Abstract
Introduction: We are reporting a case of 62 year old male patient with central obesity presented with nonspecific gastrointestinal symptoms which was misdiagnosed and treated according to its symptomology. After complete evaluation found to have a large right Renal Cell Carcinoma (RCC) with involvement of right renal vein and later he underwent right radical nephrectomy. The presentation of RCC is variable and most of the times it presents with nonspecific symptoms. The classic triad of loin pain, hematuria and abdominal mass is found only in 4-17 % of cases. The incidence of RCC is 2.2% and mortality rate is 1.8% worldwide, in 2018. In India, its incidence is around 1.3% and mortality is 1.3% in 2018. It occurs predominantly in the 6th to 8th decade of life with median age at diagnosis around 64 years. Due to earlier detection of these tumors, the incidence has increased threefold than the mortality. One of the established risk factor for RCC is cigarette smoking. As the use of cigarette smoking increases the stage of RCC advances further.
Keywords: Clear cell renal cell carcinoma; Gastrointestinal; Non-specific; Radical nephrectomy; Targeted therapy
Introduction
The incidence of Renal Cell Carcinoma (RCC) varies from region to region worldwide with observation of highest rates in the Czech Republic and North America [1,2]. Worldwide, in 2018, there were an estimated 403,000 new cases of RCC and 175,000 deaths due to kidney cancer [3]. The incidence of RCC is 2.2% and mortality rate is 1.8% worldwide, in 2018 [4]. In India, its incidence is around 1.3% and mortality is 1.3% in 2018 [5]. It is twofold more commonly found in men as compared with women [6]. It occurs predominantly in the 6th to 8th decade of life with median age at diagnosis around 64 years [7]. From the SEER registry analysis it is found that there has been a steady decrease in the size of tumors at presentation and this may be because of increase in the numbers of detection of incidental tumors on imaging [8,9]. Over the last 60 years, the five year survival rate of patients with renal cell carcinoma has doubled from 34 percent in 1954 to 62 percent in 1996 and 75 percent from 2009 to 2015 [8,10]. Due to earlier detection of these tumors, the incidence has increased threefold than the mortality [11]. One of the established risk factor for RCC is cigarette smoking and the relative risks for RCC for all smokers, current smokers, and former smokers are 1.31, 1.36, and 1.16, respectively [12]. As the use of cigarette smoking increases, the stage of RCC advances further [13]. Some of the studies had found that excessive body weight is a risk factor for RCC in both men and women [14,15]. The presentation of RCC is variable and most of the times it presents with nonspecific symptoms. The classic triad of loin pain, hematuria and abdominal mass is found only in 4%-17% of cases [16,17]. There are case reports of gastrointestinal bleeding in patients with RCC due to infiltration of the tumor into duodenum. We are reporting a case of 62 year old male patient with central obesity presented with nonspecific gastrointestinal symptoms and after complete evaluation found to have renal cell carcinoma.
Case Presentation
A 62 year old gentleman with Eastern Cooperative Oncology Group Performance Status 1 (ECOG PS 1) had history of heart burn, anorexia, loss of weight and irregular bowel habits for the duration of 3 months. He had monitored his weight and within 3 months there was loss of 3kg. Patient had irregular bowel habits with history of passing occasional liquid and semisolid stool. There was no relevant family history, past history and surgical history. He was hypertensive but defaulted the treatment and not on any antihypertensive. He started smoking cigarette at the age of 30 years with 30 pack-year smoking history. All these complains directed him to consult a general practitioner. Giving importance to his heartburn, general practitioner started him on omeprazole and called him after 3 weeks. Patient’s symptoms were not subsided with omeprazole so he was referred to gastroenterologist for Oesophaogastroduodenoscopy (OGD). A treating gastroenterologist advised him to undergo Oesophaogastroduodenoscopy (OGD). Patient underwent OGD and there was findings of mild gastritis. Again he had advised to continue omeprazole for next one month. Along with gastritis, patient had loss of appetite so he had been prescribed with liquid appetizers. He continued the same medications for one month but there was no relief of symptoms. The treating gastroenterologist advised him to do ultrasound of abdomen and pelvis. On ultrasound a mass lesion was found arising from mid and lower pole of right kidney. With this report, he had been referred to our clinic for further evaluation and management. During the whole course of this treatment, patient did not complain of loin pain or hematuria. Clinical examination of all systems were unremarkable except presence of central obesity with feeling of vague mass on deep palpation at right flank. We evaluated him with CECT (Contrast Enhanced Computed Tomography) of thorax, abdomen and pelvis and asked him to do some blood reports. All blood reports including complete blood count, serum calcium, liver function test, renal function test, erythrocyte sedimentation rate were normal. CECT was suggestive of 12.6 x 13.4 x 11.7cm heterogeneous lesion arising from mid and lower pole of right kidney with mild surrounding fat stranding with involvement of right renal vein by the lesion (Figure 1 and 2). There were enlarged lymph nodes at right renal hilum and aortocaval region. Inferior Vena Cava (IVC) and right adrenal gland were free with no distant metastasis.
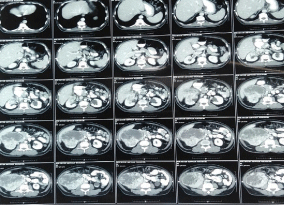
Figure 1: Axial section - Right renal mass lesion.
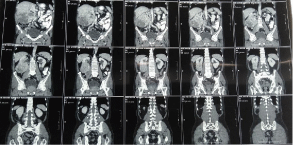
Figure 2: Coronal section - Right renal mass lesion.
The case was discussed in our multidisciplinary tumor board and board had advised to plan for right radical nephrectomy with retroperitoneal lymph node dissection in view of tumor >7cm in size, involving a more central position of the kidney with involvement of right renal vein. Patient was prepared for the surgery. First we controlled his blood pressure and fitness was obtained from anaesthetist and physian. Opposite kidney function was tested with DMSA (Dimercapto Succinic Acid) scan and it was functioning normally. Patient was planned with anterior trasnsabdominal approach. Intraoperative, we found that it was a very vascular tumor and bowel was adhered with the tumor. IVC was completely compressed by the tumor (Figure 3). We divided and ligated renal vein flush with IVC wall with macroscopic 4-5 mm tumor free margin (Figure 4). Multiple lymph nodes were enlarged at renal hilum but aortocaval lymph nodes were not significantly enlarged. Right adrenal gland was preserved. Enblock right kidney was removed with completion of retroperitoneal lymph node dissection (Figure 5). There was approximately 750ml of blood loss occurred. Post-operative course was uneventful and liquid diet was started after 12 hours of surgery. Patient was discharged on 6th postoperative day. On gross examination of the specimen (Figure 6), tumor was seen at the mid and lower pole of kidney. There were areas of haemorrhage and necrosis which were present predominantly in the center of the tumor. Tumor was present predominantly in the cortex with involvement of medullary and renal sinus. Perinephric adipose tissue and gerota’s fascia were uninvolved by the tumor. On microscopic examination under low and high magnification (Figure 7 and 8), solid or trabecular pattern, polygonal cells usually with clear cytoplasm were seen with presence of central nucleus and delicate branching vasculature. Final histopathology report was suggestive of conventional clear cell renal cell carcinoma with ISUP grade III with tumor size 13x 12.5 x 9 cm. Total nine lymph nodes were retrieved and all were free of metastasis. Lymphovascular invasion was present with involvement of renal vein. Perinephric fat, ureter, renal artery and renal vein cut margin were free of tumor. According to AJCC 8th edition, it was stage III (pT3a N0 M0). The available data regarding use of adjuvant targeted agents after complete surgical resection of RCC does not show any significant survival benefit. However, our multidisciplinary tumor board had advised him an adjuvant targeted therapy in view of locally advanced nature with risk of local or distant recurrence. But, patient and his relatives refused adjuvant treatment, hence he had been advised close surveillance. Patient is on periodic follow up with us according to our institutional protocol and after completion of one year of surgery, he is still disease free.
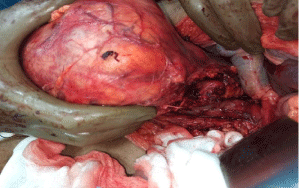
Figure 3: Dissection at right renal pedicle.
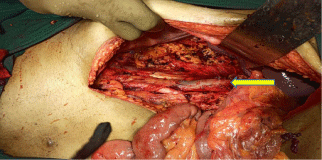
Figure 4: Post Right Radical Nephrectomy, Flush ligation of renal vein at IVC
(yellow arrow).
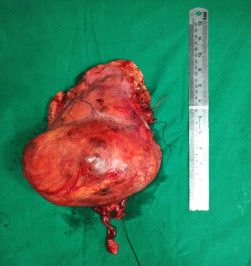
Figure 5: Specimen of Right Kidney.
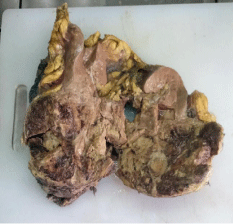
Figure 6: Cut section of specimen.
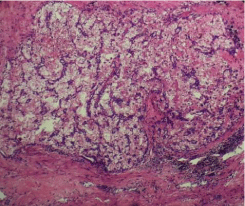
Figure 7: Microscopic view of CCRCC (Low magnification).
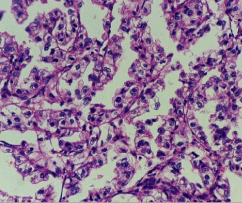
Figure 8: Microscopic view of CCRCC (High magnification).
Discussion
Renal Cell Carcinoma (RCC) is classified according to cell type, growth pattern, cell of origin, histochemical nature and on molecular basis into different types of adenocarcinomas as follows [18,19].
• Clear cell (75 to 85 percent of tumors)
• Papillary (10 to 15 percent)
• Chromophobe (5 to 10 percent)
• Oncocytic (3 to 7 percent)
• Collecting duct (Bellini duct; very rare)
Up to 5 percent of RCCs are considered unclassified. Clear cell carcinomas typically have a deletion of chromosome 3p and it arises from the proximal tubule [20]. A poor prognosis is associated with higher nuclear grade or the presence of a sarcomatoid pattern [21]. They are specifically associated with von Hippel-Lindau (VHL) disease. The present case had no history of any disease running in the family.
Von Hippel-Lindau gene
The VHL gene is found on chromosome 3 (3p25 to 26) and plays a pivotal role in the development of clear cell RCC in patients with VHL disease. VHL gene alterations appear to be important in the pathogenesis of sporadic RCC [22,23]. Common genetic abnormalities in sporadic VHL RCC includes the following:
• Loss of 3p (94 percent), it contains several genes associated with RCC which includes the VHL, BRCA1 associated protein 1 (BAP-1), and protein polybromo 1 (PBRM1) genes.
• Gain of 5q (69 percent)
• Monosomy or partial loss of 14q (42 percent)
• 7q gain (20 percent)
• 8p deletion (32 percent)
• 9p loss (29 percent)
The initial approach to deal with presumed RCC depends on the extent of disease, patient’s age and comorbidity. Extent of disease defines whether it is localized disease or advanced disease. The present case had localized RCC as disease was limited to kidney with involvement of renal vein with enlarged renal hilar and aortocaval lymph nodes which belongs to stage III of AJCC 8th edition. Surgery is curative in the majority of patients with localized RCC or who do not have metastases. Surgery is therefore the preferred treatment for patients with stages I, II, and III disease through a conventional approach or by a minimally-invasive approach such as laparoscopy. Radical nephrectomy is preferred in patients with any of the following presentation.
• Tumors >7 cm in size or those involving a more central position of the kidney.
• Suspected lymph node involvement.
• Tumor with associated renal vein or Inferior Vena Cava (IVC) thrombus.
• Direct extension into the ipsilateral adrenal gland.
Patients with a tumor ≤7cm should undergo a partial nephrectomy when it is technically feasible in order to preserve renal function with any of the following presentation.
• A solitary kidney.
• Multiple, small, and/or bilateral tumors.
• Patients with or at risk for chronic renal disease.
The present case had involvement of renal vein with lymphadenopathy so, we planned radical nephrectomy for him. The cancer specific survival rate for radical nephrectomy is around 80-90% [24,25]. But these patients are at long term risk of renal dysfunction. As the incidence of adrenal metastasis is very rare (<10%), adrenal gland should be preserved when it is uninvolved [26]. We had preserved the right adrenal gland in this patient. We had ligated vascular pedicle earlier to prevent tumor dissemination during surgery and then mobilized the kidney. We preferred anterior trasnabdominal approach to get the clear picture of involved renal vein with its thrombus through a small incision. Other approaches available are thoracoabdominal, extrapleural and through flank incision which are used depending upon the location of the tumor. In most institutions, laparoscopic nephrectomy has replaced open radical nephrectomy if the tumor size is less than 10cm [27,28]. In the present case, we did retroperitoneal lymph node dissection as there were suspected nodal metastases on preoperative imaging [29]. The available data from European Organization for Research and Treatment of Cancer (EORTC) 30881 trial did not show any differences in overall survival or deaths due to cancer in the radical nephrectomy alone arm or radical nephrectomy with lymph node dissection control arm . However in this series only 4% of patients had pathologically involved lymph nodes which limits their interpretation [30]. The other data from mayo clinic of analysis of 1797 patients did not show statistically significant difference in the risk of distant metastases, cancer specific mortality, or all-cause mortality in patients with pathologically involved lymph nodes who underwent radical nephrectomy with lymph node dissection [31].
Regarding adjuvant treatment, there is no clear role for targeted systemic adjuvant therapy or immunotherapy after complete surgical resection. However, our multidisciplinary tumor board had advised the index case an adjuvant targeted therapy in view of locally advanced nature with risk of local or distant recurrence. Multiple trials has shown results of targeted agents used as an adjuvant therapy i.e. sunitinib, pazopanib, axitinib, girentuximab, everolimus. However no one has demonstrated a clear clinical benefit of disease free survival or overall survival in the adjuvant setting [32-36].
The present case was evaluated as per non-specific symptoms and treatment was directed to cure these symptoms. This case highlights that the classical triad of loin pain, hematuria and a palpable abdominal mass in RCC is not that common. RCC should be considered as differential diagnosis if the patient presents with gastrointestinal symptoms and unexplained weight loss. Always GI cause will not be the culprit behind heartburn. The reason of heartburn in this case may be due to lifting of duodenum by the retroperitoneal mass causing reflux effect. The reason of irregular bowel habits may be due to lifting and compression of right colon. GI symptoms represent underlying pathology arising from GI tract or kidney. Hence it’s treating clinician’s job to think and implement immediately as per patient’s presentation so that unnecessary delay in diagnosis and treatment can be avoided. In this case radiological imagings were not advised for two months from first consultation and it was misdiagnosed. Lesson learned from the present case is to refer patient to higher speciality when there is unclear picture of clinical presentation.
Conclusion
The clinical presentation of Renal cell carcinoma is variable as the classic presentation is not that common and non-specific gastrointestinal symptoms always needs careful attention to avoid misdiagnosis.
Disclosures
Human subject: Informed consent was obtained from the patient for being included in the study.
References
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015; 67: 519.
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010; 7: 245.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394.
- WHO. Cancer Today. Data visualization tools for exploring the global cancer burden. 2020.
- Source: Globocan. 2020.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70: 7.
- Thompson RH, Ordonez MA, Iasonos A, et al. Renal cell carcinoma in young and old patients--is there a difference? J Urol. 2008; 180: 1262.
- SEER. Stat Fact Sheets: Kidney and Renal Pelvis.
- Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008; 113: 78.
- Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001; 166: 1611.
- Ries LA, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Revie w, 1973- 1997. National Cancer Institute, Bethesda. 2000.
- Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur Urol. 2016; 70: 458.
- Tsivian M, Moreira DM, Caso JR, et al. Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol. 2011; 29: 2027.
- Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2006; 118: 728.
- Adams KF, Leitzmann MF, Albanes D, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008; 168: 268.
- Waters WB, Richie JP. Aggressive surgical approach to renal cell carcinoma: review of 130 cases. J Urol. 1979; 122: 306-308.
- Griffiths LH, Thackray AC. Parenchymal carcinoma of the kidney. Br J Urol. 1949; 21: 128-151.
- Störkel S, van den Berg E. Morphological classification of renal cancer. World J Urol. 1995; 13: 153.
- Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005; 23: 2763.
- Presti JC Jr, Rao PH, Chen Q, et al. Histopathological, cytogenetic, and molecular characterization of renal cortical tumors. Cancer Res. 1991; 51: 1544.
- Oda H, Machinami R. Sarcomatoid renal cell carcinoma. A study of its proliferative activity. Cancer. 1993; 71: 2292.
- Yao M, Yoshida M, Kishida T, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002; 94: 1569.
- Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel- Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008; 14: 4726.
- Colombo JR Jr, Haber GP, Jelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology. 2008; 71: 1149.
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011; 59: 543.
- O’Malley RL, Godoy G, Kanofsky JA, Taneja SS. The necessity of adrenalectomy at the time of radical nephrectomy: a systematic review. J Urol. 2009; 181: 2009-2017.
- Burgess NA, Koo BC, Calvert RC, et al. Randomized trial of laparoscopic v open nephrectomy. J Endourol. 2007; 21: 610.
- Berger A, Brandina R, Atalla MA, et al. Laparoscopic radical nephrectomy for renal cell carcinoma: oncological outcomes at 10 years or more. J Urol. 2009; 182: 2172.
- Crispen PL, Breau RH, Allmer C, et al. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. Eur Urol. 2011; 59: 18.
- Blom JH, van Poppel H, Maréchal JM, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009; 55: 28.
- Gershman P, Thompson RH, Moreira DM, et al. Radical nephrectomy with or without lymph node dissection for nonmetastatic renal cell carcinoma: a propensity score-based analysis. Eur Urol. 2016.
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med. 2016; 375: 2246.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for highrisk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a doubleblind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016; 387: 2008- 2016.
- Mamtani R, Wang XV, Gyawali B, et al. Association between age and sex and mortality after adjuvant therapy for renal cancer. Cancer. 2019; 125: 1637.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized Phase III Trial of Adjuvant Pazopanib versus Placebo after Nephrectomy in Patients with Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol. 2017; 35: 3916.
