
Review Article
Austin J Surg. 2021; 8(2): 1267.
Systematic Review and Meta-Analysis of the Impact of Intra-Operative Ultrasound Guidance Breast-Conserving Surgery in Early Breast Cancer
Tin SMM1, Kurup V2, Cheema I1, Viswanath YKS3* and Razdan S3
¹Department of Surgery, South Tees University Hospitals NHS Foundation Trust, United Kingdom
²Department of Surgery, North Tees University Hospitals NHS Foundation trust, United Kingdom
³Department of Surgery, James Cook University Hospital, South Tees University Hospitals NHS Foundation Trust, United Kingdom
*Corresponding author: Viswanath YKS, Department of Surgery, James Cook University Hospital, South Tees University Hospitals NHS Foundation Trust, Middlesbrough, TS22 5QE, United Kingdom
Received: March 17, 2021; Accepted: April 03, 2021; Published: April 10, 2021
Abstract
Systematic review and meta-analysis of the impact of intra-operative ultrasound guided breast-conserving surgery in early breast cancer.
Background: Breast Conservation (BCS) is the standard surgical procedure for early breast cancer. It is challenging for surgeons to achieve adequate excision of the lesion with clear margins and acceptable cosmesis. A continuous Intra-Operative Ultrasound (IOUS) is used during BCS in volume precision surgery. We reviewed its effectiveness to obtain clear margins, low excision volume and better cosmetic outcome during BCS.
Methods: We searched three bibliographic databases (MEDLINE, CINAHL, Cochrane Library online) for relevant published and unpublished literature from their inception until December 2019. The randomized controlled trials of the impact of IOUS on excision volume, margin status and cosmetic outcome was assessed, and meta-analysis carried out for margin status with narrative summary was done for other results.
Results: This study included four articles in the systematic review. A total of 207 patients with IOUS and 192 patients with Palpation Guided (PGS) BCS was studied in this review. The standardised mean difference of excision volume for 2 trials was -0.31 (-0.62, -0.00) and -0.50 (-0.85, -0.16) with p-value of 0.048 and 0.004. There was no significant volume difference in the remaining two studies. The positive margin rate reduced significantly with IOUS guidance with the pooled OR was 0.19 (95% CI: 0.09, 0.41) with no heterogeneity among studies (p=0.72, I2= 0%). The overall cosmetic outcome favoured satisfaction in both ultrasound-guided and palpation guided BCS groups without significant difference.
Conclusion: This study suggests that the use of IOUS provides a statistically significant, less positive margin without a considerable difference in excisional volume. Overall, satisfaction exceeds dissatisfaction with ultrasound-guided Breast-conserving surgery. However, there is insufficient evidence to support the better cosmetic outcome in the IOUS group.
Keywords: Breast cancer; Breast-conserving surgery; Image guidance; Satisfaction; Ultrasound
Abbreviations
BCS: Breast Conserving Surgery; IOUS: Intra-Operative Ultrasound
Introduction
Breast cancer is the most common cancer in the UK, with approximately 54,000 new cases diagnosed each year [1]. Overall, survival has improved steadily over time due to increasing awareness, early diagnosis, advances in adjuvant treatment and widespread use of the NHS breast screening program. Early-stage (79-87 % at Stage 1-2) breast cancer patients are diagnosed than a late stage (13-21% at stage 3 or 4) [1].
Breast-Conserving Therapy (BCT) is an established standard of care for women with early breast cancer. The patient’s overall survival with BCT is comparable to those treated with mastectomy [2]. This study concluded as BCS had an improved overall survival rate compared to mastectomy in N0-N1 tumour but no significant difference in N2-3 patients. Overall survival was better with BCT (HR=1.42, 95% CI 1.16-1.74) in the young age group. The large retrospective cohort study of 5335 patients reported 3, 5, and 10- year overall survival was 96.5% vs 93.4%, 92.9% vs 88.3% and 80.9% vs 67.2%, respectively [3]. The meta-analysis of Breast-conserving surgery and mastectomy for locally advanced breast cancer reported no significant difference in local and regional recurrence rate (OR=0.83, 0.60, 1.15, p=0.26) and higher disease-free survival rate (OR=2.35, 1.84, 3.01, p=<0.01) in Breast-conserving surgery [4].
Breast-Conserving Surgery (BCS) aims to complete tumour excision with clear margins while maintaining a reasonable cosmetic outcome [5]. To fulfil this principle, various techniques have been used to localize the tumour, such as wire localization, seed localization, MarginProbe, ultrasound and specimen X ray, intra-operative MRI margin, and margin assessment 3D specimen evaluation, Clear-Edge imaging and I Knife. The wire or seed localization and specimen X-ray is the gold standard for nonpalpable breast cancer. For palpable breast cancer, surgeons typically rely on pre-operative imaging and their skills and experience. This technique may be insufficient in differentiating tumour from surrounding tissue, especially in highly glandular breasts. It can lead to a high involved margin rate. Reexcision of the positive margin will affect the patient’s emotional status, increase wound infection rate, delay adjuvant treatment, and increase scar formation [6,7].
Consequently, it may necessitate further cosmetic procedure and incur supplementary healthcare cost. Use of Intra-operative ultrasound aids to estimate the size of the tumour more accurately and improve the negative margin rate with a better cosmetic outcome [8,9]. It is a cost-effective technology compared to re-excision and other real-time imaging.
The rate of the negative margin of breast cancer was significantly higher with the IOUS group than PGS (OR= 2.75, 95% CI: 1.66-4.55, p= 0.193) for both palpable and nonpalpable breast cancer [10]. The localisation accuracy was 100%, and the negative margin rate was 91.01% with continuous intra-operative ultrasound monitoring by the surgeon [9]. The relative risk for re-excision in the ultrasound group due to positive margin was 0.82 (95% CI: 0.23, 2.93), and clear margins were achieved in 88% (IOUS) and 86% (PGS) (p=0.91) for both palpable and nonpalpable breast cancer [11]. Houssami and colleagues performed a study-level meta-analysis that included 33 eligible studies and over 28,000 women with early-stage breast cancer. A positive margin was associated with increased Local Recurrence (LR) (OR= 2.44; 95% CI 1.97-3.03; p<0.001), even after adjusting for the use of a radiation boost or adjuvant endocrine therapy [12]. Notably, there was no evidence of a decreased LR risk with increasing negative margin widths from 1mm to 2mm to 5mm (p=0.90). Analysis of 10-years data from the Breast cancer quality assurance project showed an involved margin or margin of 1mm increased risk of Locoregional Recurrence (LRR) (HR 3.24,95% CI 1.46-7.17, p=0.004) whilst margin 2mm or greater had no effect on LRR [13]. These data confirm that even with modern multimodality treatment, a negative margin reduces the risk of LR; however, increasing the size of a negative margin is not significantly associated with improvement in local control.
The cosmetic outcome depends on the volume of tissue excision and secondary treatment with radiotherapy. Poor cosmetic outcome was observed in up to 30% of patients after BCS [14]. In an extensive survey among 963 women treated with BCS for breast cancer, cosmetic results were scored as 3.4 on a 5-point scale with from 1 (very dissatisfied) to 5 (very satisfied) [15]. COBALT trial reported IOUS resulted in improvement of cosmetic outcomes within one year follow up point. Poor cosmetic outcome rated at the end of follow up was 11% for USS and 21% for PGS [9,16].
This study will investigate the margin involvement, excision volume of breast tissue and cosmetic outcome and patient satisfaction in a breast cancer patient who were treated with Intra-operative ultrasound guided Breast conserving surgery.
Methods
Search strategy
After the scoping searches, three bibliographic databases (MEDLINE, CINAHL, Cochrane Library online) were searched for relevant published and unpublished literature from their inception until December 2019. In order to reduce the bias, we tabulated searches without search filters or procedure specific keywords that would limit results to specific study design or diagnostic groups [17]. To fulfil the criteria for structured literature search, Boolean logic was used. In addition to searching bibliographic databases, hand searching, and citation chaining were performed. This search was performed independently by 2 reviewers to increase the validity of the results. Duplicating the study selection process reduces both the risk of making mistakes and the possibility that selection is influenced by a single person’s biases [18]. Region was not limited but language was limited (English). This study emphasized effect of ultrasound guided BCS on excision volume, margin status and cosmetic outcome compared with conventional palpation guided BCS.
Inclusion and Exclusion criteria
Two reviewers independently screened all the titles and abstracts. The full-text papers of relevant abstract were obtained and assessed according to inclusion criteria. The inclusion criteria were as follow: the patients with early palpable breast cancer (T1-T2, N0-N1), who were treated with Intra-Operative Ultrasound Guided BCS (IOUS) or Palpation Guided BCS (PGS) to evaluate the excisional volume, margin status and cosmetic outcome. The randomized controlled trials were included in the study.
This review excluded the papers which assessed the nonpalpable breast cancer or locally advanced breast cancer, other image- guided localization techniques and procedures other than BCS. The studies investigating cavity shaving or oncoplastic breast surgery were also excluded.
Quality assessment and data extraction
Each paper’s quality was assessed with quality assessment tools for randomized controlled trial. The Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) and Centre for Research Dissemination (CRD) check list were used for individual paper. The information was careful extracted from individual studies independently. Thereafter, the data extraction was redone at different time and both datasets were cross-checked to ensure accuracy and completeness. The following variables were extracted from each study: first named author, year of publication, study design, type of breast cancer, number of cases and control, number of patients with positive margins, specimen volume and cosmetic outcomes and sponsorship.
Statistical analysis
After extracting the relevant data according to study inclusion criteria, the Cochrane Collaboration review Manager (Rev Man 5) statistical software were used for data analysis. Continuous data (Excision volume) was presented separately with mean, standard deviation, mean difference and p value to interpret the final outcome. The Standardized mean difference was used to assess the excised volume between two groups. It estimates the amount by which the experimental intervention (IOUS) changes the outcome on average compared with the control (PGS).
For margin positivity, the Odd Ratio (OR) and Risk difference with its variance and 95% Confidence Interval (CI) were estimated to assess the association between two techniques. The heterogenicity was evaluated by I2 test. I2 value was 0% for positive margin, which represents no heterogeneity among these studies. Therefore, fixedeffect model (The Mantel-Haenszed Method) was used to calculate the pooled OR. Forest plots were used to present the outcomes of meta-analysis. Publication bias was investigated by Funnel plot.
The cosmetic outcome (patient satisfaction) was estimated with OR of having a worse cosmetic outcome based on the propotional odds model for ordinal responses. The patient and assessor satisfaction were presented with percentage.
Results
After scope electronic and hand search, 84 citations were identified. Their titles were assessed for the relevance to the review and duplication were removed, resulting in 24 potential citations being retained. The abstracts were reviewed for these studies and 7 were selected for full article reviews according to inclusion and exclusion criteria of the study. One article was rejected after full text review because of study protocol. Two relevant poster presentations of RCT were identified and requested to authors for full text paper. These two papers were not accessible. Hence, these poster presentations with insufficient information were rejected from the review. Therefore, 4 articles were included in the systematic review. Identification of included studies is shown (Figure 1). Overall, 207 patients in IOUS and 192 patients in PGS were included in the study.
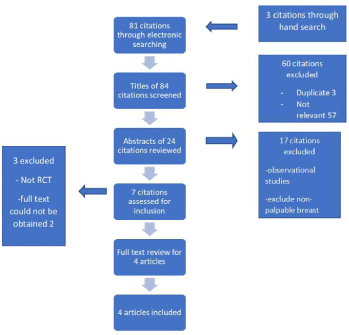
Figure 1: Identification of included studies in a systematic review. Articles
were selected according to inclusion and exclusion criteria. Full publication of
Randomised controlled trial was selected for review.
This study will investigate the three outcomes including excised volume, margin involvement and cosmetic outcome after Breast conserving surgery between intra-operative ultrasound guided and conventional palpation method.
Effect of IOUS on excised volume
The detail of eligibility of studies about the effect of IOUS on excised volume of breast tissue in Breast conserving surgery is shown in Table 1. All the eligible studies focused on the investigation of amount of tissue excised when IOUS was used during surgery, compared with surgery without IOUS. 2 out of 4 studies [16,19] presented the excisional volume with means (SD) but Vispute et al. [20] presented with median, mean and range and Moore et al [21] presented with range. Therefore, standardized mean difference could not be calculated from the result and unable to combine the study for meta-analysis. The remaining 2 studies also had high heterogeneity for combined study (Chi2=9.77, p= 0.008, I2= 80%).
Study
Outcome (IOUS)(excision volume)
Outcome (PGS)
Summary of findings
Std Mean difference
IV, Fixed, 95% CIP values
Moore [20]
104+/-8 cm3
114+/-5.6 cm3
Not estimable
Karanilk [18]
89.9 (53.9) cm3
108.1 (63.4) cm3
-0.31 (-0.62, -0.00)
0.048
Mean (SD)
Mean (SD)
Volder [19]
38 (26) cc
53 (33) cc
-0.50 (-0.85, -0.16)
0.004
Mean (SD)
Mean (SD)
Vispute [21]
92.3cm3
80.6cm3
Not estimable
0.101
Mean
Mean
This table showed excision volume of intra-operative ultrasound guidance and palpation guidance Breast conserving surgery. Each study's outcome was presented with mean (standard deviation), range and standard mean difference, and p-value.
SD: Standard Deviation; IV: Inverse Variance; Fixed: Fixed Effect Meta-Analysis: 95% CI=95% Confidence Interval
Table 1: The study results table for Excised volume.
There was statistically significant in less volume excision in two studies [18,19] (p=0.048 and p=0.004). The mean excisional volume was 89.9 cm³ (53.9) vs 108 cm³ (63.4) and 38 cc (26) 53 cc (33) respectively. This was confirmed by Standardised Mean Difference (SMD -0.31, 95% CI: -0.62, -0.00 and -0.50, -0.85, -0.16).The SMD less than zero favoured experiment group (IOUS) and above zero favoured control group (PGS).There was no significant volume difference in remaining two studies [20,21], (104 +/- 8 vs 114 +/- 5.6 cm³ vs 92.3 (6-275 cm³) vs 80.6 (43-350 cm³). All the studies showed that volume excised by guidance of ultrasound was less than conventional palpation guided excision except in one study. Vispute et al. [20] reported no statistically significant difference between IOUS and PGS (p=0.101). The author reflected this finding as a possibly contributed by tumour size of participants (average 3.18 cm, 2-5 cm for IOUS, 1.5-5 cm for PGS) being larger in ultrasound guided BCS group.
Effect of IOUS on margin status
The study result Table 2 shows the detailed eligibility studies of the effect of IOUS on margin status. There was a total of 299 patients included in the study. A total of 46 patients was reported as positive margin involvement. There was a higher rate of positive margin in the conventional group (37/192) than in the IOUS group (9/207).
Study
Outcomes (Positive margin)
IOUS
Number (percentage)Outcome (Positive margin)
PGS
Number (percentage)Summary of finding
OR (95% CI)
RR (95% CI)
(MH, Fixed, 95% CI)Moore [20]
1 (3.5%)
7 (29%)
0.09 (0.10, 0.87)
0.13 (0.02,0.96)
Karanilk [18]
5 (6%)
14 (17%)
0.30 (0.10,0.87)
0.34 (0.13,0.90)
Volder [19]
2 (3%)
12 (17%)
0.13 (0.03,0.60)
0.16 (.04,.67)
Vispute [21]
1 (3.22%)
4 (14.28%)
0.19 (0.02,1.85)
0.22 (0.03,1.84)
This table showed the number (Percentage) of patients with a positive margin after Breast-conserving surgery guided by ultrasound and palpation. Summarized the finding with Odd Ratio (OR) and Relative Risk Ration (RR). OR: Odd Ratio; RR: Relative Risk; MH: Mantel-Haenszel Odds Ratio for Dichotomous Data; Fixed: Fixed effect Meta-Analysis for Generic Inverse Variance Outcome; 95% CI: 95% Confidence Interval
Table 2: The Study Result table for Margin status.
The Forrest plot in Figure 2 shows an advantage for the experimental group (IUOS) over control (PGS). The point estimates are all to the vertical axis left, indicating that all studies favoured the experimental group. The diamond representing the pooled effect is also to the left of the axis. This effect can be seen in the odds ratios reported on the left, all less than 1. The result in fixed-effect model (OR=0.19, 95% CI: 0.09, 0.41, p=<0.0001) indicated less positive margin rate in IOUS group (Figure 2). The odds ratio of 0.19 means an 81 per cent reduction in odds for patients in the experimental group (IOUS). The 95 per cent confidence intervals all overlap, suggesting that the degree of heterogeneity is small. This is proved by Chi2= 1.32, p=0.72 and I2 =0%, which shows no heterogeneity between studies.
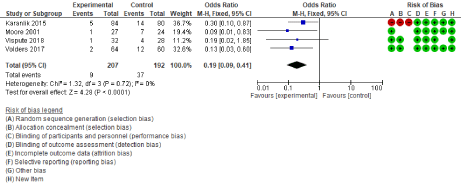
Figure 2: Effect of IOUS on positive margin rate (Pooled OR). Comparison of positive margin in intra-operative ultrasound and palpation guided Breast-conserving
surgery. The value represents OR (95% CI), and the diamond representing the pooled effect is to the left of the axis, indicated a reduction in positive margin rate
in IOUS. I2= Heterogeneity, CI= Confidence Interval.
The overall risk difference indicated that the risk of positive margin was lower in the IOUS group than in the conventional group (Risk difference= -0.15, 95% CI: -0.21, -0.02, p=<0.0001). The risk of a positive margin in the IOUS group is 15% lower than the conventional PGS group. All 95% confidence interval overlap and Chi2 test confirmed that there is no heterogeneity among these studies (p=0.58, I2= 0%) (Figure 3).
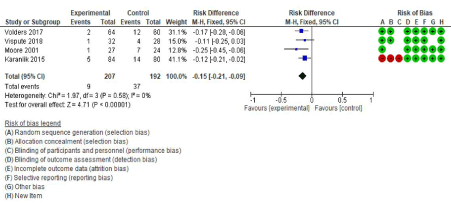
Figure 3: Effect of IOUS on positive margin rate (Risk Difference). Comparison of positive margin in intra-operative ultrasound and palpation guided Breastconserving
surgery. The value represents RD (95% CI), and the diamond representing the pooled effect is to the left of the axis indicated a reduction in positive
margin rate in IOUS. The risk of bias is reported on the right of the Forrest plot. I2= Heterogeneity, CI= Confidence Interval.
The asymmetry of the funnel plot Figure 4 shows bias among the studies. A small number of studies and their sample variation may lead to an association between intervention effect and standard error. ‘Tests for funnel plot asymmetry should not be used when there are fewer than ten studies in the meta-analysis because test power is usually too low to distinguish chance from fundamental asymmetry [22]. Therefore, a further evaluation of asymmetry was not performed.
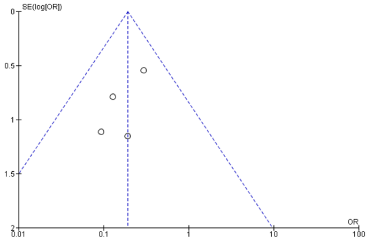
Figure 4: Funnel plot of the studies reported a positive margin in Breastconserving
surgery; investigating for bias among studies.
Effect of IOUS on cosmetic outcome
Table 3 showed the summary results of all the studies for the cosmetic outcome. Although all the studies investigated satisfaction and cosmetic outcome by assessor and patient, the result was reported at different time points. Therefore, combining the data has not been appropriate for a meta-analysis. The overall cosmetic outcome was statistically significant in Volders et al. [16] study (OR=0.53, 95% CI: 0.28, 0.99, p=0.048) when a comparison made between the two methods. This study showed the odds of the worse cosmetic outcome were no difference after one year (OR= 1, 95% CI: 0.74, 1.36, p= 0.988). The remaining three studies, the p-value of >0.05, suggested no significant difference in satisfaction between IOUS and PGS. At two weeks, the cosmetic outcome reported not statistically significant between IOUS and PGS (p=0.90) [21]. At 3 months, COBALT study reported statistically significant better outcome with IOUS (OR=0.39, 95% CI: 0.29, 0.54, p=<0.001) [22]. But Vispute et al. [20] reported no difference in outcome (p=0.83). The assessors and patients’ agreement were assessed by Kappa (0.3252), which showed strong agreement between the two. At 6 months, one study reported statistically significant better cosmetic outcome with IOUS (OR=0.35, 95% CI: 0.35, 0.65, p=<0.001) [21]. Karanilk et al. [19] reported good/ excellent outcome by assessor as 94% vs 92%. However, there was no statistically significant difference (p=0.54) between IOUS and PGS at six months. Patients were not involved in outcome analysis. In principle, the cosmetic outcome is directly associated with the excisional volume and timing of assessment. In these studies [20,21], there was no significant difference in cosmetic outcome due to an insignificant difference in volume excision between IOUS and PGS. Early evaluation of patient satisfaction does not reflect the long-term cosmetic outcome [5]. The cosmetic outcome evaluation at a two-week post-operative period cannot be generalized for the breast cancer population undergoing breast-conserving surgery. The patients’ satisfaction was not performed in Karanilk et al. study [19], while the cosmetic outcome was not assessed by professionals in Moore et al. study [21]. There were different scoring systems for each study showed in Table 4. Table 5 showed overall satisfaction by assessor was higher with an ultrasound-guided procedure in all studies. The Cosmetic appearance had no statistically difference at three year after exclusion of patients who underwent mastectomy (OR=1.1, 95% CI 0.65, 1.94, p= 0.67) [16].
Study
Outcomes
Time to event
Post opSatisfaction
IOUS
N (%)Satisfaction
PGS
N (%)Summary of finding
OR (95% CI)P value
Moore [20]
2 weeks
25 (92.6%)
22 (91.7%)
0.9
Karanilk [18]
6 months
67 (94%)
55 (92%)
0.54
Volder [19]
36 months
-74%
-65%
0.53 (0.28-0.99)
0.048
6 months
0.48 (0.35-0.65)
<0.001
3 months
0.39 (0.29-0.54)
<0.001
Vispute [21]
30 (85.6%)
24 (86.6%)
0.83
Comparison of overall satisfaction of cosmetic outcome between IOUS and palpation guided BCS groups. The significance of statistical difference showed with p-value.
N: Number of patients; %: Percentage of Patients; OR: Odd Ratio; 95% CI: 95% Confidence Interval
Table 3: The study Result table for the cosmetic outcome.
Study
Patients satisfaction
Assessor
Method for Patient’s satisfaction
Method for cosmetic outcome
Yes/no
No of assessor
Moore [20]
yes
No
-
Questionnaire Scale 1-5
-
Karanilk [18]
no
yes
2 (Blinded)
-
Harvard Cosmetic scales
Poor/fair/good/excellent
Volders [19]
yes
yes
3 (Blinded)
Likert score
Likert score
BCCT. core
(very satisfied, satisfied, dissatisfied, very dissatisfied)
Poor/fair/good/excellent
Vispute [21]
yes
yes
2 (not Blinded)
Visual Analog scale
Fair/good/ excellent
(very satisfied, satisfied, dissatisfied, very dissatisfied)
This table showed cosmetic assessment methods by patients and assessors for each study.
Table 4: The methods of cosmetic assessment.
Study Name
Study Name
Study Name
Cosmetic outcome
Vispute (2018) at 3 months
Volders (2017) at 3 years
Karanlik (2015) at 6 months
IOUS
PGS
IOUS
PGS
IOUS
PGS
Excellent
43.80%
30.80%
38%
42%
94%
92%
Good
43.80%
55.80%
24%
12%
Fair
12.50%
13.50%
27%
25%
Poor
NA
NA
11%
21%
Comparison of percentage of satisfaction of cosmetic outcome accessed by assessors between IOUS and palpation guided BCS.
Table 5: Cosmetic outcome by an assessor.
There was 12%, 51%, 47% and 13% reduction in odds for worse cosmetic outcome in patients from IOUS group [16,20,21] (Table 6). There was no statistically significant difference in patients’ satisfaction between 2 groups at different time points at all studies. Patients had more satisfaction with the cosmetic outcome than dissatisfaction in both IOUS and PGS group. Volders et al. reported that the dissatisfaction rate was higher in patients who needed further procedure for the involved margin (p=<0.001). Comparison of patients and tumour characteristics between IOUS and PGS showed no significant difference (Table 7).
Study Name
Study Name
Study Name
Study Name
Cosmetic outcome
Vispute (2018) at 3 months
Volders (2017) at 12 months
Volders (2017) at 3 years
Moore (2011) at 2 weeks
IOUS
PGS
IOUS
PGS
IOUS
PGS
IOUS
PGS
Very satisfied
38%
46.90%
43%
26%
45%
38.50%
92.60%
91.70%
Satisfied
24%
46.90%
47%
54%
29%
53.80%
Dissatisfied
27%
6.30%
8%
7%
23%
7.70%
Very dissatisfied
11%
-
3%
13%
3%
-
Overall OR (worse outcome)
0.87 (0.11,6.59)
0.49 (0.22,1.10)
0.53 (0.28,0.99)
0.88 (0.17,2.56)
Comparison of percentage of patient's satisfaction with cosmetic outcome between studies. Each study reported the worse outcome with an overall Odds Ratio (OR) with a 95% confidence interval.
Table 6: Cosmetic outcome by Patients.
Study
Age
BMI
Stage of tumour
Type of tumour
Moore [20]
Not specified
Not specified
Not specified
Not specified
Karanilk [18]
0.25
0.8
0.26
0.62
Volders [19]
0.124
0.685
0.523
0.42
Vispute [21]
-
-
Not significant
-
Age, BMI, stage of tumour and type of tumour for each study was compared, and statistically significant was demonstrated with a p-value.
Table 7: Overall patient characteristics, tumour characteristic and outcome.
Discussion
Breast-conserving surgery is the preferred first treatment for early breast cancer to remove the tumour with negative margins altogether while achieving a reasonable cosmetic outcome [5,6]. Wire localization breast-conserving surgery is a widely accepted procedure for impalpable breast cancer. For palpable breast cancer, surgeons rely on pre-operative imaging and their surgical skill and experience. During either of these procedures, obtaining a negative margin while excising the small volume of breast tissue is a challenge for surgeons as they strive to achieve good oncological and cosmetic outcome at the same time. The removal of a large volume of breast tissue can result in poor cosmetic outcome [5,6]. Histologically involved margins at the first operation remain a significant issue with no pre-operative assessment tool being available to help minimize this risk. There is no standardized technique for assessment of the margin status intraoperatively for palpable breast cancer. The most effective method to minimize the positive margin rate during first operation has not been agreed upon.
Direct visualization of the tumour with intra-operative ultrasound can help surgeons obtain a clear surgical margin [9]. Ultrasound is readily available and less expensive when compared with other modern imaging technologies. The ultrasound technology has advanced in recent years. This study used systematic review methodology to investigate and compare the effect on excision volume, margin involvement and cosmetic outcome in intra-operative ultrasoundguided Breast-conserving surgery and conventional palpation guided Breast-conserving surgery in patients with early palpable invasive breast cancer.
The rate of a negative margin of breast cancer was significantly higher with the IOUS group than PGS (OR= 2.75, 95% CI: 1.66, 4.55, p= 0.193) for both palpable and nonpalpable breast cancer [10]. The re-excision rate was lower with ultrasound-guided surgery (6%, 4.8%) when compared with palpation guided surgery (17%, 15.2%) [19]. Our systematic review and meta-analysis demonstrated that the positive margin rate is significantly less in intra-operative ultrasound guidance Breast-conserving surgery.
A positive margin, defined as ink on the tumour, is associated with a significant increase in Local Recurrence (LR) risk and warrants consideration of further surgery. The adjusted re-operation rate among 156 NHS Trusts for BCS was 12.2% and 30.2% [23]. Patients undergoing re-excision for closed or involved margins have only a 30% incidence of residual cancer at re-excised tissue [24].
This study reviewed the impact of intra-operative ultrasound on excisional volume. Overall excision volume is less in the IOUS group. Excess breast tissue excision was better determined by the Calculated Resection Ratio (CRR). There is striking evidence that CRR was reduced in IOUS guided Breast-conserving surgery [21,24,25]. However, this study could not evaluate that because the CRR was not presented in most studies, and there was a variation of data presentation among assignments.
Methods to assess cosmesis following breast-conserving surgery are varied, and it is difficult to compare the scores. The cosmetic outcome was better with IOUS at time point follow up period of 3, 6, 9 and 12 months [26]. This finding agreed with the COBALT trial result [9,16,24]; however, there is conflicting evidence among studies regarding the cosmetic outcome. The postoperative follow up was varied from 2 weeks to 41 months among included RCTs. Therefore, interpretation of combined cosmetic outcome was not possible. In our study, the overall percentage of patients and assessors’ satisfaction is still higher than dissatisfaction in the IOUS group.
Ultrasound is accurate, simple with low procedure-related risk when compared to other localization techniques. With the proper training and supervised practice, surgeons can learn to perform an ultrasound guidance procedure to incorporate into their daily routine. This comprehensive skill can reduce the positive margin rate, increased surgical accuracy and reduce the re-excision rate [26]. This will reduce the pressure on the theatre list and financial burden to the hospitals and positively impact patients. The use of intra-operative ultrasound by breast surgeons is low. Breast surgeons should be trained to perform an ultrasound to become confident to use it during surgery in suitable cases to reduce positive margin rate and re-excision rate.
The ultrasound can be used alongside other intra-operative margin assessment methods (MarginProbe, specimen X-ray) to reduce the positive margin rate, especially in the tumour associated with DCIS. The Oncoplastic Breast Surgery (OPBS) alone showed 11.9% positive margins and a 91% breast conservation rate [27-29]. Combined IOUS with OPBS can reduce the positive margin rate; this needs a better design randomized control trial to investigate the outcome.
There are some limitations to this systematic review and metaanalysis. Firstly, only a limited number of articles was reviewed in this study due to a lack of RCT regarding the focus question. Secondly, the sample size of some studies was small and not fulfilled its sample size requirement. Thirdly, a meta-analysis could not be performed for excisional volume and cosmetic outcome due to high heterogeneity among studies. Finally, findings from this review only represented an early palpable breast cancer population.
In summary, the relevant data was extracted for synthesis and analysis using appropriate methods to conclude. This study supported the better oncology outcome due to a less positive margin using intraoperative ultrasound in breast cancer patients. There is no statistically significant difference in excisional volume, although less volume was excised in the IOUS group. Overall, satisfaction exceeds dissatisfaction with ultrasound-guided Breast-conserving surgery. However, there is insufficient evidence to support the better cosmetic outcome in the IOUS group. Further research will be needed to compare the actual cosmetic outcome differences between groups.
Acknowledgement
Authors would like to thank Mrs Vicki Whittaker, senior lecturer, Teesside University to support this work and Librarian at Friarage Hospital for providing us with necessary literatures.
Author Contributions
• Conception and design: Su Min Min Tin.
• Administrative Support: Imtiaz Cheema, Vijay Kurup, YKS Viswanath.
• Provisions of study materials or patients: Su Min Min Tin, Shiveta Razdan.
• Collection and assembly of data: Su Min Min Tin, Shiveta Razdan.
• Data analysis and interpretation: Su Min Min Tin.
• Manuscript writing: All Authors.
• Final approval of manuscript: All Authors.
References
- Cancer Research UK. 2019.
- Chen K, Liu J, Zhu L, Su F, Song E, Jacobs L. Comparative effectiveness study of breast-conserving surgery and mastectomy in the general population: A NCDB analysis. Oncotarget. 2015; 6.
- Onitilo A, Engel J, Stankowski R, Doi S. Survival Comparisons for Breast- Conserving Surgery and Mastectomy Revisited: Community Experience and the Role of Radiation Therapy. Clinical Medicine & Research. 2014; 13: 65- 73.
- Sun Y, Liao M, He L, Zhu C. Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after a good response to neoadjuvant chemotherapy. Medicine. 2017; 96: e8367.
- Lebovic G. Oncoplastic and Reconstructive Breast Surgery. 2nd ed. Switzerland: Springer. 2019; 7-11.
- Tang S, Kaptanis S, Haddow J, Mondani G, Elsberger B, Tasoulis M, et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. European Journal of Cancer. 2017; 84: 315- 324.
- Dixon J. Breast surgery. 5th edition. Edinburgh: Saunders/Elsevier. 2014; 26-34.
- Krekel N, Lopes Cardozo A, Muller S, Bergers E, Meijer S, van den Tol M. Optimizing surgical accuracy in palpable breast cancer with intra-operative breast ultrasound-Feasibility and surgeons’ learning curve. European Journal of Surgical Oncology (EJSO). 2011; 37: 1044-1050.
- Krekel N, Zonderhuis B, Schreurs H, Lopes Cardozo A, Rijna H, van der Veen H, et al. Ultrasound-guided breast-sparing surgery to improve cosmetic outcomes and quality of life. A prospective multicentre randomized controlled clinical trial comparing ultrasound-guided surgery to traditional palpationguided surgery (COBALT trial). BMC Surgery. 2011; 11.
- Pan H, Wu N, Ding H, Ding Q, Dai J, Ling L, et al. Intraoperative Ultrasound Guidance Is Associated with Clear Lumpectomy Margins for Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE. 2013; 8: e74028.
- Slijkhuis W, Noorda E, van der Zaag-Loonen H, Eenennaam M, Greve K, Lastdrager W, et al. Ultrasound-guided breast-conserving surgery for earlystage palpable and nonpalpable invasive breast cancer: decreased excision volume at unchanged tumour-free resection margin. Breast Cancer Research and Treatment. 2016; 158: 535-541.
- Houssami N, Macaskill P, Marinovich M, Morrow M. The Association of Surgical Margins and Local Recurrence in Women with Early Stage Invasive Breast Cancer Treated with Breast-Conserving Surgery: Meta-Analysis. Annals of Surgical Oncology. 2014; 21: 717-730.
- Behm E, Beckmann K, Dahlstrom J, Zhang Y, Cho C, Stuart-Harris R, et al. Surgical margins and risk of locoregional recurrence in invasive breast cancer: An analysis of 10-year data from the Breast Cancer Treatment Quality Assurance Project. The Breast. 2013; 22: 839-844.
- Vrieling C, Collette L, Fourquet A, Hoogenraad W, Horiot J, Jager J, et al. The influence of patient, tumor and treatment factors on the cosmetic results after breast-conserving therapy in the EORTC ‘boost vs. no boost’ trial. Radiotherapy and Oncology. 2000; 55: 219-232.
- Jagsi R, Li Y, Morrow M, Janz N, Alderman A, Graff J, et al. Patient-reported Quality of Life and Satisfaction With Cosmetic Outcomes After Breast Conservation and Mastectomy With and Without Reconstruction. Annals of Surgery. 2015; 261: 1198-1206.
- Volders J, Haloua M, Krekel N, Negenborn V, Kolk R, Lopes Cardozo A, et al. Intraoperative ultrasound guidance in breast-conserving surgery shows superiority in oncological outcome, long-term cosmetic and patient-reported outcomes. European Journal of Surgical Oncology (EJSO). 2017; 43: 649- 657.
- Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. Editors. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Cochrane. 2019.
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. Editors. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Cochrane. 2019.
- Karanlik H, Ozgur I, Sahin D, Fayda M, Onder S, Yavuz E. Intraoperative ultrasound reduces the need for re-excision in breast-conserving surgery. World Journal of Surgical Oncology. 2015; 13.
- Vispute T, Seenu V, Suhani Parshad R, Hari S, Thulkar S, Mathur S. Comparison of resection margins and cosmetic outcome following intraoperative ultrasound-guided excision versus conventional palpationguided breast conservation surgery in breast cancer: A randomized controlled trial. Indian Journal of Cancer. 2018; 55: 361-365.
- Moore M, Whitney L, Cerilli L, Imbrie J, Bunch M, Simpson V, Hanks A. Intraoperative Ultrasound Is Associated With Clear Lumpectomy Margins for Palpable Infiltrating Ductal Breast Cancer. Annals of Surgery. 2001; 233: 761-768.
- Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ. 2011; 343: d4002.
- Leidenius M. Surgery to the Breast: Breast Conservation Techniques. Editors. In: Wyld L, Markopoulos C, Leidenius M, Senkus-Konefka E. Breast Cancer Management for Surgeons. Springer, Cham. 2018.
- Haloua M, Volders J, Krekel N, Lopes Cardozo A, de Roos W, de Widt- Levert L, et al. Intraoperative Ultrasound Guidance in Breast-Conserving Surgery Improves Cosmetic Outcomes and Patient Satisfaction: Results of a Multicenter Randomized Controlled Trial (COBALT). Annals of Surgical Oncology. 2015; 23: 30-37.
- Karadeniz Cakmak G, Emre A, Tascilar O, Bahadir B, Ozkan S. Surgeon performed continuous intraoperative ultrasound guidance decreases reexcisions and mastectomy rates in breast cancer. The Breast. 2017; 33: 23- 28.
- Shivran N, Dev K, Kurpad V, Gurawalia J, Veeredrakumar K, Brar G. Intraoperative ultra-sonography in Breast conserving surgery: Better re-excision rate or cosmetic outcome. European Journal of Cancer. 2018; 92: S63-S64.
- Clough K, Gouveia P, Benyahi D, Massey E, Russ E, Sarfati I, et al. Positive Margins After Oncoplastic Surgery for Breast Cancer. Annals of Surgical Oncology. 2015; 22: 4247-4253.
- Jeevan R, Cromwell D, Trivella M, Lawrence G, Kearins O, Pereira J, et al. Reoperation rates after Breast conserving surgery for breast cancer among women in England: a retrospective study of hospital episode statistics. BMJ. 2012; 345: e4505.
- Charfare H, MacLatchie E, Cordier C, Bradley M, Eadie C, Byrtus A, et al. A Comparison Of Different Methods Of Assessing Cosmetic Outcome Following Breast-Conserving Surgery And Factors Influencing Cosmetic Outcome | British Journal Of Medical Practitioners. 2010; 3: 310.