Abstract
Background: Lip cancer is a malignancy that results from the autonomous and uncontrolled cell growth in the lips. Lip cancer is a part of head and neck cancers and may occur together with oral cancer or may be one symptom of oral cancer. Lip cancers come in the form of squamous cell carcinoma with abnormal growth of the flat cells on the lips.
Aim of the Work: To evaluate the relation and impact of the depth of invasion in mm of tumor to number of lymph nodes infiltrated.
Material and Methods: 42 patients had lip cancer proved by biopsy or clinically to be malignant, undergone wide excision with safety margin with reconstruction either by primary repair or by loco regional flap with cervical nodes block dissection to evaluate the positive nodes number in relation to the depth of tumor.
Results: Patients under went excision of lesion with lymph nodes with the increase in number of nodes infiltrated in proportion to the depth of invasion with increase recurrence rate irrespective to age of patients.
Conclusion: Depth of tumor and number of lymph nodes infiltrated are important prognostic factors in lip cancer.
Keywords: Squamous Cell Carcinoma; Cancer Lip; Depth of Invasion; Cervical Lymph Node Metastases
Abbreviations
SCC: Squamous Cell Carcinoma; LN: Lymph Node; LVI: Lymph Vascular Invasion; PNI: Peri Neural Invasion; cN0: Clinically Negative Cervical Nodes; LNM: Lymph Node Metastases; LLNM: Late Lymph Node Metastases; END: Elective Neck Dissection
Introduction
Lip cancer is a disease in which malignant (cancer) cells form in the lips. Tobacco and alcohol use can affect the risk of lip cancer. Cancers related to the oral cavity and lip are a major public health issue everywhere, with tobacco and betel chewing being the significant risk factors specially in India. Approximately, 77,000 new cases and 52,000 deaths are reported annually [1]. Squamous Cell Carcinomas (SCCs) encompass at least 90% of all oral malignancies [2]. With the World Health Organization expecting a worldwide increase in oral SCC incidence in the next decade, [2] oral cancer, predominantly SCC, is the major malignancy in India and South East Asia, accounting for up to 50% of all cancers [3]. The overall 5-year survival rate of oral cancers, including all the stages, has shown little improvement over the past several decades, ranging around 50% [4,5]. Manifestations of lip cancer include a sore or lump or ulcer on the lips. Tests that examine the mouth and throat are used to detect diagnosis and staging of lip cancer. Most lip and oral cavity cancers start in squamous cells, the thin flat cells lining the inside of the lips and oral cavity. These are called squamous cell carcinomas. Lip Cancer cells may spread into deeper tissue. Lip cancer account s up to 15% of oral cancers in origin. Lip cancer is responsible for about 4,000 cases of oral cancer yearly.
The prognosis of Squamous Cell Carcinoma (SCC) of the lip is good if early diagnosis and adequate treatment is established, with a mean survival rate at 5 years of 90% [6], the most important prognostic factor of survival for these patients is the cervical Lymph Node Metastasis (LNM). The frequency of LNM in lip SCC ranges from 6 to 37% [7-11] and only 25%-50% of these patients are still alive after 5 years [9,12,13]. Therefore, it is important to detect patients with high risk of occult LNM to prevent an unfavorable clinical evolution.
Many prognostic factors for LNM in lip SCC such as tumor size; grading of differentiation; vascular invasion, depth of invasion; perineural invasion; mitotic activity, mode of tumor invasion and positive surgical margins [7-19], also However, the criteria for Elective Neck Dissection (END) according to predictive factors are still controversial. There are studies indicating END in almost all cN0 patients [20,21] whereas others suggest a “wait and see” management and neck dissection only when the neck relapses [22-25].
Tumor thickness is a relatively new prognostic factor that has been investigated for lower lip cancer. Prognostic significance of tumor thickness was first shown by Breslow [26] and Clark et al [27] in malignant melanoma of the skin. Later, tumor thickness was demonstrated to be a factor prognostically related in cancer of the colon, cervix, rectum, tongue, floor of the mouth, soft palate, and oropharynx. Frierson and Cooper [28] were first to prove the prognostic importance of thickness in cancer of the lower lip. This study was conducted to investigate whether tumor thickness could be used as a predictor of neck metastasis in squamous cell carcinoma of the lower lip.
This study was a retrospective analysis of the clinic pathologic factors related to Late Lymph Node Metastasis (LLNM) occurrence and to identify patients at risk, to improve neck management and prognostic outcomes in stage I and II SCC of the lip.
Patients and Methods
Retrospective study designed in Surgical oncology department, Tanta Cancer Center and General surgery department, Faculty of medicine, Fayoum university as tertiary centers for cancer lip in between start of 2015 to the end of 2018, 42 cases were diagnosed for lip cancer including lower lip, commissure and upper lip with Squamous Cell Carcinoma (SCC) and examined for the age and gender of patients, site of lesion, size, biopsy taken and method of diagnosis with the detection of cervical Lymph nodes status both clinically and radio logically and also pathologically in some cases.
Before going into surgery all cases had US of neck and sometimes CT scan of neck and mandible to detect local infiltration or distant metastases together with full laboratory investigations including full blood picture, kidney and Liver functions and coagulation profile to detect any bleeding tendency.
All harvested specimens had examined for the mitotic activity, degree of differentiation, and depth of invasion in millimeters (mm) and the number of LNs dissected with number of infiltrated LNs and capsular invasion then the patients followed up for detection of local or nodal recurrence and complications with the methods of treatment (Figure 1,2).
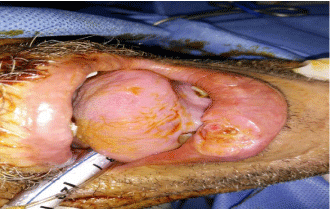
Figure 1: Lesion in lower lip proved by pathology to be squamous cell
carcinoma GII.
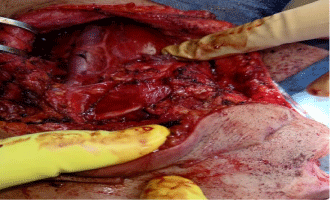
Figure 2: Neck dissection in a case of lower lip squamous cell carcinoma.
Results
42 cases of cancer lip were diagnosed in Tanta cancer center surgical oncology department and General surgery department, faculty of medicine, Fayoum university, EGYPT, in a period between start of 2014 and end of 2018, all cases came to examination complaining of ulcer either in upper or lower lip resisting to medical treatment with dermatologists and suspected to have malignant ulcers.
All cases examined locally and diagnosed clinically as cancer lip then approved by pathological confirmation to be squamous cell carcinoma and proceed to investigations for metastases detection and decided for surgical interference as excision with safety margin and lip reconstruction according to the lesion site with one case had hemi mandibulectomy and another one had maxillectomy due to infiltrative aggressive lesion.
During examination, the mean age was 58.5yars old (ranged from 40-77years), there was 33/42 (78.57%) male patients and 9/42 (21.43%) female patients.
9/42(21.43%) located in upper lip and 30/42(71.43%) located at lower lip with 3/42(7.14%) located at commissure, of them 18/42 (42.86%) was on Right side and 18/42(42.86%) on left side and 6/42 (14.28%) located on the middle of lip.
Pathological results
All cases had pathological diagnosis 15/42(35.72%) diagnosed by punch biopsy, 6/42(14.28%) diagnosed by incisional biopsy and 21/42(50%) proceeded for excisional biopsy direct with all cases approved to be squamous cell carcinoma GII of lip.
Different sizes for the lesion was detected ranging from least size was 1.5cm *1cm to largest one was 6.5cm *2.5cm, with the neck evaluation by clinical examination for groups of neck nodes and detect positive nodes in 15/42(35.71%) patients that approved by Ultrasound neck or CT scan for neck in some cases with 27/42 (64.29%) was node negative on clinical examination of neck nodal groups.
All specimens of lip lesions was examined and confirmed as squamous cell carcinoma GII with focus examination on depth of invasion of the tumor through the tissue and layers of lip that measured in millimeters with counting the number of Lymph Nodes (LNs) harvested during dissection with the tumor excision and examined for number of infiltrated nodes together with capsular invasion, size of node infiltrated and also the presence of Lymph Vascular Invasion (LVI) or Per Neural Invasion (PNI) with examination of the least safety margin and largest margin during evaluation of the tumor for safety to detect the behavior of the lesion and factors affecting possibility of recurrence.
Study detected least safety margin as 0.2cm with the largest was 3cm safety and had 6/42 (14.28%) cases detected positive safety margin that required for wider excision to obtain negative margin, with depth of invasion in the tumor ranging from 1mm to 15mm for different cases, number of LNs dissected in cases was 2-41 LNs and number of LNs infiltrated was 0-9 nodes infiltrated, with no PNI or LVI detected in any case of the study while capsular invasion was detected in 3/42 (7.14%) cases of the study, 6/42 (14.28%) was admitted to have post-operative radiotherapy as an adjuvant therapy.
During follow up of cases after surgery, Local recurrence was detected in 9/42(21.42%) while distant recurrence detected in 6/42 (14.28%) and 3/42(7.14%) had both local and distant recurrence, 4/42(9.5%) cases was complicated by 2ry infection and abscess formation and 2 cases had wound dehiscence and all of them was conservatively managed with good results with another one case developed cancer head of pancreas that approved pathologically (Figure 3 and Table 1).
Characteristics
Case (n=42)
Lymph node metastasis
Yes (%)
No (%)
Gender
Male
33
12(36.36)
21(63.63)
Female
9
6(66.66)
3(33.33)
Age
<60
18
12(66.66)
6(33.33)
=60
24
15(62.5)
9(37.5)
Primary site
Upper lip
9
6(66.66)
3(33.33)
Lower lip
30
21(70)
9(30)
Commissure
3
0(0)
3(100)
Rt. side lip
18
9(50)
9(50)
Lt. side lip
18
12(66.66)
6(33.33)
Middle
6
3(50)
3(50)
T stage
T1
12
2(16.6)
10(83.4)
T2
22
4(18)
18(82)
T3
5
1(20)
4(80)
T4
3
1(33)
2(66)
Depth of invasion
<4mm
12
0(0)
12(100)
=4mm
30
24(80)
6(20)
Growth type
Exogenesis
6
3(50)
3(50)
Ulcerative
30
18(60)
12(40)
Infiltrating
6
6(100)
0(0)
Differentiation
Well
0
0
0
Medium
42
27(64.3)
15(35.7)
Poor
0
0
0
Capsular invasion
Yes
3
3(100)
0
No
39
24(61.5)
15(38.5)
Perivascular or /and perineural invasion
No
42
27(64.3)
15(35.7)
Yes
0
0
0
TNM stage
I
3
0(0)
3(100)
II
27
21(77.77)
6(22.22)
III
9
7(77.77)
2(22.22)
IVA
3
3(100)
0
Table 1: Analysis of risk factors of the cervical lymph node metastasis in SCC lip patients.
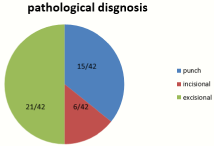
Figure 3: Methods of pathological diagnosis.
Discussion
Lymphatic spread is the most important mechanism in the spread of head and neck squamous cell carcinomas. The rate of metastases probably reflects the aggressiveness of the primary tumor, and is an important prognostic factor [29]. Cervical lymph node metastasis is the main mode of metastasis in various stages of lip cancer. The number and size of metastatic lymph nodes, whether or not with capsular invasion, and the extent of involvement of the neck region and the depth of invasion of the tumor will directly affect the prognosis of patients [30].
Studies have shown that 50% of patients with oral cancer have lymph node metastasis at the first visit [31]. Researcher from Hunan Medical University analyzed 262 oral cancer patients and found that 5.3% patients had cervical lymph node metastasis as the first symptom [32]. Akhter M and colleagues found that the prognosis of patients with oral cancer with cervical lymph node metastasis is worse than that without lymph node metastasis and the probability of distant metastasis increases when cervical lymph nodes metastasize [33].
Clinically, many options are available in the management of cervical lymph nodes for patients with early (Clinically negative Lymph nodes) cN0 in oral and lip squamous cell carcinoma. Some researchers advocate the policy of “wait-and-see”. The “wait-andsee” strategy suggests that lymph node dissection is performed when obvious lymph node metastases are confirmed, but this treatment program significantly reduces the survival rate of the patients. The resection of the primary tumor combined with cervical lymph node dissection has a high cure rate. However, another believe that it has extended the scope of surgery, causing shape damage and loss of some of the early functions of patients, affecting patients’ quality of life [34]. Therefore, this study focuses on the importance of lymph nodes metastases as a prognostic factor and the impact of depth of the tumor invasion on the number of lymph node affected to improve the quality of life of patient.
The results of this study found that growth pattern, differentiation degree, depth of invasion are important factors affecting the cervical lymph node metastasis of oral squamous cell carcinoma of cN0; and further analysis found that the growth pattern, degree of differentiation, depth of invasion were all independent risk factors for cervical lymph node metastasis.
T stage express the size of the tumor in TNM staging, in this study, as the size of the tumor increase, the number of lymph node metastases increase with affection of the surrounding structures by large size of the tumor as when the size of tumor increases, it invade the surrounding structures and more lymphatic affection. So, in general, the larger the tumor size, the more large size of the lymph node and infiltration.
Many studies done to approve this, a study of Monroe MM et al [35] from the Oxford University Evidence-Based Medicine Center found that patients with oral cN0 stage T1 have an occult metastasis rate of 18% to 30%, and the chance of occult metastases in T2 patients rises to 24%-53%. Koo et al [36] reported a retrospective analysis of 66 patients with stage N0-2 oral cancer and revealed that the rate of recessive metastasis was 8% in patients with T2, 25% in T3, 18% in T4, and no metastasis in T1.
In this study , the incidence of lymph node metastases in T1 series patients was 16%, T2 series had 18% lymph node metastases , T3 patients was associated with 20% metastases in cervical nodes and T4 series was 33% .
The depth of invasion of oral squamous cell carcinoma affects the prognosis of patients. Many literatures [37] had confirmed that the depth of tumor infiltration was significantly correlated with cervical lymph node metastasis. In multivariate analysis, tumor infiltration depth is an independent prognostic factor and associated with tumor size, especially for the patients with T1-T2 stage. Kane SV et al [38] retrospectively studied 48 patients with early oral cancer who had undergone primary tumor resection and selective cervical lymph node dissection. The relationship between T size, tumor depth and thickness, degree of differentiation, invasion patterns, inflammatory response and lymph vascular infiltration and cervical lymph node metastasis was analyzed. Fukano H et al [39] believed that the depth of invasion is the most important predictor of early oral squamous cell carcinoma. Pentenero et al [40] reviewed 55 articles with nearly 6,000 oral cancer patients with different clinical stages, sites and different methods of measurement in different studies. The threshold of infiltration depth varied from 1.5 to 10mm in different studies, and the mode and median values were both at 4mm. The infiltration depth threshold of 4mm was accepted as the criteria to determine whether or not the patients with cN0 undergo selective neck treatment. Melchers et al [41] obtained a threshold of invasive depth of 4.59mm by ROC curve analysis, in which the infiltration depth of 4mm can be used as a critical value for judging cervical lymph node metastasis in early oral cancer. Patients with a tumor infiltration depth greater than or equal to 4mm have an increased risk of lymph node metastasis, radical or elective neck dissection for initial therapy or post-operative radiotherapy without neck dissection is highly recommended. Many authors reported that tumors invading less than 2mm do not metastatize [41,42], whereas those greater than 5mm have a high risk of developing LNM [43-45]. Our results were similar and a tumor depth of invasion cutoff > 5mm was identified as the best value associated with LLNM.
In this study we considered the depth of invasion of 5mm is the cutoff point for dissection of nodes and found as the tumor with invasion more than 5mm is associated with more positive lymph node infiltration with lesion that has depth of invasion less than 5mm found to have less Ln metastases and those lesions has depth of invasion more than 5mm are associated with more LNs invasion and worse prognosis, also noted in this study, the cases associated with depth of invasion more than 5mm and large number of LNs invasion was significantly the cases detected with local recurrence and distant or nodal recurrence and need post- operative radiotherapy as an adjuvant treatment, in this study, there was 2 cases with aggressive infiltrative lesion, one case infiltrating the mandible and treated with hemi mandibulectomy and another case infiltrating the maxilla and treated with associated maxillectomy together with excision of lip and neck dissection and were associated with depth of invasion more than 7mm and large number of LNs infiltration.
Conclusion
Lip cancer is a malignancy that results from the autonomous and uncontrolled cell growth in the lips. Lip cancer is a part of head and neck cancers and may occur together with oral cancer or may be one symptom of oral cancer. Lip cancers come in the form of squamous cell carcinoma, the abnormal growth of the flat cells on the lips, neck nodes may be negative or positive clinically and detected by imaging with cases under went cervical block dissection with detection of depth of invasion of tumor and number of lymph nodes dissected, as the increase of depth measured in millimeters as the increase of number and size of lymph node infiltrated and incidence of local or nodal metastases.
References
- Laprise C, Madathil SA, Allison P, Abraham P, Raghavendran A, Shahul HP, et al. No role for human papillomavirus infection in oral cancers in a region in Southern India. Int J Cancer. 2016; 138: 912-917.
- Barnes L, Eveson JW, editors. World Health Organization Classification of Tumours: Pathology & Genetics Head and Neck Tumours. Lyon: Peterreichart, David Sidransky, International Agency for Research on Cancer (IARC). 2005.
- Johnson NW. A Global View of the Epidemiology of Oral Cancer. Cambridge UK: Cambridge University Press; 1991.p.3-326.
- Bogenrieder T, Herlyn M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncog/8ene. 2003; 22: 6524-6536.
- Kapoor C, Vaidya S, Wadhwan V, Malik S. Lymph node metastasis: A bearing on prognosis in squamous cell carcinoma. Indian J Cancer. 2015; 52: 417-424.
- Hasson O. Squamous cell carcinoma of the lower lip. J Oral Maxillofac Surg. 2008; 66: 1259-1262.
- Frierson HF Jr, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol. 1986; 17: 346-354.
- Heller KS, Shah JP. Carcinoma of the lip. Am J Surg. 1979; 138: 600-603.
- Jørgensen K, Elbrønd O, Andersen AP. Carcinoma of the lip: a series of 869 patients. Acta Otolaryngol. 1973; 75: 312-313.
- Martin H, MacComb WS, Blady JV. Cancer of the lip: part I. Ann Surg. 1941; 114: 226-242.
- Salgarelli AC, Sartorelli F, Cangiano A, Pagani R, Collini M. Surgical treatment of lip cancer: our experience with 106 cases. J Oral Maxillofac Surg. 2009; 67:840-845.
- Baker SR, Krause CJ. Carcinoma of the lip. Laryngoscope. 1980; 90: 19-27.
- Bilkay U, Kerem H, Ozek C, Gurler T et al. Management of lower lip cancer: a retrospective analysis of 118 patients and review of the literature. Ann Plast Surg. 2003; 50: 43-50.
- De Visscher JG, van den Elsaker K, Grond AJ, van der Wal JE, van der Waal I. Surgical treatment of squamous cell carcinoma of the lower lip: evaluation of long-term results and prognostic factors-a retrospective analysis of 184 patients. J Oral Maxillofac Surg. 1998; 56: 814-820.
- Santos HB, Silva AL, Cavalcante LH, Alves PM, Godoy GP, Nonaka CF. Histopathological grading systems and their relationship with clinical parameters in lower lip squamous cell carcinoma. Int J Oral Maxillofac Surg. 2014; 43: 539-545.
- Strieder Camilo LCM, Costa V, da Cruz Perez DE, Kowalski LP, Kaminakura E. Comparative analysis of three histologic grading methods for squamous cell carcinoma of the lip. Oral Dis. 2017; 23: 120-125.
- Vartanian JG, Carvalho AL, de Araújo Filho MJ, Junior MH, Magrin J, Kowalski LP. Predictive factors and distribution of lymph node metastasis in lip cancer patients and their implications on the treatment of the neck. Oral Oncol. 2004; 40: 223-227.
- Wermker K, Belok F, Schipmann S, Klein M, Schulze HJ, Hallermann C. Prediction model for lymph node metastasis and recommendations for elective neck dissection in lip cancer. J Craniomaxillofac Surg. 2015; 43: 545- 552.
- Zitsch RP 3rd, Parc CW, Renner GJ, Rea JL. Outcome analysis for lip carcinoma. Otolaryngol Head Neck Surg. 1995; 113: 589-596.
- Altinyollar H, Bulut H, Berberoglu U. Is suprahyoid dissection a diagnostic operation in lower lip carcinoma? J Exp Clin Cancer Res. 2002; 21: 29-30.
- Bucur A, Stefanescu L. Management of patients with squamous cell carcinoma of the lower lip and N0-neck. J Craniomaxillofac Surg. 2004; 32: 16-18.
- Bhandari K, Wang DC, Li SC et al. Primary cN0 lip squamous cell carcinoma and elective neck dissection: systematic review and meta-analysis. Head Neck. 2015; 37: 1392-1400.
- Califano L, Zupi A, Massari PS, Giardino C. Lymph-node metastasis in squamous cell carcinoma of the lip. A restrospective analysis of 105 cases. Int J Oral Maxillofac Surg. 1994; 23: 351-355.
- Eskiizmir G, Ozgur E, Karaca G, Temiz P, Yanar NH, Ozyurt BC. Stage is a prognostic factor for surgically treated patients with early-stage lip cancer for whom a ‘wait and see’ policy in terms of neck status has been implemented. J Laryngol Otol. 2017; 131: 889-894.
- Zitsch RP 3rd, Lee BW, Smith RB. Cervical lymph node metastases and squamous cell carcinoma of the lip. Head Neck. 1999; 21: 447-453.
- Breslow A. Thickness, cross-sectional area and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970; 172: 902-908.
- Clark WH Jr,From L,Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969; 29: 705-726.
- Frierson HF Jr, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Human Pathol. 1986; 17: 346-354.
- Hoang JK, Vanka J, Ludwig BJ, Glastonbury CM. Evaluation of cervical lymph nodes in head and neck cancer with CT and MRI: tips, traps, and a systematic approach. AJR Am J Roentgenol. 2013; 200: 17-25.
- Bobdey S, Sathwara J, Jain A, Saoba S, Balasubramaniam G. Squamous cell carcinoma of buccal mucosa: An analysis of prognostic factors. South Asian J Cancer. 2018; 7: 49-54.
- O’Donnell RK, Kupferman M, Wei SJ, Singhal S, Weber R, O’Malley B. et al. Gene expression signature predicts lymphatic metastasis in squamous cell carcinoma of the oral cavity. Oncogene. 2005; 24: 1244-1251.
- Jiang CH, Jian XC, Cheng HQ. [Clinical analysis of cervical lymph node metastasis as primary manifestation of oral cancer]. Hunan Yi Ke Da Xue Xue Bao. 2003; 28: 657-658.
- Akhter M, Hossain S, Rahman QB, Molla MR. A study on histological grading of oral squamous cell carcinoma and its co-relationship with regional metastasis. Journal of oral and maxillofacial pathology: JOMFP. 2011; 15: 168-176.
- Farhan-Alanie OM, McMahon J, McMillan DC. Systemic inflammatory response and survival in patients undergoing curative resection of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 2015; 53: 126-131.
- Monroe MM, Gross ND. Evidence-based practice: management of the clinical node-negative neck in early-stage oral cavity squamous cell carcinoma. Otolaryngol Clin North Am. 2012; 45: 1181-1193.
- Koo BS, Choi EC, Yoon YH, Kim DH, Kim EH, Lim YC. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009; 249: 840-844.
- Yuen AP, Lam KY, Wei WI, Lam KY, Ho CM, Chow TL. et al. A comparison of the prognostic significance of tumor diameter, length, width, thickness, area, volume, and clinicopathological features of oral tongue carcinoma. Am J Surg. 2000; 180: 139-143.
- Kane SV, Gupta M, Kakade AC, A DC. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006; 32: 795-803.
- Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997; 19: 205-210.
- Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005; 27: 1080-1091.
- Melchers LJ, Schuuring E, van Dijk BA, de Bock GH, Witjes MJ, van der Laan BF et al. Tumor infiltration depth >/=4 mm is an indication for an elective neck dissection in pT1cN0 oral squamous cell carcinoma. Oral Oncol. 2012; 48: 337-342.
- Mehregan DA, Roenigk RK. Management of superficial squamous cell carcinoma of the lip with Mohs micrographic surgery. Cancer. 1990; 66: 463- 468.
- Frierson HF Jr, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol. 1986; 17: 346-354.
- Salgarelli AC, Sartorelli F, Cangiano A, Pagani R, Collini M. Surgical treatment of lip cancer: our experience with 106 cases. J Oral Maxillofac Surg. 2009; 67: 840-845.
- Wermker K, Belok F, Schipmann S, Klein M, Schulze HJ, Hallermann C. Prediction model for lymph node metastasis and recommendations for elective neck dissection in lip cancer. J Craniomaxillofac Surg. 2015; 43: 545- 552.
