
Research Article
Austin J Surg. 2022; 9(2): 1288.
Panlongqi Tablet (PLQT) Inhibits LPS-Induced Abnormal Proliferation of RA-HFLS by Regulating PI3K/ AKT and MAPK Signaling Pathways
Niu X¹, Yang Y¹, Yu J¹, Song H¹, Huang Q¹, Yu J¹, Liu Y¹, Zhang D², Han T²* and Li W¹*
¹School of Pharmacy, Xi’an Jiaotong University, China
²Shaanxi Panlong Pharmaceutical Group Limited by Share LTD, China
*Corresponding author: Weifeng Li, School of Pharmacy, Xi’an Jiaotong University, No.76 Western Yanta Road, Xi’an, Shaanxi Province 710061, P.R China
Tengfei Han, Shaanxi Panlong Pharmaceutical Group Limited by Share LTD, No. 2801Baliu Second Road, Xi’an, Shaanxi Province 710061, P.R China
Received: July 18, 2022; Accepted: August 18, 2022; Published: August 25, 2022
Abstract
Panlongqi Tablet (PLQT), a traditional Chinese formula, is effective in the clinical treatment of Rheumatoid Arthritis (RA) in China for several decades. However, the underlying mechanism of PLQT’s therapeutic effect remains unclear. The main chemical constituents of PLQT were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The human fibroblast-like synovial cells in rheumatoid arthritis (RA-HFLS) were stimulated with LPS (10 μg/mL) to establish a inflammatory model in vitro. The cells were then treated with PLQT-medicated serum to verify the protective effect of the herbal compound. MTT assay was used to detect the viability of RAHFLS and abnormal cell proliferation. The inflammatory factors levels including interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-17 (IL-17) in RAHFLS were measured by ELISA. In addition, the cell cycle progression was analyzed by flow cytometry and protein expression of Proliferating Cell Nuclear Antigen (PCNA) was detected using immunohistochemistry and western blot. The protein expression levels associated with PI3K/AKT and MAPK signaling pathways in RA-HFLS were detected by western blot analyses. Our results revealed that PLQT treatment effectively inhibited the abnormal proliferation of RA-HFLS and decreased the inflammatory factors levels. The proportion of cells in S phase was significantly increased with the treatment of PLQT. Meanwhile, the expression levels of PCNA, p-PI3K, p-AKT, p-mTOR, p-JNK/JNK, p-ERK/ ERK, and p-P38/P38 proteins from RA-HFLS were dramatically inhibited by PLQT treatment. PLQT inhibits LPS-induced abnormal proliferation of RAHFLS, which may be related to the down regulation of PCNA, and the inhibition of abnormally activated PI3K/AKT and MAPK signaling pathways.
Keywords: Panlongqi tablet; Rheumatoid arthritis; Human fibroblast-like synovial cells; PI3K/AKT signaling pathway; MAPK signaling pathway
Abbreviations
PLQT: Panlongqi Tablet; RA: Rheumatoid Arthritis; RA-HFLS: Human Fibroblast-Like Synovial Cells in Rheumatoid Arthritis; LPS: Lipopolysaccharide; LC-MS/MS: Liquid Chromatography-Tandem Mass Spectrometry; FBS: Fetal Bovine Serum; MTT: Methyl Thiazolyl Tetrazolium; IL-1β: Interleukin-1β; IL-6: Interleukin-6; IL-17: Interleukin-17; TNF-a: Tumor Necrosis Factor-a; ELISA: Enzyme Linked Immunosorbent Assay; PCNA: Proliferating Cell Nuclear Antigen; PI3K/AKT: Phosphatidylinositol 3-Kinase/Protein Kinase B; MAPK: Mitogen-Activated Protein Kinase; mTOR: Mammalian Target of Rapamycin; NIH: National Institutes of Health; S.E.M: Standard Error of Mean; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; DMARDs: Disease-Modifying Anti-Rheumatic Drugs
Introduction
Rheumatoid Arthritis (RA), as an autoimmune disease, seriously threatens human health. The occurrence of the disease is often accompanied by a series of symptoms, such as synovial hyperplasia, cartilage erosion, joint swelling and deformation, limited movement and so on. What’s worse, some serious complications, such as osteoporosis and cardiovascular disease, make the situation of patients with RA more difficult [1,2]. RA always brings a huge burden to individuals and society, soit is very paramount to find excellent therapeutic drugs to prevent the deterioration of RA.
Human fibroblast-like synovial cells in rheumatoid arthritis (RA-HFLS) are derived from the synovial tissue of human joints and play an important role in the pathogenesis of RA. Previous studies had confirmed that the abnormally active proliferation of RAHFLS could secrete a variety of inflammatory factors, chemokines, metalloproteinases, which could cause synovial hyperplasia and joint inflammation. The chronic inflammation gradually erodes joints and cartilage, eventually leading to RA [3]. In this pathological process, interleukin interleukin-1β (IL-1β) and interleukin-6 (IL-6) are two main pro-inflammatory cytokines in synovial membrane of joints that contribute to the cartilagedestruction and joint disruption [4]. Interleukin-17 (IL-17), as a proinflammatory cytokine, is involved in the pathogenesis of RA. The level of IL-17 in the body is high, which often aggravates synovial inflammation, joint injury and accelerates the onset of RA [5]. Thus, inhibiting the abnormal proliferation of RA-HFLS and reducing the expression of proinflammatory factors may be the potential strategies for treating RA. PI3K/AKT signaling pathway was found to be constitutionally activated in different malignant tumors, which exactly showed the importance of this signaling pathway in abnormal cell proliferation [6]. At the same time, more and more studies implicated that MAPK pathway was activated in RA-HFLS, inhibition of the MAPK signaling pathway can suppress the proliferation, migration and invasion of RA-HFLS [7-10]. Therefore, the research of PI3K/AKT and MAPK signaling pathway on RA-HFLS may be quite valuable and essential.
At present, there are many drugs for RA. However, these drugs can only alleviate the symptoms of RA to some extent [11]. Long term use of these drugs will lead to gastrointestinal diseases, cardiovascular diseases or reproductive system toxicities, thus limiting their safety and effectiveness [12]. Nowadays, traditional Chinese herbal medicines, whether extracted from natural plants or classical prescriptions, showed the advantages of low toxicity, high efficiency and moderate price. These advantages suggest that traditional herbal medicines may be a potentially alternative agent for RA.
Panlongqi Tablet (PLQT) is a listed Chinese patent medicine approved by National Medical Products Administration (No. Z61020050).PLQT is composed of 29 Chinese herbal medicines, mainly including Polygonum taipaishanense Kung (Panlongqi, rhizome), Angelica sinensis (Oliv.) Diels (Danggui, root), Salvia miltiorrhiza Bge (Danshen, root and rhizome), Carthamus tinctorius L (Honghua, flower), Gentiana macrophylla Pall (Qinjiao, root), Iris tectorum Maxim (Qingwaqi, rhizome), Eucommia ulmoides Oliv (Duzhong, root bark). The detailed composition and proportion of PLQT are shown in Table 1. It was found that PLQT displayed pleiotropic roles in the treatment of osteoarthritis, such as antiinflammatory and analgesic, regulating synovitis, promoting blood circulation and improving joint function [13,14]. Recent study had found that PLQT may inhibit the proliferation of synovial cell, reduce the synthesis of proinflammatory factors and alleviate inflammatory response in rats with adjuvant arthritis by inhibiting the activation of NF-κB signaling pathway [15]. In addition, clinical studies had found that PLQT could alleviate the symptoms of RA, improve joint pain caused by synovitis, alleviate swelling and stiffness, and reduce joint dysfunction in RA patients [16-18]. Nevertheless, the underlying mechanism of anti-RA still remains unknown. Liquid chromatography-tandemmass spectrometry (LC-MS/MS) can effectively separate and identify the active components in traditional Chinese medicine formula for subsequent pharmacological activity analysis. In this paper, the LC-MS/MS was employed and the abnormal proliferations of RA-HFLS were used as a cell model for RA to systematically investigate the therapeutic effect of PLQT, and explore its pharmacological mechanism.
Chinese name
Pinying(Chinese phonetic alphabet)
Plant name
Part used
Proportionof ingredients(100%)
Dragon Seven
Pan Long Qi
Polygonum taipaishanense Kung
Dired rhizome
5.93
Strong Tendon Pill
Zhuang Jin Dan
Myosoton aquaticum (L.) Moench
Dired the whole grass
1.17
Wujiapi
Wu Jia Pi
Acanthopanax gracilistylus W. W. Smith
Dired root bark
5.93
Eucommia
Du Zhong
Eucommia ulmoides Oliv
Dired root bark
5.93
Ginseng
Zhu Zi Shen
Panax japonicus C. A. Mey. var. maior (Burkill)
Dired rhizome
0.36
C. Y. Wu & K. M. Feng
frog seven
Qing Wa Qing
Iris tectorum Maxim
Dired rhizome
0.36
Mountain Dragon
Guo Shan Long
Ampelopsis aconitifolia Bunge
Dired root bark
2.95
Gentiana
Qin Jiao
Gentiana macrophylla Pall
Dired root
5.93
Zu Sima
Zu Si Ma
Daphne giraldii Nitsche
Dried stem bark and root bark
0.36
Woody
Mu Xiang
Aucklandia lappa Decne
Dired root
2.95
Caterpillar vine
Luo Shi Teng
Trachelos permum jasminoides(Lindl.)Lem
Dired rattan stem
2.95
Chuanwu
Chuan Wu
Aconitum carmichaelii Debx
Dired root
0.36
white hair seven
Bai Mao Qi
Chloranthus multistachys Pei.
Dried the whole grass,root
1.17
and rhizome
iron rod hammer
Tie Bang Chui
Aconitum pendulum Busch
Dired root tubers
0.36
myrrh
Mo Yao
Commiphora myrrha (T.Nees) Engl.
Dried resin
2.95
valerian
Xie Cao
Valeriana officinalis L.
Dried root
2.95
extensor grass
Shen Jin Cao
Lycopodium japonicum Thunb.
Dried the whole grass
1.17
Achyranthes knee
Niu Xi
Achyranthes bidentata Blume
Dried root
2.95
Danshen
Dan Shen
Salvia miltiorrhiza Bge
Dried root and rhizome
8.89
heavy building
Chong Lou
Paris polyphylla Smith var. yunnanensis (Franch.)Hand. -Mazz.
Dried rhizome
8.89
mastic
Ru Xiang
Boswellia carteri Birdw.
Dried resin
2.95
angelica
Dang Gui
Angelica sinensis (Oliv.) Diels
Dried root
14.81
mouse seven
Lao Shu Qi
Hylomecon japonica (Thunb.)Prantl
Dried rhizome
1.17
Pillar Polygonum
Zhi Zhu Liao
Bistorta suffulta (Maxim.) Greene ex H. Gross
Dried rhizome
2.95
bamboo root seven
Zhu Gen Qi
Disporopsis fuscopicta Hance
Dried rhizome
2.95
Seven horns
Yang Jiao Qi
Tricyrtis macropoda Miq.
Dried root tubers
2.95
Bali Ma
Ba Li Ma
Sambucus javanica Blume
Dried the whole grass
2.95
Aconitum grass
Cao Wu
Aconitum kusnezoffii Rchb
Dried root tubers
0.36
safflower
Hong Hua
Carthamus tinctorius L
Dried flower
2.95
Table 1: The composition of PLQT.
Materials and Methods
Materials and Reagents
PLQT was provided by Pan Long Pharmaceutical Co., Ltd. (Shanxi, China). Lipopolysaccharide(LPS) and Dimethyl Sulfoxide(DMSO) were purchased from Sigma-Aldrich (St. Louis, USA). Methyl Thiazolyl Tetrazolium (MTT) was purchased from Nanjing Jiancheng Technology Co., Ltd. (Nanjing, China). Dexamethasone(DXM) was provided by Jintaiyang Biochemical Pharmaceutical Co., Ltd (Anhui, China). DMEM/F12 incomplete medium and Phosphate Buffer Saline(PBS) were purchased from Keygen Bio Tech (Nanjing, China). Fetal Bovine Serum(FBS) was supplied by Honbiotech (Jinan, China). The cell cycle analysis kit, and cell apoptosis analysis kit were purchased from Keygen Bio Tech (Nanjing, China). The Enzyme Linked Immunosorbent Assay (ELISA) kits for human IL-6, IL-1βand IL-17 were purchased from BioSwamp (Wuhan, China). The reference substances of (-)-Epicatechin, Gentiopicroside, Astragalin, Salvianolic acid B, Hydroxysafflor yellow A, Tectoridin, and Imperatorin were supplied by Desite (Chengdu, China). All antibodies were obtained from Abclonal(Wuhan, China). Other reagents were commercial analytical grade or better.
The Specific Composition of PLQT
PLQT consists 29 herbs according toa dosage ratio in (Table 1). Polygonum taipaishanense Kung (Panlongqi,rhizome), Salvia miltiorrhiza Bge (Danshen, root and rhizome), Paris polyphylla Smith var. yunnanensis(Franch.) Hand.–Mazz (Chonglou, rhizome) and Angelica sinensis (Oliv.) Diels (Danggui, root) are the main herbs, which was provided by Panlong pharmaceutical Co., Ltd. (shaanxi, China). It was identified by Professor Xiaofeng Niu from School of Pharmacy, Xi’an Jiaotong University. The voucher specimen was deposited in Panlong pharmaceutical Co., Ltd (shaanxi, China). In the preparation process, the 29 herbs were powdered and decocted twice with water. Then, these extracts were collected and concentrated to the required density. An appropriate amount of excipients were added and mixed to form granules. At last, the granules were pressed into tablets and coated
Analysis of Index Components in PLQT
The chemical constituents of PLQT were analyzed by LC-MS/MS consisting of LC-20ADXR system with WondaSil C18 column. The mobile phase was composed of 0.1% formic acid aqueous solution (A)-acetonitrile (B) for gradient elution (5~50% B in 0~23 min, 50%~80% B in 23~43 min, 80%~80% B in 43~53 min, 80%~95% B in 53~56 min, 95%~5% B in 56~58 min, 5%~5% B in 58~60 min). The flow rate was 0.3 mL/min with column temperature of 35°C. The amount of injection was 15 μL. On the conditions of mass spectrometry, Electron Spray Ionization (ESI) source was adopted. The desolvent pipeline temperature was 250°C, the heating block temperature was 400°C, the atomization gas flow rate was 3 L/min, and the drying gas flow rate was 15 L/min.
Animals
C57/BL6 male mice (20–22 g) were obtained from the Experimental Animal Centre of Xi’an Jiaotong University. All mice were housed in standard experimental conditions (temperature: 24±1°C, relative humidity: 40–80%, 12 light/12 dark cycle). The whole procedures were based on the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publication No. 85–23, revised 1996) and approved by the Ethics Committee of Xi’an Jiaotong University.
Preparation of PLQT-Medicated Serum
Male C57/BL6 mice were divided into three groups randomly: Control group, PLQT group (180mg/kg, dissolved in saline) and DXM group (5mg/kg, dissolved in saline). The dosed of drug given by intragastric administration in PLQT group and DXM group were 1 mL/0.018 g and 1 mL/0.0005 g, respectively. The control group was given the same doses of saline. All mice were given drugs for 7 consecutive days (1 time/1 day). Two hours after the last administration, mice were killed. Serum samples of the mice were collected aliquoted and stored at -80°C.
Cell Culture
RA-HFLS were purchased from Fenghui Biotechnology Co., Ltd (Hunan, China) and cultured in DMEM/F12containing 10% common FBS, and incubated in an incubator at 37°C with 95% air and 5% CO2. RA–HFLS at passages 5-10 were used.
For the preparation of LPS, LPS dry powder is dissolved in sterile PBS to prepare a stock solution in1 mg/mL. DMEM/F12 was used to dilute LPS solution to the working concentration of 10 μg/mLand store at -20°C for later use.
Cell Viability Assay
MTT assay was adopted to determine the safety threshold of blank serum, PLQT-medicated serum and DXM-medicated serum. Their effects on cell proliferation can be also verified at a later stage. First, RA-HFLS was inoculated in 96-well plates and incubated overnight in DMEM/F12 with 10% FBS. When the cells were confluent to 70%, serum starvation (incubated with blank DMEM/F12) was performed for 24h. After that, different proportions of DMEM/F12 containing blank serum, PLQT-medicated serum, and DXM-medicated serum were inoculated, and then incubated for 24h. In the cell proliferation experiment, LPS was used for stimulation after incubation with specific concentrations of the above three serums for 24h. The cell supernatant was removed and MTT was added and incubated at 37oC for 4 h. Finally, 150 μL DMSO was added to each well to dissolve formazan crystals. The absorbance was measured with a microplate reader at 490 nm.
IL-1β, IL-6 and IL-17 Detection
RA-HFLS were seeded into 24-well culture plates at a density of 1×104 cells/well, and incubated in DMEM/F12 containing 10% common FBS. After the cells were grown and fused to 70%, the culture solution was discarded and the serum was starved for 24h. Then cells were incubated with blank serum, PLQT-medicated serum, and DXM-medicated serum for 24h. Subsequently, RA-HFLS were irritated with LPS for 24h and the supernatants were obtained by centrifugation at 1000×g for 10 min. The centrifuged supernatants were stored at -80°C for quantification of IL-1β, IL-6 and IL-17. The levels of IL-1β, IL-6 and IL-17 in cell supernatants were detected by ELISA according to the manufacturer’s protocol (BioSwamp, Wuhan, China).
Cell Cycle Progression Assay
RA-HFLS were seeded into 6-well culture plates and incubated in DMEM/F12 containing 10% common FBS. After the cells were grown and fused to 80%, the culture solution was removed and the serum was starved for 24h. Then cells were incubated with blank serum, PLQT-medicated serum, and DXM-medicated serum for 24h, and stimulated by LPS for another 24h. The cells were collected and stained at room temperature in the dark and then analyzed by flow cytometry (ACEA Biosciences, China). The data were analyzed by the ModFit LT 5.0.
Immunohistochemistry
Immunohistochemistry was employed to detect PCNA protein expression in RA-HFLS. Firstly, the cells were fixed in 3% H2O2 and 3% normal goat serum, and were then incubated with PCNA primary antibodies (diluted with PBS at a ratio of 1:100) overnight at 4°C. The cells were incubated with HRP-labeled secondary antibody and stained with DBA. At the end, photos of RA-HFLS were taken under a microscope at 40× magnification. Quantitative average optical density analysis was performed using the Image Pro Plus Analysis system (Media Cybernetics Inc, USA).
Western Blot Analysis
RA-HFLS were collected after treatment with blank serum, PLQT-medicated serum, DXM-medicated serum and LPS. The cells were lysed, and the lysate and loading buffer were mixed at a ratio of 4:1 and then boiled for 10 min. The protein samples were separated and incubated with primary antibody (p-PI3K, p-AKT, p- mTOR, p38, p-p38, JNK, p-JNK, ERK, p-ERK and β-actin) overnight at 4°C. The next day, the membrane was incubated with HRP-labeled secondary antibody (diluted with TBST at a ratio of 1:20000) at 37°C for 1h. The protein was developed with ECL reagent and displayed by an imaging system (Tanon, Shanghai, China). The relative intensity of each protein was analyzed by Image J software.
Statistical Analysis
The data were statistically analyzed by one-way ANOVA followed by Student-Newman-Keuls test employing Prism 5.01 (California, USA). Data are expressed as S.E.M. Statistical significance was accepted at the P value of p < 0.05 (n=3).
Result
PLQT Index Component Analysis
Based on the LC-MS/MS analysis results, seven main chemical components were preliminarily identified according to the m/z of primary fragment ions, retention time, molecular ion peaks and main secondary fragment ions (Table 2). In detail, the identified components were Hydroxysafflor yellow A, Gentiopicroside, (-)-Epicatechin, Tectoridin, Astragalin, Salvianolic acid B, and Imperatorin. Of which the mass spectrum total ion flow diagram was shown in (Figure 1), the chemical structure of the main chemical components was shown in (Figure 2).
Peak
Compound
tR(min)
Fractions in a positive ion mode(m/z)
Fractions in a negative ion mode(m/z)
1
Hydroxysafflor yellow A
21.545
613.20[M+H]+
611.15[M-H]-
2
Gentiopicroside
23.319
357.15[M+H] +
355.25[M-H] -
3
(-)-Epicatechin
23.653
291.15 [M+H] +
289.15[M-H] -
4
Tectoridin
26.807
301.15[M+H] +
299.15[M-H] -
5
Astragalin
27.083
449.15[M+H] +
447.15[M-H] -
6
Salvianolic acid B
29.093
-
717.20[M-H] -
7
Imperatorin
50.381
271.15[M+H] +
269.15[M-H] -
Table 2: Chemical components of PLQT identified by LC-MS/MS.
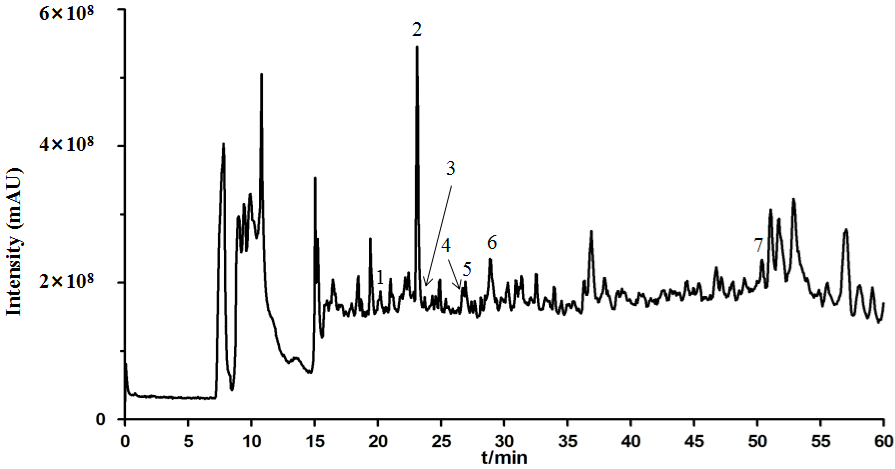
Figure 1: The total ion current (TIC) chromatogram of PLQT in positive mode. (1) Hydroxysafflor yellow A, (2) Gentiopicroside, (3) (-)-Epicatechin, (4) Tectoridin,
(5) Astragalin, (6) Salvianolic acid B, (7) Imperatorin.
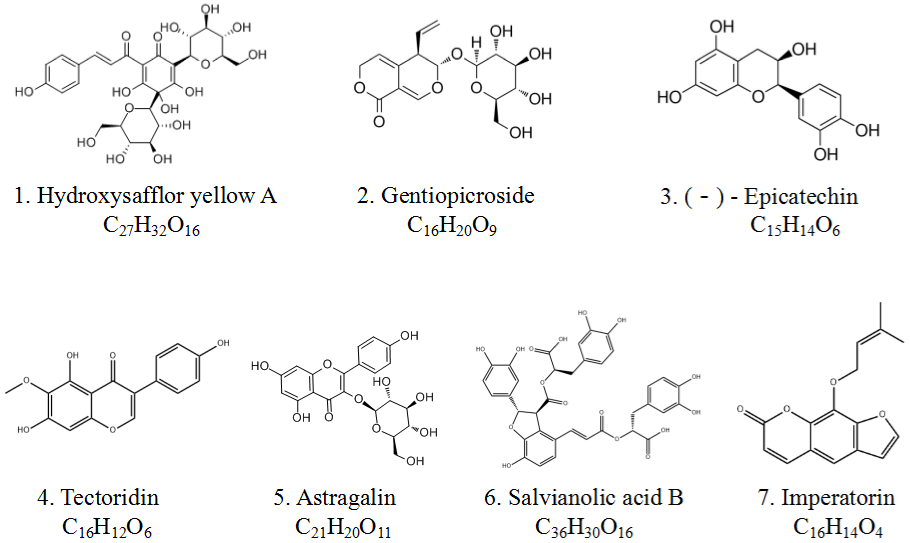
Figure 2: The chemical structure of the main chemical components of PLQT. The numbers 1-7 correspond to the numbers in Figure 1, respectively.
Cytotoxicity Assay
RA-HFLS were identified by immunohistochemistry with Vimentin antibody after trypsin digestion. More than 95% of RAHFLS were positive for the antibody and were identified as RAHFLS (Figure 3A). As shown in (Figure 3B), the MTT results showed that after blank serum and DXM-medicated serum (addition ratio/ concentration 20%) were administered, the normal growth of RAHFLS were inhibited (P<0.01). However, after administration of (1%, 2.5%, 5%, 10%and 20%) PLQT-medicated serum, (1%, 2.5%, 5% and 10%) blank serum, and (1%, 2.5%, 5% and 10%) DXM-medicated, the survival rate of RA-HFLS were not change significantly, compared with the control group. It indicated that these concentrations of serums did not affect the normal growth of cells. Therefore, 2.5% blank serum, 2.5% DXM-medicated serum and 1%, 2.5% PLQTmedicated serum were selected to detect the protective effects of PLQT in the present experiment due to their best cost performance.
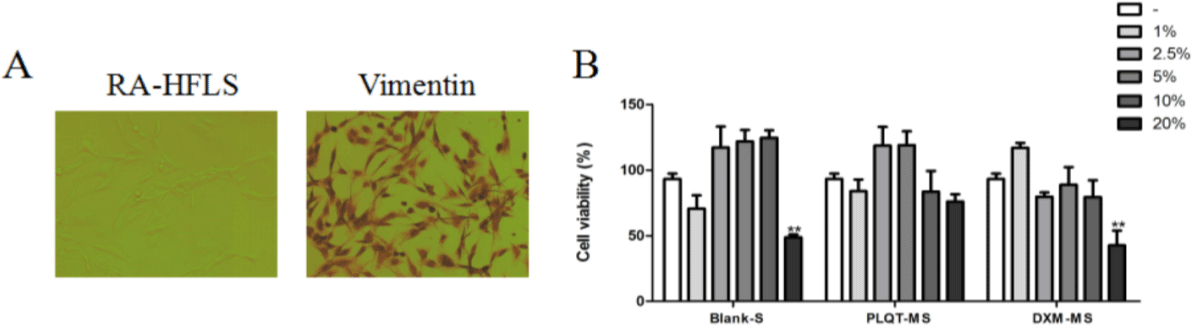
Figure 3: The safe concentration range of Blank-serum, PLQT-medicated serum and DXM-medicated serum for normal growth of RA-HFLS were screened. (A)
Observation and identification of RA-HFLS under microscope (20 ×). (B) After RA-HFLS were incubated with different proportions of Blank-serum, PLQT-medicated
serum and DXM-medicated serum (1%, 2.5%, 5%, 10% and 20%). The cell viability was detected by MTT assay. All the values are represented by the means ±
S.E.M. **P< 0.01 compared with the control group. Blank-S: Blank-serum; PLQT-MS: Panlongqi Tablet-medicated serum; DXM-MS: Dexamethasone-medicated
serum (n=3).
PLQT-Medicated Serum Inhibits LPS-Induced Inflammatory Factors in RA-HFLS
As shown in Fig. 4, the expression levels of the three inflammatory factors, including IL-1β, IL-6 and IL-17, were significantly increased in the LPS-induced(10μg/mL) groups compared with those in the control groups (P< 0.05). However, pretreatment of the cells with PLQT-medicated serum reduced expression levels of the three inflammatory factors, especially the high-dose PLQT-medicated serum which can sharply down-regulate the expression level of IL-17 (P< 0.001 versus the LPS-induced group). In addition, the expression levels of these three inflammatory factors were also reduced in the DXM-medicated serum groups (P<0.05 versus the LPS-induced group), but the levels of these three inflammatory factors were not reduced in the blank serum groups.
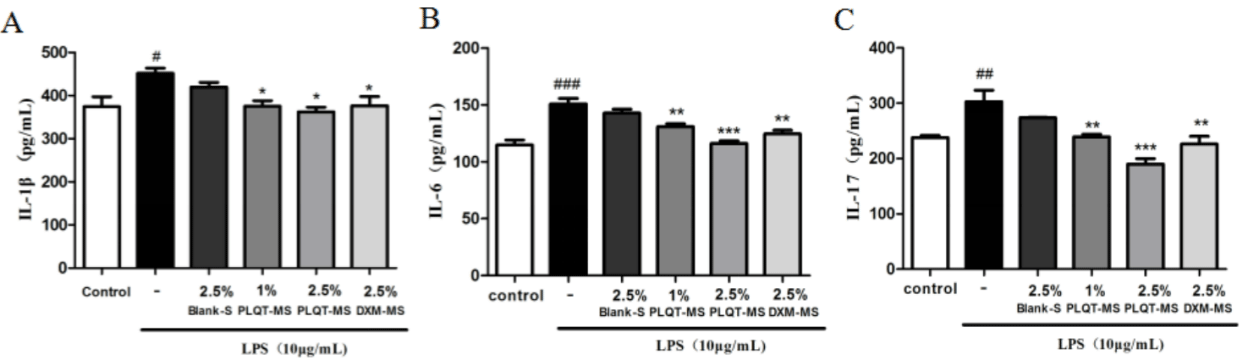
Figure 4: PLQT-medicated serum inhibited the production of IL-1β, IL-6 and IL-17 in RA-HFLS induced by LPS. (A), (B) and (C) RA-HFLS were pretreated with
2.5% Blank-serum, 1% and 2.5% PLQT-medicated serum, and 2.5% DXM-medicated serum for 24h, and then stimulated with LPS (10 μg/mL) for 24h. The levels
of IL-1β, IL-6 and IL-17 were determined by ELISA. All the values are represented by the means ± S.E.M. #P< 0.05, ##P< 0.01 or ###P< 0.001 compared with the
control group. *P< 0.01, **P< 0.01 or ***P< 0.001 compared with the LPS-induced group. Blank-S: Blank-serum; PLQT-MS: Panlongqi Tablet-medicated serum;
DXM-MS: Dexamethasone-medicated serum (n=3).
PLQT-Medicated Serum Inhibits LPS-Induced RA-HFLS Proliferation
As shown in Figure 5A, compared with the control group, LPS induction caused RA-HFLS to exhibit severe abnormal proliferation (P < 0.05). While, the PLQT-medicated serum reversed this situation, and the abnormal proliferation level of RA-HFLS returned to the normal cell level at higher PLQT dose. The DXM-medicated serum group slightly inhibited the abnormal proliferation of RA-HFLS, but no significant inhibition was observed in blank serum group.
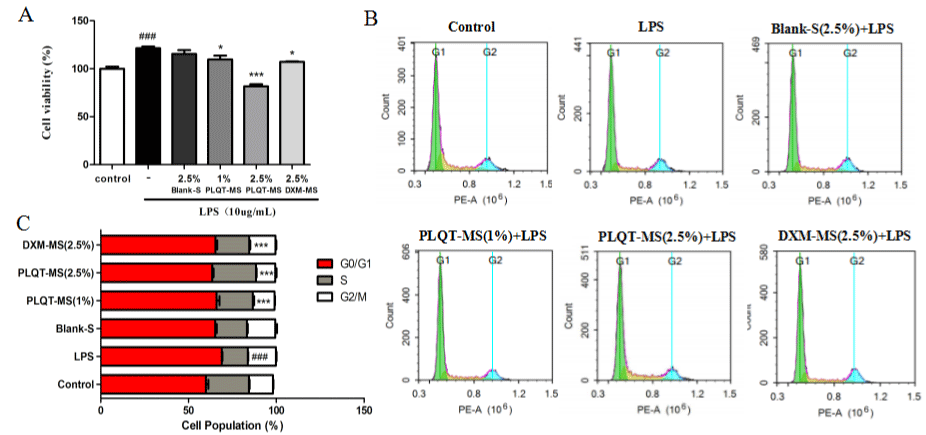
Figure 5: PLQT-medicated serum inhibited LPS induced RA-HFLS proliferation. RA-HFLS were incubated with 2.5% Blank-serum, 1% and 2.5% PLQT-medicated
serum, and 2.5% DXM-medicated serum before stimulated with 10 μg/mL LPS for 24h. (A) The proliferation of RA-HFLS was measured by the MTT assay. (B)
Cell cycle distribution was determined by flow cytometry. (C) Percentage of cells at G0/G1, S and G2/M phases indicated by flow cytometry. Values are expressed
as mean ± S.E.M., n = 3 independent experiments. ###P< 0.001 compared with the control group. *P< 0.01 or ***P< 0.001 compared with the LPS-induced group.
Blank-S: Blank-serum; PLQT-MS: Panlongqi Tablet-medicated serum; DXM-MS: Dexamethasone-medicated serum (n=3).
Flow cytometry results (Figure 5B and C) showed the cell cycle distribution in the control group and the percentage was approximately 24.55% in the S phase. The percentage of cells in S phase was significantly decreased from 24.55% to 14.88% (P<0.001 versus the control group) in the LPS-induced group. By contrast, the percentage of cells in S phase was increased after PLQT-medicated serum intervention. Especially, the percentage of cells in S phase in 2.5% PLQT-medicated serum group was significantly increased to 24.94% (P<0.001 versus the LPS-induced group) almost reached the level in control group. Moreover, the percentage of cells in S phase in DXM-medicated group was increased to 19.27% (P<0.001 versus the LPS-induced group). The percentage of cells in S phase in blank serum group also increased although statistically insignificant compared with LPS-induced group.
In addition, PCNA protein expressions in RA-HFLS were examined as we. The results of immunohistochemistry showed that PLQT-medicated serum and DXM-medicated serum significantly reduced the expression of PCNA and reduced the area of positive staining (P<0.001 versus the LPS-induced group) (Figure 6A and C), but this is not the case for blank serum. Western blot analysis also showed that PLQT-medicated serum and DXM-medicated serum can inhibit the expression of PCNA in RA-HFLS stimulated by LPS (P<0.001 versus the LPS-induced group) (Figure 6B and D), which was not observed in blank serum group. Therefore, these results indicate that PLQT-medicated serum mayinhibit the RAHFLS abnormal proliferation by arresting the cell at the S phase and decreasing the expression of PCNA.
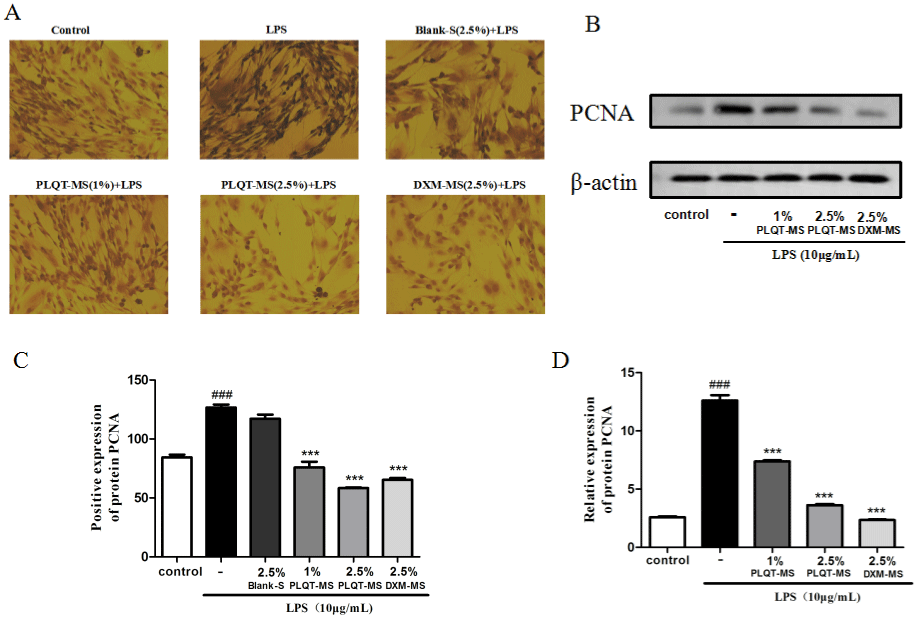
Figure 6: PLQT-medicated serum inhibited the cell cycle-related proteins in RA-HFLS induced by LPS. (A) RA-HFLS were pretreated with 2.5% Blank-serum, 1%
and 2.5% PLQT-medicated serum, and 2.5% DXM-medicated serum for 24h, and then stimulated with 10 μg/mL LPS for 24h. The PCNA expression was measured
by immunohistochemistry. (C) The quantification of PCNA positive expressions in immunohistochemistry. (B) RA-HFLS were pretreated with 1% and 2.5% PLQTmedicated
serum, and 2.5% DXM-medicated serum for 24h, and then stimulated with 10 μg/mL LPS for 24h. The expression of PCNA in RA-HFLS was detected
by Western blot. (D) Normalized quantitative data for PCNA protein expressions. Values are expressed as mean ± S.E.M., n = 3 independent experiments. ###P<
0.001 compared with the control group. ***P< 0.001 compared with the LPS-induced group. Blank-S: Blank-serum; PLQT-MS: Panlongqi Tablet-medicated serum;
DXM-MS: Dexamethasone-medicated serum (n=3).
Effects of PLQT-Medicated Serum on PI3K/AKT and MAPK Signaling Pathways in RA-HFLS Stimulated By LPS
Recent studies have shown that phosphoinositide 3-kinase (PI3K)/ AKT kinase, Extracellular Signal-Regulated Kinases (ERKs), and p38 mitogen-activated protein kinase (P38-MAPK) are associated with RA angiogenesis [19,20]. So the effects of PLQT-medicated serum on PI3K/AKT and MAPK pathways in LPS stimulated RA-HFLS were explored. Results in Fig. 7 showed that after treatment with PLQTmedicated serum and DXM-medicated serum, a significant inhibition of increased p-PI3K, p-AKT, p-mTOR protein levels due to LPSinduced upregulation was achieved (P< 0.001 versus the LPS-induced group) (Figure 7A, B, C, and E). Moreover, the results showed that the expression levels of p-P38, p-ERK1/2 and p-JNK protein in RA-HFLS were significantly up-regulation by LPS (P< 0.001 versus the control group). After PLQT-medicated serum and DXM-medicated serum treatment, p-P38, p-ERK1/2 and p-JNK protein expressions generally showed a downward trend (P< 0.001 versus the LPS-induced group) (Figure 7D, F, G and H). Taken together, these results indicated that PLQT-medicated serum may inhibit the abnormal activation of PI3K/ AKT and MAPK signaling pathways in RA-HFLS stimulated by LPS.
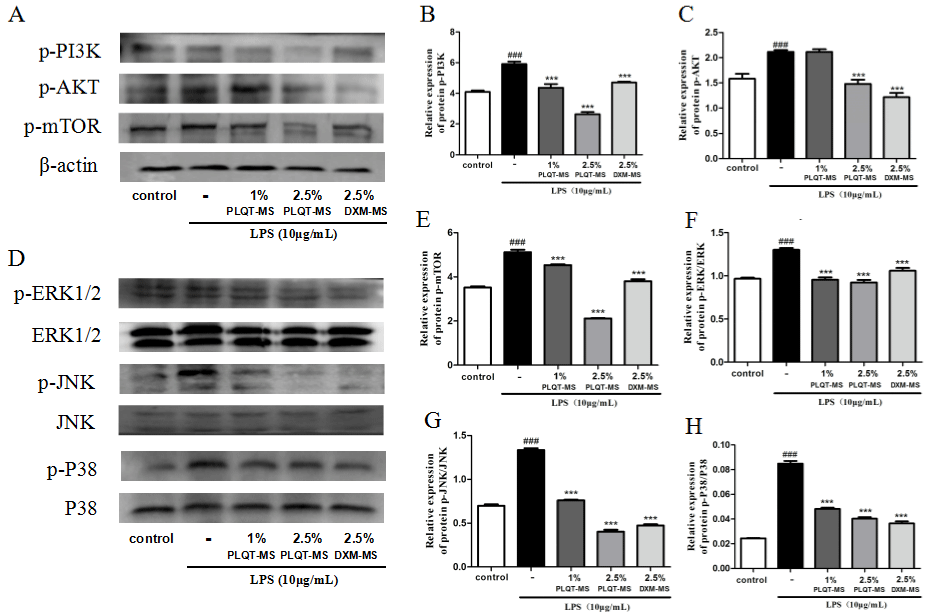
Figure 7: PLQT-medicated serum blocked PI3K/AKT and MAPK signaling pathways induced by LPS in RA-HFLS. RA-HFLS were pretreated with 1% and 2.5%
PLQT-medicated serum, and 2.5% DXM-medicated serum for 24h, and then stimulated with 10 μg/mL LPS for 24h. (A) and (D) Expressions of p-PI3K, p-AKT,
p-mTOR, ERK1/2, p-ERK1/2, JNK, p-JNK, P38 and p-P38 proteins were detected by Western blot. (B), (C), (E), (F), (G) and (H) Standardized quantitative data
of p-PI3K, p-AKT, and p-mTOR protein expression, β-actin was used for standardization, p-ERK1/2, p-JNK, and p-P38 protein expression, ERK1/2, JNK and P38
were used for standardization, respectively. Values are expressed as mean ± S.E.M., n = 3 independent experiments. ###P< 0.001 compared with the control group.
***P< 0.001 compared with the LPS-induced group. Blank-S: Blank-serum; PLQT-MS: Panlongqi Tablet-medicated serum; DXM-MS: Dexamethasone-medicated
serum (n=3).
Discussion
Rheumatoid Arthritis (RA), a systemic autoimmune disease with high morbidity and disability, is influenced by genetic and environmental factors [21]. PLQT, one of Chinese traditional herb compound, has a long history of clinical treatment in China. Clinical trial showed that PLQT was effective in the treatment of RA [18]. It can improve the blood circulation around joints, reduce vascular permeability and promote the absorption of inflammatory exudates [17].
In order to clarify the possible effective components of PLQT in anti-RA, we built a method which can detect 7 components of 29 herbs at the same time in PLQT by LC-MS/MS analysis (Figure 1). Of the 7 compounds (Figure 2), previous studies have shown that (-)-Epicatechin, Salvianolic acid B, Hydroxysafflor yellow A, Gentiopicroside, Imperatorin, Tectoridin, Astragalin had the activity to relieve joint pain and inflammation in RA [22].
There are many factors causing RA, and some cell abnormalities are related to the pathogenesis of RA, such as T cells, B cells and macrophages. Fibroblast-Like Synoviocytes (FLS) are the key effect or cells in RA. Cytokines and proteases are continuously secreted in FLS, leading to inflammation permanence and cartilage destruction. Rheumatoid arthritis FLS has a unique invasive phenotype, which increases the invasiveness to extracellular matrix and further aggravates joint injury [23]. Therefore, a RA-HFLS model was established to investigate the anti-RA effect of PLQT and its mechanism. LPS is a heat-stable endotoxin derived from gram-negative bacteria used to induce inflammation, which can mediate immune responses and cause various biological effects in vitro and in vivo (Lee et al., 2013; Niu et al., 2019). On the other hand, pro-inflammatory cytokines play an important role in RA-HFLS proliferation and survival, which including: TNFa, IL-1β and IL-17 [24].
The immune and inflammatory reactions run through the occurrence and development of RA. It is mainly reflected in the antagonistic balance of anti-inflammatory and pro-inflammatory factors [25]. Over expression of pro-inflammatory factors (IL-1β, IL-6, and IL-17) have been demonstrated to play important roles in synovial infiltration, cartilage degradation and subsequent joint damage [22,26-28]. In our experiment, the levels of IL-1β, IL-6 and IL-17 in RA-HFLS were increased significantly after the stimulation of LPS, which is consistent with previous reports. Whereas administration of PLQT dramatically decreased the levels of IL-1β, IL-6, and IL-17 in RA-HFLS, which indicated that PLQT might play a positive part in inflammatory response, and it is exactly vital for the treatment of RA.
In this study, it is found that RA-HFLS proliferated abnormally after LPS stimulation, which may be related to the release of inflammatory factors. After treatment with PLQT, this phenomenon was significantly alleviated, which indicated that PLQT may inhibit the abnormal proliferation of RA-HFLS by reducing the release of inflammatory factors. Proliferating Cell Nuclear Antigen (PCNA) is closely related to DNA replication, repair, and plays an essential role in the initiation of cell proliferation [29]. Hence, we detected the expression of PCNA by immunohistochemistry and western blot experiments, our results revealed that PLQT reduced the expression level of PCNA, which may be another important reason for the inhibition of RA-HFL Sabnormal proliferation by PLQT. More importantly, the flow cytometry results showed that PLQT may arrest the cell in S phase to inhibit LPS-induced abnormal proliferation.
It was found that the PI3K/AKT signaling pathway plays an important role in the abnormal proliferation and inflammation of FLS cells [24]. The phosphorylation and activation of mTOR are related to PI3K and AKT, which are key upstream molecules [30]. Typically, when the PI3K pathway is activated, Phosphatidylinositol 3 – 5 triphosphate (PIP3) is produced, which transfers inactive AKT from the cytosol to the plasma membrane. AKT was activated by dual phosphorylation of thr308 and ser473. Then activated AKT promotes the phosphorylation of mTOR and its downstream signal molecules for protein translation [31]. In this study, the proteins expression of PI3K/AKT signaling pathway in RA-HFLS was detected by western blot experiment, after LPS stimulation, the contents of p-PI3K, p-AKT and p-mTOR in RA-HFLS were significantly increased. However, after PLQT treatment, the expression levels of these proteins were significantly reduced.
The proteins in MAPK family mainly includes ERK, JNK and p38. Increased phosphorylation of these proteins was observed in human RA synovium tissue, indicating that the occurrence of RA is linked to the increased activity of MAPK pathway [32,33]. Previous study indicated that the proliferation, migration and invasion of FLS might be achieved by activating the ERK-MAPK signaling pathway [10]. In our study, the protein expressions of JNK, P-JNK, ERK, p-ERK, P38 and p-P38 were analyzed and verified. Interestingly, the western blot results showed that P-JNK/JNK, p-ERK/ERK, and p-P38/P38 proteins were actively expressed in RA-HFLS induced by LPS, and the relative expression levels of them in RA-HFLS were down-regulated after treatment with PLQT. These results suggested that PLQT may protect RA-HFLS by inhibiting the abnormal activation of PI3K/AKT and MAPK signaling pathway (Figure 8).
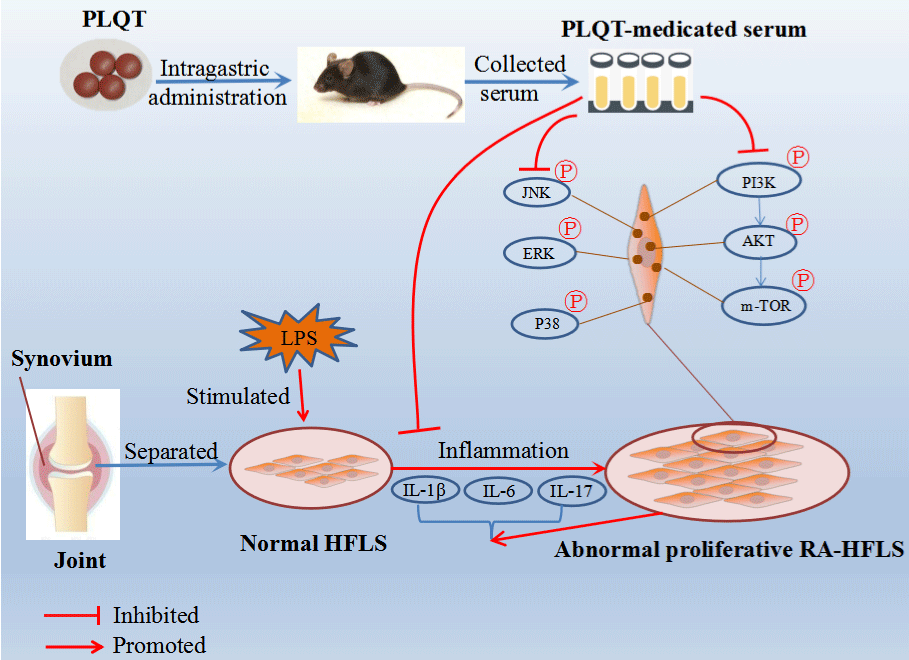
Figure 8: The potential mechanism of action of PLQT in the treatment of RA by inhibiting the PI3K/AKT and MAPK pathways. PLQT could inhibit the abnormal
proliferation of RA-HFLS by reducing the release of IL-1β, IL-6, and IL-17. Furthermore, PLQT could down-regulate the phosphorylation of PI3K, AKT, mTOR, JNK,
ERK, and P38 in RA-HFLS.
Conclusion
In summary, this study showed that PLQT had emerged anti- RA effect on RA-HFLS, and revealed the possible pharmacological mechanism. The anti-RA mechanism of PLQT on RA-HFLS may be related to inhibiting inflammatory response and inhibiting abnormal proliferation of cells by regulating PI3K/AKT and MAPK signaling pathways.
Acknowledgements
This work was supported by Project of Shaanxi Traditional Chinese Medicine Administration (no.: 2019-13), and Local projects under the guidance of the central government of Shaanxi Provincial Department of science and technology (no.: 2021ZY-CG-04) (Shaanxi Province, PR China).
Author Contributions
Data collection: X.F. Niu, Y.J. Yang; design of the study: X.F. Niu, Y.J. Yang, W.F. Li; statistical analysis: Y.J. Yang, J.J. Yu; analysis and interpretation of the data: H.X. Song, Q.X. Huang, Y. Liu; drafting the manuscript: Y.J. Yang; critical revision of the manuscript: J.B. Yu, T.F. Han, D.Z. Zhang.“
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet. 2016; 388: 2023-2038.
- Pan L, Zhang T, Yu M, Shi M, Jia X, Jia X, et al. Bioactive-guided isolation and identification of oligostilbenes as anti-rheumatoid arthritis constituents from the roots of Caragana stenophylla. Journal of ethnopharmacology. 2021; 280: 114134.
- Hu P, Dong Z, Zheng S, Guan X, Zhang L, Li L, et al. The effects of miR- 26b-5p on fibroblast-like synovial cells in rheumatoid arthritis (RA-FLS) via targeting EZH2. Tissue & cell. 2021; 72: 101591.
- Ahsan H, Irfan HM, Alamgeer, Jahan S, Shahzad M, Asim MH. Potential of ephedrine to suppress the gene expression of TNF-a, IL-1β, IL-6 and PGE2: A novel approach towards management of rheumatoid arthritis. Life sciences. 2021; 282: 119825.
- Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL- 22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine. 2015; 74: 101-107.
- Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR Pathway – Beyond Rapalogs. Oncotarget. 2010; 1: 530-543.
- Lv Q, Zhu X, Xia Y, Dai Y, Wei Z. Tetrandrine inhibits migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes through down-regulating the expressions of Rac1, Cdc42, and RhoA GTPases and activation of the PI3K/Akt and JNK signaling pathways. Chinese journal of natural medicines. 2015; 13: 831-841.
- Dulos J, Wijnands FPG, Alebeek JAJVDH, Vugt MJHV, Rullmann JAC, Schot JG, et al. p38 inhibition and not MK2 inhibition enhances the secretion of chemokines from TNF-a activated rheumatoid arthritis fibroblast-like synoviocytes. Clinical and experimental rheumatology. 2013; 31: 515-25.
- Zeng S, Wang K, Huang M, Qiu Q, Xiao Y, Shi M, et al. Halofuginone inhibits TNF-a-induced the migration and proliferation of fibroblast-like synoviocytes from rheumatoid arthritis patients. Int Immunopharmacol. 2017; 43: 187-194.
- Guo X, Zhang D, Zhang X, Jiang J, Xue P, Wu C, et al. Dyrk1A promotes the proliferation, migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis via down-regulating Spry2 and activating the ERK MAPK pathway. Tissue & cell. 2018; 55: 63-70.
- Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. The Lancet. 2017; 389: 2338-2348.
- Svanström H, Lund M, Melbye M, Pasternak B. Concomitant use of low-dose methotrexate and NSAIDs and the risk of serious adverse events among patients with rheumatoid arthritis. Pharmacoepidemiology and Drug Safety. 2018; 27: 885-893.
- Wang L, Xu Y, Liu C, Han T, Cui R, Lin N. Study on Compatibility Rationality of Panlongqi Tablets in Treatment of Osteoarthritis Based on Network Pharmacology. Chinese journal of Experimental Traditional Medical Formulae. 2020; 26: 166-74.
- Gong Q. Pharmacological effect and clinical application of Panlongqi Tablet (in Chinese). China Foreign Medical Treatment. 2009; 28: 167.
- Lin P, Zhou X, Gao W, Ji Y, Feng J, Li J. Panlongqi tablets protect synovial tissue in rats with adjuvant arthritis by regulating Toll-like receptor 4-myeloid differentiation factor 88-nuclear factor-κB pathway (in Chinese). Anhui Medical and Pharmaceutical Journal. 2021; 25: 1713-1717.
- Luo Q, Ji H, Cao X, Lei P. The clinical research of Panlongqi Tablets treat rheumatoid arthritis with cold dampness blocking collaterals with 43 cases (in Chinese). Shanxi Journal of Traditional Chinese Medicine. 2007: 1341-1343.
- Zhang X, Shi F, Ji H. The clinical research of Panlongqi Tablets treat rheumatoid arthritis with 60 cases (in Chinese). Modern Traditional Chinese Medicine. 2011; 31: 41-42.
- Ju Z, Xu Y. Clinical study on Panlongqi Tablets treat rheumatoid arthritis combined with celecoxib (in Chinese). Drugs & Clinic. 2018; 33: 3011-5.
- Kumar P, Amin MA, Harlow LA, Polverini PJ, Koch AE. Src and phosphatidylinositol 3-kinase mediate soluble E-selectin-induced angiogenesis. Blood. 2003; 101: 3960-3968.
- Balogh E, Biniecka M, Fearon U, Veale DJ, Szekanecz Z. Angiogenesis in Inflammatory Arthritis. The Israel Medical Association journal: IMAJ. 2019; 5: 345-352.
- Xu N, Wang Y, Zhao S, Jiao T, Xue H, Shan F, et al. Naltrexone (NTX) relieves inflammation in the collagen-induced- arthritis (CIA) rat models through regulating TLR4/NFκB signaling pathway. International immunopharmacology. 2019; 79: 106056.
- Wang Y, Chen S, Du K, Liang C, Wang S, Evans OB, et al. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. Journal of ethnopharmacology. 2021; 279: 114368.
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunological Reviews. 2010; 233: 233-255.
- Dinesh P, Rasool M. Berberine inhibits IL-21/IL-21R mediated inflammatory proliferation of fibroblast-like synoviocytes through the attenuation of PI3K/ Akt signaling pathway and ameliorates IL-21 mediated osteoclastogenesis. Cytokine. 2018; 106: 54-66.
- Liu C, Zhao Q, Zhong L, Li Q, Li R, Li S, et al. Tibetan medicine Ershiwuwei Lvxue Pill attenuates collagen-induced arthritis via inhibition of JAK2/STAT3 signaling pathway. Journal of ethnopharmacology. 2021; 270: 113820.
- Tu Y, Wang K, Liang Y, Jia X, Wang L, Wan J, et al. Glycine tabacina ethanol extract ameliorates collagen-induced arthritis in rats via inhibiting proinflammatory cytokines and oxidation. Journal of ethnopharmacology. 2019; 237: 20-27.
- Kamel KM, Gad AM, Mansour SM, Safar MM, Fawzy HM. Venlafaxine alleviates complete Freund’s adjuvant-induced arthritis in rats: Modulation of STAT-3/IL-17/RANKL axis. Life Sci. 2019; 226: 68-76.
- Zhang X, Yuan Y, Pan Z, Ma Y, Wu M, Yang J, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: A meta-analysis. Clinica chimica acta; international journal of clinical chemistry. 2019; 496: 76-83.
- Moradi-Ozarlou M, Moshari S, Agdam HR, Nomanzadeh A, Shahmohamadlou S, Razi M. High-fat diet-induced obesity amplifies HSP70-2a and HSP90 expression in testicular tissue; correlation with proliferating cell nuclear antigen (PCNA). Life sciences. 2021; 279: 119633.
- Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012; 60: 38- 42.
- Lizcano JM, Alessi DR. The insulin signalling pathway. Current Biology. 2002; 12: R236-R238.
- Nygaard G, Paolo JAD, Hammaker D, Boyle DL, Budas G, Notte GT, et al. Regulation and function of apoptosis signal-regulating kinase 1 in rheumatoid arthritis. Biochemical Pharmacology. 2018; 151: 282-290.
- Han Z, Boyle DL, Aupperle KR, Bennett B, Manning AM, Firestein GS. Jun N-terminal kinase in rheumatoid arthritis. The Journal of pharmacology and experimental therapeutics. 1999; 291: 124-30.