Abstract
Background: Pancreatic carcinoma is currently one of the deadliest of the solid malignancies and is the fourth cause of death from cancer in the United States, with a survival rate at 5 years of less than 5%. Most of cases diagnosed as advanced with vascular encasement or invasion and have chemotherapy for down staging to increase liability of resection with good results.
Materials and Methods: 20 patients with diagnosis of locally advanced pancreatic carcinoma was examined and planned prospective to detect results of treatment and curability with prognosis in a period between start of January 2016 to end of December 2017.
Conclusion: Pancreatic carcinoma may be curable with the use of MDT for treatment and neoadjuvant with surgery and vascular resection with follow up with CT, PET-CT and markers CA19.9 with good results and prognosis.
Results: 20 patients included in this study, diagnosed as pancreatic head carcinoma in 10/20 (50%) and periampullary carcinoma in 10/20 (50%) of patients, all patients were diagnosed as locally advanced pancreatic cancer by radiological staging and diagnosis, 14/20 (70%) was males and 6/20 (30%) female, the age of patients at diagnosis was 39-62 years with median age 51 years.
Keywords: Pancreatic carcinoma; Chemotherapy; Locally advanced pancreatic carcinoma; Vascular resection
Abbreviations
BMI: Body Mass Index; OR: Odds Ratio; RR: Relative Risk; PC: Pancreatic Carcinoma; SMA: Superior Mesenteric Artery; SMV: Superior Mesenteric Vein; PV: Portal Vein; HA: Hepatic Artery; VR: Venous Resection; PD: Pancreaticoduodenectomy; APC: Advanced Pancreatic Carcinoma; QOL: Quality of Life; PVR: Portal Vein Resection; IVC: Inferior Vena Cava
Introduction
Pancreas is an important abdominal and retroperitoneal organ that has both endocrine and exocrine functions, it may have either benign or malignant tumors, the Pancreatic carcinoma is currently one of the deadliest of the solid malignancies and is the fourth cause of death from cancer in the United States, with a survival rate at 5 years of less than 5% [1,2]. About 60% of tumors originate at the head of the pancreas, 15% in the body, 5% in the tail; 20% are diffuse within the pancreas [3]. At the time of diagnosis tumors located in the head are usually smaller (2.5-3cm) compared with those in the body and tail (5-7cm), as a result of earlier clinical manifestation because of the close contiguity with the bile ducts. Imaging of pancreatic carcinoma has a leading role in assessing the best options for the treatment of pancreatic carcinoma.
Surgical resection is the only curative treatment of pancreatic carcinoma. Unfortunately, at surgical exploration only 5-30% of tumors are amenable to resection [4,5]. Even with expertize surgeons, Whipple’s procedure has a mortality of up to 4% and exploratory laparotomy has a morbidity up to 25% [6].
The majority of patients with pancreatic adenocarcinoma present at an advanced stage at the time of diagnosis. The prognosis of these patients is poor, with a 1-year survival rate of 20% and a 5-year survival rate of less than 5%. While complete surgical resection may lead to long-term survival in approximately 25% of patients, only 15% are actually resectable. Therefore, the principle goal of preoperative staging is to identify all resectable disease to avoid surgical exploration in those patients with unresectable disease.
MSCT (Multislice CT Scan) is the most important diagnostic for cancer pancreas especially with pancreatic protocoal has high accuracy in staging of pancreatic carcinoma and follow up after treatment either surgical or neoadjuvant with long run follow up also, PET-CT (Positron Emission Tomography) also used for diagnosis and staging of pancreatic carcinoma and also used as an important method for follow up of treatment especially combined with computed tomography (CT), it also has an established role in differentiating benign from malignant lesions and in the staging and treatment planning of various tumors. The increased glucose metabolism of most malignant lesions results in significant uptake of FDG in primary malignant tumors and metastases that does not occur in healthy tissues and benign lesions after i.e. injection, allowing a higher conspicuity compared with that of the surrounding tissue [7,8].
This is study highlights the staging of locally advanced pancreatic carcinoma with pre-operative diagnosis to detect the resectability and operability with neoadjuvant treatment and then surgical interference and results with follow up to 24 months after surgery.
Materials and Methods
20 patients with diagnosis of locally advanced pancreatic carcinoma was examined and planned prospective to detect results of treatment and curability with prognosis in a period between start of January 2016 to end of December 2017 in multicenters including department of surgery, HPB Surgery and Liver Transplantation Department, National Liver Institute, Menoufia university., Clinical oncology department, faculty of medicine, Ain shams university, surgical Oncology department, Tanta Cancer Center and Radio diagnosis department, National Liver Institute, Menoufia university.
All cases were presented as periampullary or pancreatic head carcinoma and all of them diagnosed by Multi slice CT and confirmed by PET-CT scan to confirm the diagnosis and resectablity with staging of the disease and also had determination of serum level of markers CA19.9 and CEA before treatment and every 3 months after treatment either chemo radiation or surgery, during examination, 14/20 patients were males and 6/20 females (2.3:1) and median age was 51 years old (39-62), all patients examined for DM and Hypertension together with determination of smoking or not.
All patients had full laboratory investigations before treatment in the form of complete blood pictures, Kidney functions and serum bilirubin both total and direct with liver enzymes estimations and level of serum albumin before treatment and in every stage during, also patients screened for hepatitis B and C and found as all negative for both.
Inclusion criteria:
• All patients diagnosed with pancreatic head carcinoma or peri-ampullary carcinoma.
• All patients diagnosed as unresectable due to Superior mesenteric vessels or portal infiltration or coeliac Lymph node affection.
• All patients underwent neoadjuvant chemotherapy, radiotherapy or both and subjected to have surgery.
Presentation of Patients
Patients in the study presented by obstructive jaundice of high level with marked yellowish colouration of sclera of eye in 14/20 patients and associated with weight loss and jaundice in 2/20 cases while presented with jaundice and hematemesis in 2/20 cases and melena and jaundice in 2/20 cases, serum level of bilirubin detected with high levels in all cases 9-23 and direct bilirubin 8-2, with estimated high level of serum marker CA19.9 ranging between 167- 2454.
Results
20 patients included in this study, diagnosed as pancreatic head carcinoma in 10/20 (50%) and peri-ampullary carcinoma in 10/20 (50%) of patients, all patients were diagnosed as locally advanced pancreatic cancer by radiological staging and diagnosis, 14/20 (70%) was males and 6/20 (30%) females with male: female ratio 2.3:1, the age of patients at diagnosis was 39-62 years with median age 51 years (Figure 1).
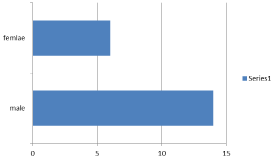
Figure 1: Types of patients diagnosed.
Risk factors including smoking detected in 12/20 patients (60%) while 8/20 (40%) nonsmokers, Diabetes Mellitus in 6/20 (30%) and hypertension detected in 6/20 patients (30%) while all patients screened for HBV and HCV and found as negative for both (Table 1).
Risk factor
Yes
No
Smoking
12
8
DM
6
14
Hypertension
6
14
HBV
0
20
HCV
0
20
Table 1: Risk factors.
Patients presented mostly with jaundice and high level of bilirubin especially direct bilirubin attending high level reaching in some cases above 20 with slight increase in indirect bilirubin due to affection of the liver by obstruction of ducts , the patients presented in jaundice only in 14/20 cases (70%), obstructive jaundice with weight loss in 2/20 cases (10%), obstructed jaundice with hematemesis in 2/20 (10%) cases and presented with melena in 2/20 (10%) of cases and all cases are provisionally diagnosed as cancer head and peri-ampullary pancreatic carcinoma before any interference (Figure 2).
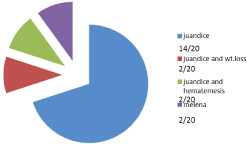
Figure 2: Presentation of patients.
Patients was diagnosed radiologically as pancreatic head cancer in 10/20 (50%) of cases and peri-ampullary carcinoma in 10/20 (50%) of cases with 4/20 (20%) had hypoprothrombonaemia less than 70% of prothrombin concentration and treated medically to improve the condition (Figure 3).
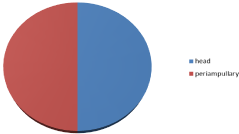
Figure 3: Diagnosis of patients.
All patients undergone laboratory investigations for detection of serum bilirubin both total, direct and indirect billirubin together with kidney function tests, liver profile and serum tumor markers to detect the prognosis and follow up stage and found high increase of serum CA19.9 that ranges between 167-2454 with the majority of patients ranging between 600-1000 and 4/20 (20%) had more than 2000 in estimation (Table 2).
Lab indicator
Number of patients (total 20)
Total bilirubin
10-Jan
4
10.1-20
14
>20
2
Direct
10-Jan
4
10.1-20
14
>20
2
Indirect
10-Jan
20
10.1-20
0
>20
0
Albumin
<4
8
>4
12
Prothrombin concentration (PT)
<70%
6
>70%
14
CA19.9
0-300
4
300-600
4
600-1000
8
>1000
4
CEA
0-10
18
>10
2
Table 2: Laboratory investigations before interference.
In this study all patients were subjected from the start to differ imaging models to confirm the diagnosis to facilitate the decision making for interference starting with ultrasound of the abdomen and pelvis and found to have dilated common bile duct (CBD) in 8/20 (40%) of cases , obstructed distal part of CBD in 10/20 (50%) of cases while liver cirrhosis was detected in 2/20 (10%) of cases by U/S that used for measurement of the size of tumor and detect 4/20 (20%) of patients ‹3cm in dimensions and 6/20 (30%) of them had 3-4cm in tumor size while cases diagnosed more than 4cm was 10/20 (50%) of cases.
Multi slice computerized tomography (MC CT) with pancreatic protocol used for all patients to detect the radiological staging of the tumor with vascular status either invaded or abutted with measurement of degree of invasion to take the decision, we were looking for the Superior Mesenteric Artery (SMA), Superior Mesenteric Vein (SMV) affection together with the Hepatic Artery (HA), Coeliac Artery (CA), inferior vena cava (IVC),coeliac Lymph nodes and para aortic lymph nodes status and infiltration and also detection of the presence of liver metastasis, all these points were detected by multi sliced CT scan for all patients then all patients had MRCP (Magnetic Resonant Cholecysto Pancreaticography) to comment on the site of the mass and CBD status together with the intrahepatic pancreatic duct dilatation and its level.
Then all patients had PET-CT scan to detect and hidden or distant metastasis and confirm the vascular status of the pancreas either infiltrated or abutted, the presence of positive or suspicious coeliac or para aortic lymph nodes and also done in all follow up stages.
During investigations, all patients had biliary drainage before the neoadjuvant treatment start to improve the general condition of patients and also state of the liver, 12/20 (60%) of patients undergone ERCP with plastic stents to drain bilirubin while 8/20 (40%) need the insertion of PTD to drain bilirubin and improve the general condition of patients. all data are listed in Table 3.
Radiological imaging
Number of patients
Ultrasound (US)
Dilated CBD
8
Obstructed lower CBD
10
Cirrhotic liver
2
Size of tumor
<3cm
4
3-4cm
6
>4cm
10
Computerized tomography (CT)
Portal vein
Encasement
14
No encasement
6
Superior mesenteric artery(SMA)
Abutting
4
Infiltration
0
No
16
Superior mesenteric vein
Infiltration
2
abutting
8
No
10
inferior vena cava (IVC)
abutting
2
No
18
Hepatic Artery
main HA
encasement>180
2
No
18
RHA
Encasement
2
No
18
coeliac and Para aortic LNS
coeliac infiltration
2
both
16
No
2
Liver metastasis
Yes
2
No
18
MRCP
Mass at head and uncinate process
12
Dilated IHBD and proximal CBD
4
Dilated CBD and pancreatic duct
4
PET-CT scan
Size of tumor
<3cm
4
3.1-4cm
14
>4cm
2
Vascular invasion
Portal vein and SMV Encasing
10
Portal vein and SMV Abutting
4
IVC abutting
2
Hepatic artery
2
PV&SMV&RHA
2
LNs
20
Liver metastases
2
Distant metastases
0
Biliary drainage
ERCP stenting
12
PTD
8
Table 3: Preoperative radiological diagnosis.
After radiological and laboratory investigations, decision taken to explore only 2/20 cases that was found advanced on exploration and biopsy was taken to confirm the diagnosis, while the rest of patients 18/20 (90%) of cases directed to have neoadjuvant chemotherapy and radiotherapy.
Chemotherapy protocol given for all patients for 6months as the protocol FOLFIRINOX: The new triple combination chemotherapy (oxaliplatin 85mg/m2, irinotecan 180mg/m2, leucovorin 400mg/m2, and 5-fluorouracil (400mg/m2) given as a bolus followed by 2,400mg/ m2 administered as a 46h continuous infusion q 2 weeks), with only 2/20 cases had radiotherapy.
Then all patients had investigated again with multi slice CT, PETCT with follow up by tumor markers serum levels and found to have marked regression of the size of the tumor to be less than 3cm in dimensions in all cases with improvement of the degree of infiltration of vascular supply by SMA, SMV, IVC , HA, CA and status of lymph nodes either the coeliac or para aortic, with marked regression of the size noted in all cases, the degree of invasion of the IVC, Coeliac Artery (CA) and SM vessels was decreased to a great level with disappearance of liver metastasis noted pre-chemotherapy and also marked regression of the level of markers noted to reach between 5-423 and lymph node infiltration noted in only 2/20 cases (Table 4).
Surgical interference
Surgical interference in the form of Whipple’s operation after neoadjuvant treatment and done for all patients resecting distal stomach, duodenum head of pancreas and proximal jejunum together with distal CBD, 12 cases had Whipple’s operation without vascular resection while 8 cases under went vascular resection of superior mesenteric vein (SMV) and portal vein (PV) and reconstruction again in all cases with also replacement of the right hepatic artery by saphenous vein graft between the aorta and HA of the liver in 2 cases (Figure 8 and 9).
Imaging
Number of cases
CT scan
Size
<3cm
20
>3cm
0
Portal vein (PV)
Encased
2
Abutted
6
No
12
SMA(superior mesenteric artery)
Abutted <90
2
No
18
SMV(superior mesenteric vein)
Infiltrated
2
Abutted
8
No
10
IVC( inferior vena cava)
Infiltrated
0
No
20
HA( hepatic artery)
Encased
2
Abutted
2
No
16
CA(Coeliac artery)
Infiltrated
20
No
2
LNs(lymph nodes)
Infiltrated
18
No
0
LM(liver metastasis)
Yes
20
No
14
PET-CT
Size
<2cm
14
>2cm
6
PV
Abutted
12
No
8
SMA
Abutted
12
no
8
SMV
Abutted
12
No
8
HA
Abutted
4
No
16
CA
Abutted
2
No
18
LNs
Infiltrated
2
No
18
Tumor markers
CA19.9
<50
8
50-200
6
200-500
6
>500
0
CEA
<2
0
4-Feb
20
>4
0
Table 4: Follow up after chemoradiotherapy.
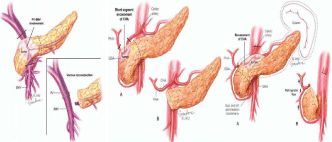
Figure 4: Infiltration of portal vein and SMV confluence/encasement of
common hepatic artery/tumor encasing coeliac, CHA and splenic artery.
Figure 5: Current CT scan for one of the cases before any treatment.
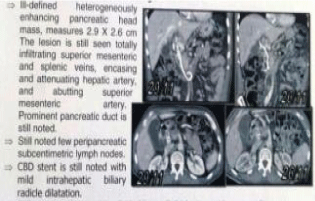
Figure 6: CT after chemotherapy.
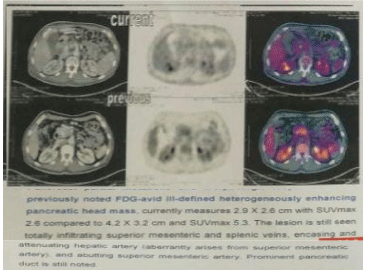
Figure 7: PET-CT pre and post chemotherapy.
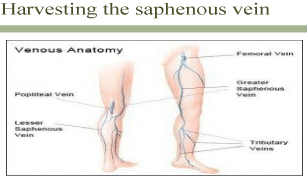
Figure 8: Saphenous vein harvested.
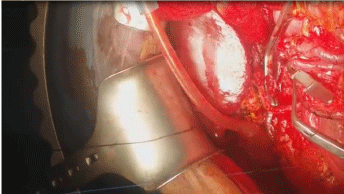
Figure 9: Portal vein resection.
The average hospital stay was 11-15 days for most majority of patients with small number exceeded this figure to reach up to 20 days with some complications that were treated conservatively.
Some cases had complications in the form of 2/20 (10%) cases had hematomas, 12/20 (60%) had post-operative wound infection, 2/20 (10%) cases had wound dehiscence in the next two weeks after surgery and 4/20 (20%) passed smoothly without complications, all the cases treated conservatively without the need for re surgery again.
Some cases had morbidity in the form of chest infection and complications noticed in 6/20 (30%) of cases, with 2/20 (10%) patients developed ascites minimal in amount post-operative and only 2/20 (10%) had wound dehiscence during the post-operative next 2 weeks while no morbidity at all recorded in this study in next 3 months following surgery and follow up and all patients were subjected to have chemotherapy for 3 months post-operative (Table 5).
Procedure
Number of patients
Surgery
Whipple’s operation
With vascular resection
12
Without vascular resection
8
Hospital stay
1-10 days
4
11-15days
12
16-20days
4
Complications
Leakage
0
Wound infection
12
Dehiscence
2
Hematoma
2
No
4
Mortality
Chest infection
6
Ascites
2
Wound dehiscence
2
No
10
Morbidity
Yes
0
No
20
Table 5: Surgery procedures and complication.
Tumor pathology
Periampullary including (pancreas, duodenum and ampulla).
The examination of the specimens after surgery showed that all cases were adenocarcinoma of periampullary with 12/20 (60%) was recorded as grade 3 and 8/20 (40%) were grade 2 in examination, with all cases had free surgical margins both proximal and distal, and the regional lymph nodes harvested ranged between 0-13 nodes and the infiltrated lymph nodes also between 0-13 nodes with all harvested nodes was 190 nodes in all cases at which 26 nodes only found to be infiltrated by the tumor (Figure 10 and 11).
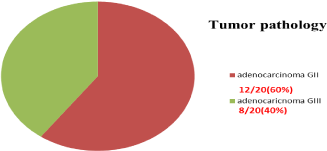
Figure 10: Tumor Grades.
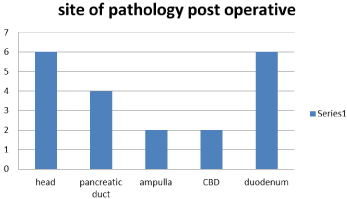
Figure 11: Site of pathology postoperative.

Figure 12: CT one month post-operative.
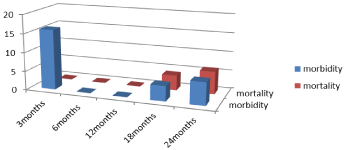
Figure 13: Mortality and morbidity during the period of follow up.
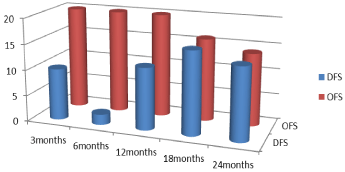
Figure 14: Over all free survival (OFS) and disease free survival (DFS)
during follow up.
Follow up of patients
All patients was under close follow up at different periods starting 3 months, 6 months, 12 months, 18 months and the after 24 months post-operative with all of them had multi slice CT, PET-CT, estimation of CA19.9 level as tumor marker during follow up and detection of any mortality or morbidity during the estimated period of follow up.
Follow up after 3 months
Follow up by CT found that there was no recurrence either regional or nodal but ascites developed in 4/20 (20%) cases detected by multi slice CT that was confirmed by examination by PET-CT to be the same with the majority of patients had CA19.9 level below 20 while small number only ranged between 40-60 and all patients had chemotherapy as an adjuvant treatment.
During the first 3 months of follow up we noticed the development of ascites in 8/20 (40%) of cases while incisional hernia at the site of incision for surgery detected in another 4/20 (20%) of cases and also chest infections and complications in 4/20 (20%) of patients but no mortality detected during this period of follow up (Table 6).
Item
Number of patients
CT
Recurrence
Yes
0
No
20
Ascites
Yes
4
No
16
PET-CT
Recurrence
Yes
0
No
20
CA19.9
0-20
10
20-40
6
40-60
4
Chemotherapy
Yes
20
No
0
Morbidity
Ascites
8
Incisional hernia
8
Chest problems
2
No
2
Mortality
Yes
0
No
20
Table 16: Follow up after 3 months.
Follow up after 6 months
After 6 months as usual all patients had the same examination by CT.PET-CT and markers and no recurrence detected by CT or PETCT and no ascites present with CA19.9 level estimation still majority of patients under 20 and no continuation of chemotherapy at any of the patients with still ascites detected in 4/20 patients and incisional hernia in 6/20 of patients and no mortality recorded in this series of patients (Table 7).
Item
Number of patients
CT
Recurrence
Yes
0
No
20
PET-CT
Recurrence
Yes
0
No
20
CA19.9
0-20
12
20-40
4
40-60
4
Chemotherapy
Yes
0
No
20
Morbidity
Ascites
4
Incisional hernia
6
No
10
Mortality
Yes
0
No
20
Table 7: Follow up after 6 months.
Follow up after 12 months
After 1 year of follow up with the same criteria and same measurements, recurrence was detected as local recurrence at the operative bed confirmed by both CT and PET-CT in 4/20 (20%) of patients while still had normal level of CA19.9 marker and 6/20 were returned again to have chemotherapy of treatment of recurrence, incisional hernia still detected in 4/20 cases and chest problems in 2/20 cases while 2/20 developed diabetes mellitus (DM) diagnosed by estimating fasting and post prandial blood sugar level and also no mortality detected (Table 8).
Item
Number of patients
CT
Recurrence
Yes
4
No
16
PET-CT
Recurrence
Yes
4
No
16
CA19.9
0-20
8
20-40
4
40-60
2
60-200
6
Chemotherapy
Yes
6
No
14
Mortality
Yes
0
No
20
Morbidity
Incisional hernia
4
Chest problems
2
DM
2
No
12
Table 8: Follow up after 1 year.
Follow up after 18 months
After 18 months of surgery, all patients had CT and PET-CT that confirmed the diagnosis of local recurrence in 2/20 cases and development of new liver distant metastasis in another 2/20 cases with the level of marker as CA19.9 start to increase gradually in some cases up to 200 and 50% of cases 10/20 had to have chemotherapy again for treatment of metastasis and recurrence or increased level of serum tumor marker and also 4/20 (20%) had cardiac problems in the form of heart failure and 4/20 (20%) also had diagnosed as pneumonia but after 18 months 4 patients out of 20 died from heart failure (Table 9).
Item
Number of patients
CT
Recurrence
2
Liver metastases
2
No
16
PET-CT
Recurrence
2
Liver metastases
2
No recurrence or metastasis
16
CA19.9
0-20
6
20-40
4
40-60
0
60-200
10
Chemotherapy
Yes
10
No
10
Morbidity
Cardiac failure
4
Pneumonia
4
No
12
Mortality
Yes
4
No
16
Table 9: Follow up after 18 months.
Follow up after 24 months
Two years follow up for 16 patients that was all examined by CT and PET-CT with no detected recurrence or ascites and the level of CA19.9 was within normal range in majority of cases but 4/16 patients undergone chemotherapy again due to graduated increase noted in serum marker level with 2/16 (12.5%) of cases had chest problems in the form of sever pneumonia and died within this period due to pneumonia (Table 10).
Item
Number of patients
CT
Recurrence
Yes
0
No
16
PET-CT
Recurrence
Yes
0
No
16
CA19.9
0-20
8
20-0
4
40-60
2
60-100
2
Chemotherapy
Yes
4
No
12
Morbidity
Chest infection
2
No
14
Mortality
Yes
2
No
14
Table 10: Follow up after 2 years.
Discussion
Pancreatic cancer is very aggressive with few symptoms before the cancer can diagnosed and usually the cancer is advanced when diagnosed. It is the most lethal type of cancer, and listed as the fourth leading cause of cancer-related death with a poor prognosis because of the late findings of the disease. To patients, the pancreatic cancer diagnosis is a life-changing disaster; however, to maximally extend the pancreatic cancer patients’ life is most important task after diagnosis, Pancreatic cancer is a common and highly aggressive type of malignancy, for which the 5-year overall survival (OS) rate is ‹6%, In recent years, the incidence of pancreatic cancer has continuously increased [9,10].
Population and family-based studies established that both environmental and inherited factors contribute to the development of pancreatic adenocarcinoma. The most common risk factor for pancreatic adenocarcinoma is cigarette smoking. Analyses of data from 12 case-control studies showed that current smokers have a 2.2-fold (95% Confidence Interval (CI) 1.71–2.83) increased risk of pancreatic cancer compared with those whom never be smokers [11]. Approximately 25% of pancreatic cancers are attributable to cigarette smoking [12]. The “finger print” of tobacco smoking can be seen in pancreatic cancers, as genetic analyses have shown that pancreatic cancers resected from smokers have more mutations than pancreatic cancers from never-smokers [13]. Importantly, smoking cessation reduces this risk. 2 Risk estimates of 1.64 (Odds Ratio (OR), 95% CI 1.36–1.97) have been reported for recent quitters (1–10 years) and of 1.12 (95% CI 0.86–1.44) for individuals who quit smoking 15–20 years ago [11]. Longstanding type 2 diabetes mellitus is also associated with an increased risk of pancreatic cancer, with patients have type 2 diabetes of ›10 years duration having a 1.51-fold (95% CI=1.16–1.96) increased risk of pancreatic cancer compared with non-diabetics [14]. In addition, new onset diabetes can be the first sign of pancreatic cancer [15]. Up to 1% of new-onset adult diabetics are diagnosed with pancreatic cancer within 3 years of their diagnosis of diabetes, suggesting that new onset diabetes could be a clue to the early diagnosis of pancreatic cancer in some people [16]. Thus, longstanding diabetes is a risk factor for pancreatic cancer, and new onset diabetes can be an early sign of the disease (Table 11).
Risk factor
Risk Estimate (95% CI)
Current Cigarette Smoking
OR= 2.20 (1.71–2.83)
Past Cigarette Smoking
1–10 years since quitting
OR=1.64 (1.36–1.97)
15–20 years since quitting
OR=1.12 (0.86–1.44)
Diabetes Mellitus
<3 years
RR=7.94 (95% CI, 4.70–12.55)
>10 years duration
OR 1.51 (95% CI=1.16–1.96)
BMI (>35 vs. 18.9–24.9)
OR =1.55 (95% CI=1.16–2.07)
Heavy Alcohol (> 6 drinks/day)
OR 1.46 (95% CI=1.16–1.83)
Pancreatitis (>2 years)
2.71 fold (95% CI=1.96–3.74)
Table 11: Pancreatic Cancer Risk Factors.
In this study we have 12/20 was smokers and 8/20 not smokers with 6/20 was diabetic and another 2/20 patients developed diabetes during follow up after one year with 12/20 had BMI was between 30- 35 but none of the patients was alcohol drinkers.
There is a group of pancreatic cancer called “borderline resectable”. Diagnosed and staged radiologically These are pancreatic cancers considered unresectable by standard treatment guidelines because of vascular involvement or encasement, but there has been recent studies performed showing benefit in median survival for patients undergoing neoadjuvant chemo radiation trial and then reevaluation using traditional staging techniques [17]. Those patients showing tumor response, which is measured by shrinkage of tumor size, will then undergo surgical resection with some success with venous resection (VR), which is necessary when there is locally advanced disease into the superior mesenteric vessels, portal vein or IVC.
Many studies were reviewed for the effect of neo adjuvant therapy on the effect of vascular resection and mortality and morbidity with the median overall survival. The analysis of two studies comparing FOLFIRINOX versus gemcitabine demonstrated an improvement in OS (P ‹ 0.001), PFS (P ‹ 0.001) and response rate (RR 3.38; P ‹ 0.001) but also significantly more neutropenia and thrombocytopenia (Conroy 2011 [18] and Singhal 2014 [19]). There was improved quality of life (QOL). These results suggest that FOLFIRINOX reduces the risk of death by 49%, reduces the risk of progression by 54% and triples the rate of response compared with gemcitabine. The absolute survival gains are still modest, with OS in the gemcitabine alone arm ranging from 6.8 months to 7.4 months and in the FOLFIRINOX arms between 10.8 months to 11.1 months.
The Miiller et al. [20] study investigates the morbidity, mortality and survival in patients with advanced pancreatic adenocarcinoma (PAC) whom have had PD (pancreaticoduodenectomy) with VR (venous resection) with study of 488 cases at Germany between October 2001 and December 2007 with 100 cases had VR, The authors found that the median survival for patients undergoing PD with VR was 14.5 months, with 1 and 3 year survival rates of 55% and 14% respectively and 15/110 under gone mortality, In Kaneoka et al. [21] retrospectively investigated the operative outcomes of portal vein resection (PVR) for portal vein (PV) or superior mesenteric vein (SMV) involvement in patients with PAC and had median survival of 12 months in patients had vascular resection, Wang et al. 2008 [22], studied 61 patients in china with locally advanced pancreatic carcinoma with vascular resection done with PD and had median survival of 13 months and 13% 5 years survival, and The Martin et al study [23] was a very small study of 36 patients, had PD and VR with median survival of 18 months.
In our study the results was similar with study done at 20 patients under gone chemotherapy for down staging to facilitate safety of vascular resection with PD done after 6 months of chemotherapy in a protocol of FOLFIRINOX: The new triplet combination chemotherapy (oxaliplatin 85mg/m2, irinotecan 180mg/m2, leucovorin 400mg/m2, and 5-fluorouracil (400mg/m2) given as a bolus followed by 2,400mg/ m2 administered as a 46h continuous infusion q 2 weeks). Then reevaluation by the use of CT and PET-CT and serum level of CA19.9 and CEA level then all patients had pancreaticoduodenectomy with vascular resection in 8/20 (40%) of cases while vascular resection done in 12/20 (60%) of patients with mortality in 6/20 by the end of 24 months follow up 2 of them from the group with no vascular resection while 4of them of the group had vascular resection with median survival rate of 22.7 months in average.
Conclusion
Pancreatic carcinoma is one of the most lethal tumor associated with bad prognosis, most cases diagnosed in advanced stage with vascular invasion or abutting but with the use of new trends in neoadjuvant chemotherapy, the stage can be changed with possibility of PD and vascular resection with good prognosis and results and improved overall survival and decreased mortality.
References
- Sahmoun AE, D’Agostino Jr RA, Bell RA, Schwenke DC. International variation in pancreatic cancer mortality for the period 1955-1998. Eur J Epidemiol. 2003; 18: 801-816.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56: 106-130.
- Zamboni G, Capelli P, Pesci A, Beghelli S, Leuttges J, Kl€oppel G. Pancreatic head mass: what can be done? Classification: the pathological point of view. JOP. 2000; 1: 77-84.
- Cooperman AM, Kini S, Snady H, Bruckner H, Chamberlain RS. Current surgical therapy for carcinoma of the pancreas. J Clin Gastroenterol. 2000; 31: 107-113.
- Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology. 2005; 128: 1626-1641.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002; 346: 1128-1137.
- Saisho H, Yamaguchi T. Diagnostic imaging for pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas. 2004; 28: 273-278.
- Buchs NC, Chilcott M, Poletti PA, Buhler LH, Morel P. Vascular invasion in pancreatic cancer: Imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol. 2010; 16: 818-831.
- Lin Q, Yang F, Jin C, et al. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015; 21: 7988–8003.
- Yu X, Lu Y. Current status on epidemiology and management of pancreatic cancer. Shanghai Med. 2014; 35: 4–6.
- Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012; 23: 1880–1888.
- Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010; 28: 645–656.
- Blackford A, Parmigiani G, Kensler TW, et al. Genetic mutations associated with cigarette smoking in pancreatic cancer. Cancer Res. 2009; 69: 3681– 3688.
- Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British journal of cancer. 2005; 92: 2076–2083.
- Chari ST, Leibson CL, Rabe KG, Ransom J, de AM, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005; 129: 504–511.
- Katz M, et al. Borderline Resectable Pancreatic Cancer: The Importance of This Emerging Stage of Disease. Journal of the American College of Surgeons. 2008; 206: 833-846.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine. 2011; 364: 1817-1825.
- Singhal MK, Kapoor A, Bagri PK, Narayan S, Singh D, Nirban RK, et al. A phase III trial comparing of FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. Annals of Oncology. 2014; 25: 210.
- Miiller S, et al. Vascular Resection in Pancreatic Cancer Surgery: Survival Determinants. Journal of Gastrointestinal Surgery. 2009; 13: 784-792.
- Kaneoka Y, Yamaguchi A, Masatoshi I. Portal or Superior Mesenteric Vein Resection for Pancreatic Head adenocarcinoma: Prognostic Value of the Length of VR. Surgery. 2009; 145: 417-425.
- Wang C, Wu H, Xiong J, Zhou F, Tao J, Liu T, et al. Pancreaticoduodenectomy with vascular resection for local advanced pancreatic head cancer: a single center retrospective study. Journal of Gastrointestinal Surgery. 2008; 12: 2183-2190.
- Martin R, et al. Arterial and VR for Pancreatic Adenocarcinoma. Archives of Surgery. 2009; 144: 154-159.
