorta, the suture was snared to tighten the implanted valve and minimize both paravalvular leak and valve migration [22]. In BAV, Glauber et al., suggested to reduce the height of the three sub-commissural triangles by a mattress suture before implanting a Perceval-S valve, if the annulus was overly scalloped [15]. In his series of 13 patients with BAV, Durdu et al., described a similar SCAP technique to ours, used in order to decrease the height of the sub-commissural triangles and to circularize the annulus [14].
The present study highlights three situations in which SCAP was particularly helpful: (i) in case of aortic annuli that were slightly too large or too flexible (ii) in case of elliptic or overly scalloped aortic annuli (frequent in BAV), (iii) and as a rescue trick in case of implantation failure. We used 34 SCAPs successfully spread over these three situations, which permitted to widen the field of use of the Perceval-S. For example, among the 25 XL-sized valves implanted (including nine BAV), 14 SCAPs were performed, otherwise we would not have succeeded to implant it. This may partially explain why we reported a remarkable proportion of XL-sized valves compared to the current literature [20,23]. SCAP also afforded an issue to address implantation failure in almost one third of patients involved. We did not notice significant reduction of the effective orifice area surface, nor an increase of severe mismatch rates in the Group SCAP. Thus, we can infer that SCAP did not significantly reduce the annulus size but rather that it improved its congruence with the valve.
Our study presents several limitations. First, this is a monocentric retrospective study not in intention to treat, as the surgeon decided to implant a Perceval-S according to his feeling. Moreover, we did not randomize patients with a group control, in which we would use a sutured valve instead of SCAP if needed. Finally, our follow-up was too short to evaluate the long-term stability of SCAP, especially in BAVs, which are known to dilate by time.
Conclusion
This study shows that SCAP is a safe and useful technique that could improve the success rate of implantation of the Perceval-S sutureless aortic valve essentially by circularizing the aortic annulus and reducing the height of sub-commissural triangles. It might be particularly useful in presence of a BAV or in case of a first implantation failure, as rescue trick. Larger studies and longer followup periods are necessary.
Author Contributions
AR and MK designed the study and operated patients. AR wrote the paper. AR, MV, MZG, PM, and VR collected data. MK performed statistical analysis. All authors critically revised and accepted the final version.
References
- Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S, et al. Sutureless Perceval Aortic Valve Versus Conventional Stented Bioprostheses: Meta- Analysis of Postoperative and Midterm Results in Isolated Aortic Valve Replacement. J Am Heart Assoc. 2018; 7: e006091.
- Mujtaba SS, Ledingham SM, Shah AR, Pillay T, Schueler S, Clark S. Aortic Valve Replacement with a Conventional Stented Bioprosthesis versus Sutureless Bioprosthesis: a Study of 763 Patients. Braz J Cardiovasc Surg. 2018; 33: 122-128.
- Powell R, Pelletier MP, Chu MWA, Bouchard D, Melvin KN, Adams C. The Perceval Sutureless Aortic Valve: Review of Outcomes, Complications, and Future Direction. Innov Phila Pa. 2017; 12: 155-173.
- Bilkhu R, Borger MA, Briffa NP, Jahangiri M. Sutureless aortic valve prostheses. Heart Br Card Soc. 2019; 105: s16-s20.
- Miceli A, Santarpino G, Pfeiffer S, Murzi M, Gilmanov D, Concistre G, et al. Minimally invasive aortic valve replacement with Perceval S sutureless valve: early outcomes and one-year survival from two European centers. J Thorac Cardiovasc Surg. 2014; 148: 2838-2843.
- Baikoussis NG, Dedeilias P, Prappa E, Argiriou M. The perceval S aortic valve implantation in patients with porcelain aorta; is this ideal option? Ann Card Anaesth. 2017; 20: S70-S72.
- Gersak B, Fischlein T, Folliguet TA, Meuris B, Teoh KH, Moten SC, et al. Sutureless, rapid deployment valves and stented bioprosthesis in aortic valve replacement: recommendations of an International Expert Consensus Panel. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2016; 49: 709-718.
- Belluschi I, Moriggia S, Giacomini A, Del Forno B, Di Sanzo S, Blasio A, et al. Can Perceval sutureless valve reduce the rate of patient-prosthesis mismatch?. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2017; 51 1093-1099.
- Repossini A, Fischlein T, Solinas M, DI Bacco L, Passaretti B, Grubitzsch H, et al. Stentless sutureless and transcatheter valves: a comparison of the hemodynamic performance of different prostheses concept. Minerva Cardioangiol. 2018; 66: 180-190.
- Santarpino G, Vogt F, Pfeiffer S, Dell’Aquila AM, Jessl J, Cuomo F, et al. Sutureless versus Transfemoral Transcatheter Aortic Valve Implant: A Propensity Score Matching Study. J Heart Valve Dis. 2017; 26: 255-261.
- Miceli A, Gilmanov D, Murzi M, Marchi F, Ferrarini M, Cerillo AG, et al. Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio- Thorac Surg. 2016; 49: 960-965.
- Lloyd D, Luc JGY, Indja BE, Leung V, Wang N, Phan K. Transcatheter, sutureless and conventional aortic-valve replacement: a network metaanalysis of 16,432 patients. J Thorac Dis. 2019; 11: 188-199.
- Perceval Sutureless Aortic Heart Valve Instructions for Use. 2019.
- Durdu MS, Gumus F, Ozcinar E, Cakici M, Bermede O, Dincer I, et al. Sutureless Valve Replacement Through a Right Anterior Mini-thoracotomy in Elderly Patients With Stenotic Bicuspid Aortic Valve. Semin Thorac Cardiovasc Surg. 2019; 31: 458-464.
- Glauber M, Ferrarini M, Lio A, Miceli A. Dealing with a stenotic bicuspid aortic valve: Is this still an off-label procedure for a sutureless valve? J Thorac Cardiovasc Surg. 2015; 150: 858-859.
- Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg. 2008; 85: 1490-1495.
- Laborde F, Fischlein T, Hakim-Meibodi K, Misfeld M, Carrel T, Zembala M, et al. Clinical and haemodynamic outcomes in 658 patients receiving the Perceval sutureless aortic valve: early results from a prospective European multicentre study (the Cavalier Trial)†. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2016; 49: 978-986.
- Shrestha M, Fischlein T, Meuris B, Flameng W, Carrel T, Madonna F, et al. European multicentre experience with the sutureless Perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2016; 49: 234-241.
- Santarpino G, Berretta P, Fischlein T, Carrel TP, Teoh K, Misfeld M, et al. Operative outcome of patients at low, intermediate, high and “very high” surgical risk undergoing isolated aortic valve replacement with sutureless and rapid deployment prostheses: results of the SURD-IR registry. Eur J Cardio- Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2019; 56: 38-43.
- Sian K, Li S, Selvakumar D, Mejia R. Early results of the Sorin® Perceval S sutureless valve: systematic review and meta-analysis. J Thorac Dis. 2017; 9: 711-724.
- Margaryan R, Kallushi E, Gilmanov D, Micelli A, Murzi M, Solinas M, et al. Sutureless Aortic Valve Prosthesis Sizing: Estimation and Prediction Using Multidetector-Row Computed Tomography. Innov Phila Pa. 2015; 10: 230- 235.
- Ferrari E, Siniscalchi G, Tozzi P, von Segesser L. Aortic Annulus Stabilization Technique for Rapid Deployment Aortic Valve Replacement. Innov Phila Pa. 2015; 10: 360-362.
- Concistre G, Chiaramonti F, Bianchi G, Cerillo A, Murzi M, Margaryan R, et al. Aortic Valve Replacement With Perceval Bioprosthesis: Single-Center Experience With 617 Implants. Ann Thorac Surg. 2018; 105: 40-46.