
Case Report
Austin J Surg. 2022; 9(2): 1290.
Solid Pseudopapillary Tumor of the Pancreas with Node Invasion: What to do? A Case Report and Literature Review
Sekkat H1,2*, Moufid A1,2, Nadouri J1,2, Mesbahi OE4, Rimani M3, Bakali Y1,2, Alaoui MM1,2, Sabbah F1,2, Hrora A1,2 and Raiss M1,2
1Digestive Surgical Department C, Ibn Sina University Hospital, Morocco
2Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco
3Hassan Pathology Center, Rabat, Morocco
4Nakhil Oncology Center, Rabat, Morocco
*Corresponding author: Hamza Sekkat, Digestive Surgical Department C, Ibn Sina University Hospital. Rabat, Morocco
Received: August 17, 2022; Accepted: September 13, 2022; Published: September 20, 2022
Abstract
Solid Pseudopapillary Neoplasms (SPN) of the pancreas are rare neoplasms representing 1-2% of all pancreatic tumors, and considered as low-grade malignancies. This pathology mainly affects young women. Its prognosis is usually excellent when the tumor is limited to the pancreas, with a cure rate greater than 95% after a complete surgical resection. Preoperative diagnosis is always difficult. SPNs can be metastatic. Hepatic and lymph node localizations are the most reported in the literature. The recurrence rate after surgical resection is 3-9%. We report the case of a 36-year-old patient who was complaining of abdominal pain for 2 months. An abdominal contrast-enhanced Computed Tomography (CT) scan showed a solido-cystic mass of the tail of the pancreas.
The patient underwent laparoscopic radical antegrade modular pancreatosplenectomy. The diagnosis of SPN with lymph node metastasis was confirmed by histopathology and immunohistochemistry, requiring adjuvant chemotherapy.
Keywords: Solid pseudopapillary tumor; Laparoscopy; Neoplasm; Node invasion; Recurrence; Pancreas
Introduction
The pseudopapillary tumor of the pancreas is an extremely rare exocrine tumor [1], representing only 1 to 2% of exocrine tumors of the pancreas, and 5% of cystic pancreatic tumors [2]. It was first described by Frantz in 1959 [3], then classified by the World Health Organization (WHO) in 1996 as a borderline tumor, called SPN (Solid pseudopapillary neoplasms) [43]. It generally affects young women, [5]. The body and tail of the pancreas are the most common sites of SPN [6]. The majority of SPN have a low-grade malignancy, with a good prognosis [7]. Most patients have localized tumors, and only 9-15% are being locally advanced or metastatic [3]. Due to the rarity of the tumor, there are no well-defined recommendations regarding the extent of tumor resection, lymph node dissection or management of metastasis [2].
We report a case of young woman with a Frantz tumor of the pancreatic tail, treated laparoscopically with lymph node metastasis in the pathological study. We discuss the appropriate therapeutic strategy and we report a literature review.
Case Report
A thirty six years old woman appeared in consultation for atypical pain in the left upper quadrant for 2 months. No not other associated digestive or extra-digestive signs were reported . The patient had no medical history otherwise and the physical examination was normal.
Abdominal ultrasound and an injected CT scan showed a tissue nodular formation adhering to the tail of the pancreas measuring 41 x 45mm, that respects the spleen and the left kidney, suspecting a SPN in the first place. No locoregional lymphadenopathy or ascites were identified (Figure 1). Tumor markers were normal.
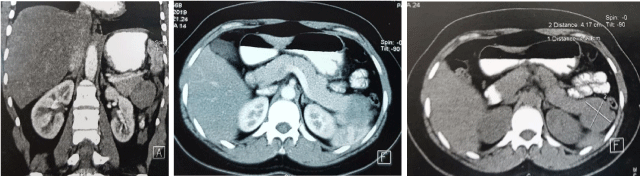
Figure 1: Abdomen CT scans showing a well-defined; tissue nodular formation adhered to the pancreas tail measuring 41X45 mm.
The patient was operated on laparoscopically. Exploration found a mass measuring 4 cm, developed on the inferior border of the pancreas tail with contact to splenic hilum (Figure 2).
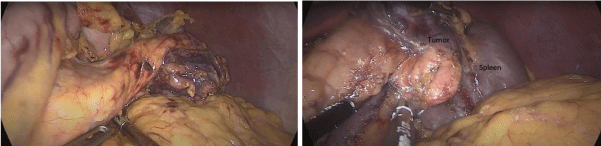
Figure 2: Intraoperative pictures. A: A preoperative picture of the tumor after releasing the pancreas tail. B: A preoperative picture showing the tumor adhering to
the spleen.
In order to preserve the spleen, a splenic vessels liberation of the the pancreas was carried out, but suspicion of splenic invasion forced us to perform an associated splenectomy.
The specimen was extracted into a bag through a small pfannenstiel-type incision.
The postoperative period passed smoothly and the patient was discharged after four days.
The diagnosis of a solid tumor pseudopapillary pancreas was confirmed by histological study. One nine nodes of the splenic hilum dissection was metastatic. The tumor was classified pT3N1 (Figure 3).
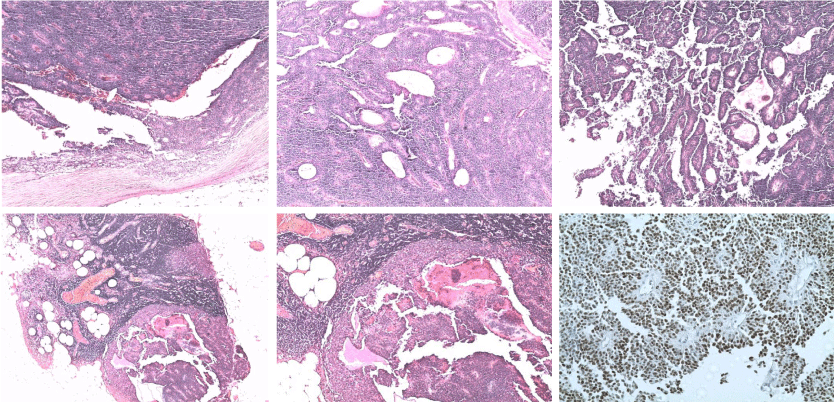
Figure 3: Histopathological examination of the surgical specimen. A, B: Hematoxylin Eosin Safran (HES Coloration): Solid and cystic tumor. C: Papillary and
pseudopapillary architecture. D, E: Metastatic lymph node. F: Immunohistochemistry: Progesterone (+): Intense and diffuse nuclear expression.
Adjuvant chemotherapy was decided in a multidisciplinary meeting. The patient received 6 chemotherapy sessions, based on 5-fluoro-uracile, Irinotecan et Oxaliplatin. A Close follow-up was initiated for this patient. At 40 months after surgery, the patient is still alive, and has not shown any recurrence.
Discussion
The solid pseudopapillary tumor of the pancreas is a rare tumor that accounts for less than 2% of exocrine tumors of the pancreas [1]. It more frequently affects females between 20 and 30 years old. The rapid progression of the disease during pregnancy prompted the idea to study the relationship between SPN and women sex hormones [8]. It generally has a low malignant potential, but in males, the course is fatal and the prognosis is worse [9].
SPN went unrecognized for a long time. Up to the year 1999, only 334 cases were reported in the literature [10]. The more frequent use of CT and MR imaging, and the better knowledge of the pathology during the last two decades, has allowed the continuous diagnosis of this kind of tumor [11]. However, and despite this development, and technological revolution, the diagnosis of SPN is not always obvious. The non-specificity of the symptoms, [3] the similarity of imaging results between the cystic lesions [12], and the non-existence of specific markers of this tumor [13] always poses a problem of preoperative diagnosis. Some teams propose a preoperative radioguided biopsy for histological proof before surgical procedure, but this attitude is avoided by several others because of possible propagation risk of the tumor [14].
Of all laparoscopic pancreatic resections, distal pancreatectomy is the most commonly performed [10]. Radical Antegrade Modular Pancreatosplenectomy (RAMPS) is the procedure of choice for body and tail of the pancreas tumor resections. Although this technique has not shown superiority over the Retrograde Pancreatosplenectomy standard in terms of survival rate and recurrence-free survival, it allows to achieve high rates of a negative margin and resected metastatic lymph nodes [15,16]. Laparoscopic pancreatectomies for SPN frequency remains low, even if the procedure is considered relatively simple given that it does not require complex reconstructions of the digestive tract or anastomosis [3,17,18]. A series of studies have demonstrated the benefit of laparoscopic distal pancreatectomy over laparotomy. There are no significant differences in postoperative morbidity and mortality. Resection margins, lymph node yield and long-term survival are also the same [12]. However, laparoscopy allows postoperative pain reduction and therefore a lower need for analgesics, a shorter hospital stay and a faster return to normal activity. It also causes less wound complications, blood loss, and shorten operative time [19,20]. Furthermore, better aesthetic results are observed [21].
SPN exceptionally gives rise to metastases. The most common metastatic sites are liver and lymph nodes [12]. Several studies with the number of patients varying between 03 and 115 patients reported no cases of lymph node invasion after resection of a papillary pseudo solid tumor of the pancreas [2,6,8,10,22,23]. Larger series or literature reviews have reported lymphatic invasion rates varying between 0.5 and 15 percent [1,3,24-28] Yao et al in his study which contained 2450 cases cited in English and Chinese literature before 2020, found a percentage of lymph node metastases of 0.4% (12 patients) [29].
Lymph node metastases are considered as a risk factor for recurrence, and are one of the criteria for malignancy of SPNs [25,26].
However, the need for lymphadenectomy remains a controversial problem. Several authors consider it unnecessary to perform lymph node dissection associated with curative resection for a pseudo-solid tumor of the pancreas [2,23,30], while others strongly recommend it, as patients who have had a lymph dissection have a better prognosis [25,31].
There is no consensus neither on the adjuvant treatment protocol, in case of a solid pseudopapillary tumor of the pancreas with a high malignancy potential. The role of chemotherapy or radiotherapy remains vague and their effectiveness is poorly understood [32]. Several chemotherapy protocols have been used on different patients, with some good results [33]. Chemotherapy based on 5-fluorouracil and gemcitabine is the most used for a solid pseudopapillary malignant tumor [4,34]. This same treatment was a failure for Tajima in India, and the progression of liver metastases under chemotherapy prompted the team to propose Hepatic Arterial Infusion (HAI) chemotherapy, which was more effective [35]. Shimizu et al also reported a case that was sensitive to gemcitabine, docetaxel, paclitaxel, epirubicin, and mitomycin C [36], and it is quite the opposite of what Matsuda described in his case report, or this protocol was not very useful [37]. Shunrong Ji’s patient received 2 cycles of floxuridine and oxaliplatin after liver metastasis [33]. Other molecules like tamoxifen, cisplatin, ifosfamide, etoposide, and vincristine have been described by other teams [34,38,39], as well as several therapeutic methods, such as radiotherapy, transcatheter arterial chemoembolization, selective internal radiotherapy or even liver transplantation [4] In our case, the use of 5-fluoro-uracile, Irinotecan et Oxaliplatine, had a very good result, and our patient showed no recurrence 3 years and half after the surgery.
Conclusion
Solid pseudopapillary tumors of the pancreas are rare tumors with low potential for malignancy. Metastasis appearance, hepatic or lymph node in the majority of cases, impairs the prognosis. Surgery remains the treatment of choice for this type of tumor, but the lack of consensus on what to do in metastatic cases, should push us to carry out multicenter studies on an international scale in order to find the best way to take charge of these patients especially since it is a pathology that affects young healthy subjects.
Data Availability Statement
The data that support the findings of this article are available from the corresponding author upon reasonable request.
Competing Interests
The authors have no conflicts of interest and source of funding. The subject of the article had no commercial interest, no financial or material support.
Ethics Statement
Drs Hamza Sekkat, Abdellah Moufid, Jaouad Nadour, Omar El Mesbahi, Mouna Rimani, Younes Bakali , Mouna Mhamdi Alaoui, Farid Sabbah, Abdelmalek Hrora , Mohammed Raiss declare no conflict of interest.
References
- Tang X, Zhang J, Che X, Chen Y, Wang C. Peripancreatic lymphadenopathy on preoperative radiologic images predicts malignancy in pancreatic solid pseudopapillary neoplasm. Int J Clin Exp Med. 2015; 8: 16315-16321.
- Zhang C, Liu F, Chang H, Li H, Zhou X, et al. Less Aggressive Surgical Procedure for Treatment of Solid Pseudopapillary Tumor: Limited Experience from a Single Institute. PLoS One. 2015; 10: e0143452.
- Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014; 43: 331-337.
- You L, Yang F, Fu DL. Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas. World J Gastrointest Oncol. 2018; 10: 184-193.
- Al Qattan AS, Alshaqaq HM, Al Abdrabalnabi AA, Alnamlah M, Alanazi AA, Alqahtani MS. Huge solid pseudopapillary tumor of the pancreas “Frantz tumor”: a case report. J Gastrointest Oncol. 2020; 11: 1098-1104.
- Song H, Dong M, Zhou J, Sheng W, Zhong B, Gao W. Solid Pseudopapillary Neoplasm of the Pancreas: Clinicopathologic Feature, Risk Factors of Malignancy, and Survival Analysis of 53 Cases from a Single Center. Biomed Res Int. 2017; 2017: 5465261.
- Hao EIU, Hwang HK, Yoon DS, Lee WJ, Kang CM. Aggressiveness of solid pseudopapillary neoplasm of the pancreas: A literature review and metaanalysis. Medicine. 2018; 97: e13147.
- Torres OJM, Rezende MB de, Waechter FL, Neiva RF, Moraes-Junior JM, et al. Pancreatoduodenectomy for Solid Pseudopapillary Tumor of the Pancreas: A Multi-institution Study. Arq Bras Cir Dig. 2019; 32: e1442.
- Lin MYC, Stabile BE. Solid pseudopapillary neoplasm of the pancreas: a rare and atypically aggressive disease among male patients. Am Surg. 2010; 76: 1075-1078.
- Coelho JCU, da Costa MAR, Ramos EJB, Torres AR, Savio MC, Claus CMP. Surgical Management of Solid Pseudopapillary Tumor of the Pancreas. JSLS. 2018; 22: e2018.00032.
- Tan HL, Tan EK, Teo JY, Kam JH, Lee SY, et al. Outcome of minimallyinvasive versus open pancreatectomies for solid pseudopapillary neoplasms of the pancreas: A 2:1 matched case-control study. Ann Hepatobiliary Pancreat Surg. 2019; 23: 252-257.
- Yagci A, Yakan S, Coskun A, Erkan N, Yildirum M, et al. Diagnosis and treatment of solid pseudopapillary tumor of the pancreas: experience of one single institution from Turkey. World J Surg Oncol. 2013; 11: 308.
- Chen H, Huang Y, Yang N, Yan W, Yang R, et al. Solid-Pseudopapillary Neoplasm of the Pancreas: A 63-Case Analysis of Clinicopathologic and Immunohistochemical Features and Risk Factors of Malignancy. Cancer Manag Res. 2021; 13: 3335-3343.
- Bardales RH, Centeno B, Mallery JS, Lai R, Pochapin M, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solidpseudopapillary tumor of the pancreas: a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol. 2004; 121: 654- 662.
- Yin J, Huang XM, Lu ZP, Zhang K, Wu PF, et al. Comparison of radical antegrade modular pancreatosplenectomy with conventional distal pancreatectomy for pancreatic adenocarcinoma of the body and tail. Zhonghua Wai Ke Za Zhi. 2020; 58: 505-511.
- Kim HS, Hong TH, You YK, Park JS, Yoon DS. Radical antegrade modular pancreatosplenectomy (RAMPS) versus conventional distal pancreatectomy for left-sided pancreatic cancer: findings of a multicenter, retrospective, propensity score matching study. Surg Today. 2021; 51: 1775-1786.
- Cavallini A, Butturini G, Daskalaki D, Salvia R, Melotti G, et al. Laparoscopic pancreatectomy for solid pseudo-papillary tumors of the pancreas is a suitable technique; our experience with long-term follow-up and review of the literature. Ann Surg Oncol. 2011; 18: 352-357.
- Soares-Junior C, Gomes CA, Peixoto R de O, Gomes CC, Juste LA. Spleenpreserving distal pancreatectomy in the management of solid papillary-cystic tumor of the pancreas: case report and literature review. Arq Bras Cir Dig. 2010; 23: 206-208.
- Iacobone M, Citton M, Nitti D. Laparoscopic distal pancreatectomy: up-todate and literature review. World J Gastroenterol. 2012; 18: 5329-5337.
- Senthilnathan P, Dhaker KC, Kaje V, Babu Naidu S, Sarvani M, et al. Laparoscopic management of solid pseudo papillary neoplasm of pancreas in tertiary care center from south India. Pancreatology. 2017; 17: 927-930.
- Lee SY, Allen PJ, Sadot E, D’Angelica MI, DeMatteo RP, et al. Distal pancreatectomy: a single institution’s experience in open, laparoscopic, and robotic approaches. J Am Coll Surg. 2015; 220: 18-27.
- Cai Y, Ran X, Xie S, Wang X, Peng B, et al. Surgical management and longterm follow-up of solid pseudopapillary tumor of pancreas: a large series from a single institution. J Gastrointest Surg. 2014; 18: 935-940.
- Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006; 93: 733-737.
- Yu PF, Hu ZH, Wang XB, et al. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010; 16: 1209-1214.
- Wu J, Mao Y, Jiang Y, Song Y, Yu P, et al. Sex differences in solid pseudopapillary neoplasm of the pancreas: A population-based study. Cancer Med. 2020; 9: 6030-6041.
- Lee G, Sung YN, Kim SJ, Lee JH, Song KB, et al. Large tumor size, lymphovascular invasion, and synchronous metastasis are associated with the recurrence of solid pseudopapillary neoplasms of the pancreas. HPB. 2021; 23: 220-230.
- Estrella JS, Li L, Rashid A, Wang H, Katz MH, et al. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol. 2014; 38: 147-157.
- Zhang H, Wang W, Yu S, Xiao Y, Chen J. The prognosis and clinical characteristics of advanced (malignant) solid pseudopapillary neoplasm of the pancreas. Tumour Biol. 2016; 37: 5347-5353.
- Yao J, Song H. A Review of Clinicopathological Characteristics and Treatment of Solid Pseudopapillary Tumor of the Pancreas with 2450 Cases in Chinese Population. Biomed Res Int. 2020; 2020: 2829647.
- Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000; 17: 66-80.
- Kim MJ, Choi DW, Choi SH, Heo JS, Sung JY. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg. 2014; 101: 1266-1271.
- Wu H, Huang YF, Liu XH, Xu MH. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: Case report. World J Gastrointest Oncol. 2017; 9: 497-501.
- Ji S, Xu J, Zhang B, Xu Y, Liu C, Long J, et al. Management of a malignant case of solid pseudopapillary tumor of pancreas: a case report and literature review. Pancreas. 2012; 41: 1336-1340.
- Brunetti O, Aprile G, Marchetti P, Vasile E, Gardini AC, et al. Systemic Chemotherapy for Advanced Rare Pancreatic Histotype Tumors: A Retrospective Multicenter Analysis. Pancreas. 2018; 47: 759-771.
- Tajima H, Takamura H, Kitagawa H, Nakayama A, Shoji M, et al. Multiple liver metastases of pancreatic solid pseudopapillary tumor treated with resection following chemotherapy and transcatheter arterial embolization: A case report. Oncol Lett. 2015; 9: 1733-1738.
- Shimizu T, Murata S, Mekata E, Miyake T, Abe H, et al. Clinical potential of an antitumor drug sensitivity test and diffusion-weighted MRI in a patient with a recurrent solid pseudopapillary tumor of the pancreas. J Gastroenterol. 2007; 42: 918-922.
- Matsuda Y, Imai Y, Kawata S, Nishikawa M, Miyoshi S, et al. Papillary-cystic neoplasm of the pancreas with multiple hepatic metastases: a case report. Gastroenterol Jpn. 1987; 22: 379-384.
- Sclafani LM, Reuter VE, Coit DG, Brennan MF. The malignant nature of papillary and cystic neoplasm of the pancreas. Cancer. 1991; 68: 153-158.
- Hah JO, Park WK, Lee NH, Choi JH. Preoperative chemotherapy and intraoperative radiofrequency ablation for unresectable solid pseudopapillary tumor of the pancreas. J Pediatr Hematol Oncol. 2007; 29: 851-853.