
Mini Review
Adv Res Text Eng. 2019; 4(1): 1037.
Influence of Chemistry of Dispersing Agent on the Properties of Textile Inkjet Inks for Polyester Printing
Abd El-Wahab H1*, Abd elBary HM1, Abd Elrahman M2, Hasanein M2
¹Chemistry Department, Faculty of Science Al-Azhar University, Egypt
²Degla Chemicals Company, Cairo, Egypt
*Corresponding author: Abd El-Wahab H Chemistry Department, Faculty of Science Al-Azhar University, Egypt
Received: April 29, 2019; Accepted: July 02, 2019; Published: July 09, 2019
Abstract
Recent years have seen a significant increase in the use of inkjet technology for printing on textile fabrics. Typical inkjet printed textile products included curtains, large advertising posters, flags and banners. So, in this research we focused on using different formulations of Textile inkjet inks based on different types of dispersing agent which prepared, evaluated and applied on the polyester fabric. The properties of the prepared ink formulations were analyzed by measuring viscosity, surface tension, and particle size. The purpose of this study was to investigate the influence of the dispersing agent chemistry on the behavior of inkjet inks characteristics used for inkjet printing on polyester textiles, the study including the effect of using different doses of the different dispersing agents and study of the time of milling. Difference of chemistries of dispersing agents give viscosity, Static Surface Tension, Dynamic Surface Tension in accepted range but it largely cleared in the particle size distribution which tend to performance of the inks on the print head and prevent clogging of nozzles. Also the study extend to evaluate the polyester printed by using the prepared inks according to light fastness, washing fastness, Alkali Perspiration Fastness and Crock Fastness which have been have good results agreement with this type of inkjet inks for textile printing.
Keywords: Textile; Inkjet; Dispersing agent; Inks; Particle size; Viscosity; Surface tension; Clogging nozzles
Introduction
Inkjet printing was found to be an incredibly powerful tool for its economic and time efficient performance in short run jobs and was adapted to print other functional materials such as conductive inks, Light Emitting Diodes (LED’s) [1], and even three-dimensional objects [2]. The inkjet printing process is very complicated, and their inks must meet the requirements for storage stability, jetting performance, color management, wetting and adhesion on substrate [1,3]. Due to it being a non-impact process, inkjet printing offers great versatility in terms of the variety of printable substrates. Emerging markets for inkjet printing include printing of plastics, circuit boards and electronic displays. For industrial applications, inkjet printing is currently used for graphics, signs, labels, textiles and flags and banners [4], While continuous inkjet is used for fast, quick change processes such as printing use by dates on produce, it is generally accepted that for most other applications, drop-on-demand inkjet printing is preferred. Two major types of drop-on-demand print heads namely, thermal and piezo jet exist. Piezo inkjet print heads are more widely used in wide format industrial applications. [5]. Recent years have also seen a significant increase in the research and development activities into inkjet printing on textiles. It is apparent to the authors that large, industrial scale inkjet printing facilities will soon be available to textile printers. There are two ink types which can be chosen from depending on the application. Traditionally, dye-based inks have been used due to their high color quality [5,6]. Inkjet inks are mainly classified according to medium system used into (solvent based inks – water-based inks – eco-solvent inks – UV inks – latex inks) and according to the colorant used into (pigment based – dye based). To be suitable for inkjet printing, an ink must also have the required viscosity profile for a specific print head. Although affected by pigment loading, the viscosity profile of an ink is largely governed by polymeric components of the ink such as binders and humectants. Other factors affecting the suitability of an ink for jet printing include pH, surface tension and thermal stability [7].
Dispersing agents are additive used in coating materials to facilitate the dispersion of the solid constituents in the liquid phase during manufacture, enhance storage, stability. Dispersion process is a homogeneous distribution of solids in a liquid medium. In the dispersion process, the adhesive strengths between the finest solid particles must be overcome [8]. The dispersion process occurs on three steps: wetting, separation and stabilization. Figure 1 represents the dispersion process. The wetting process of pigment means displacement of air from the surface of pigment with the vehicle (resin) and wet the particles. The separation of pigment occurs by milling or grinding process in which the pigment agglomerates are broken down into smaller agglomerates and/or individual particles which are wetted by the carrier medium [9]. Polymeric dispersants have been adopted by the manufacturer of surface coatings for a variety of reasons, all generally associated with improvements in the state of pigment dispersion. The benefits of using polymeric dispersants are: (1) Dispersion of high pigment loading without increased viscosity. (2) Reduction in milling time. (3) Improve the ink quality especially flow and gloss. (4) Superior color development. (5)Enhance the flocculation resistance. (6)Improved color stability. Polymeric dispersants have two key components in their structure: anchoring groups that adsorb on to the pigment surface and polymeric chains that provide the stabilization barrier around the pigment particle [10- 12]. Dispersing agents are usually sophisticated polymers which have a pigment affinic groups such as (OH-, COOH-, NH2-, NR2-, aryl-, nitrile-, amide-, etc..) which adsorbs on pigment particle surface [13- 15], and a solvent affinic chain (tail) such as (polyether – polyacrylate – polyester – etc..) which extends in solution to provide steric stabilization against re-agglomeration [16]. Thickeners and binders play a paramount role in pigment printing and rheological properties of the printing pastes during application and to obtain sharp and clean drawing patterns by preventing dye migration. The synthesis and application of thermally stable dyes for ink-jet printed color filters was investigated [17-19]. Conventional textile printing can apply dyes that are fixed by saturated steam and different processes have been developed for textile printing, depending on the kind of the fabric used (cellulosic, polyester, acrylic, protein), on the nature of the dyestuff applied (reactive dye, vat dye) and on the expected quality of the final product [20]. Water is one of the preferred vehicles for jet inks because of its viscosity, ionic nature, and conductivity. A water-based ink has key advantages over an organic-based ink, as it is less toxic, and does not denature enzymes; this allows for a one step printing process which greatly reduces the cost of production [21]. The balance of the inkjet ink composition may be water, at least 80 per cent of the ink composition is water. Inkjet inks may also include a pigment. Generally, the amount of pigment ranges from about 2 wt% to about 6 wt% [22]. In spite of the superior thickening properties of kerosene/water emulsion which also contributes towards a soft hand of the print and good fastness properties, environmental and economic factors have compelled the search for a replacement for kerosene [23]. Various modified ink formulations for inkjet printing on nylon 66 carpet materials were prepared and evaluated. A comparison between ink formulations with and without thickeners, in terms of jetting properties, storage stability and qualities of the printed images on nylon 66 carpet materials, such as optical density, drop size, and penetration properties [24].
Experimental
Materials
In this study we used Disperse Red 60 from Rialco -UK as a Colorant, Mergal K14 from Troy – USA as a biocide, BYK 019 from BYK-GERMANY as a defoamer, N-Methyl Pyrrolidone from Sigma–Aldrich as Solvent, Di Ethylene Glycol from Sigma–Aldrich as humectants, Deionized water, Dispersing Agents commercial (DISPERSING A,C (BYK -Germany) DISPERSING B (BASF- USA) – DISPERSING D (Degla Chemicals Egypt), Zirconia beads 0.8 mm from China.
Method
Synthesis of dispersing agent D: (1 mol) of alpha naphthol mixed to (1mol) of polyether amine until complete solubility And then add (2 mol) of glutaraldehyde under stirring for 10 hours at 100 0C
Active ingredients 100% Yellowish clear viscose liquid. The chemical structure of the prepared dispersing agent are shown in scheme 1.

scheme 1: Chemical structure of the prepared dispersing agent D.
Preparation of inkjet printing ink based on four different dispersing agents: The ink configuration process was as follows: deionized water, Disperse Red 60, Solvents, Humectants, Defoamer and Biocide were selected according to the ink formulations.
The procedure of the preparation of inkjet ink: 5gm of NMP mixed to (2.4, 3.2, 4, 4.8gm) (30%, 40%, 50%, 60%) respectively of Dispersing agent to generate mixed solution and then added to 8 gm of Disperse Red 60, 20 gm of DEG, 1gm of Byk019 and 0.1gm of Mergal k14 then added to previous mixed solution until completely Mixing solution then added 70gm of zirconia bead 0.8 mm in 100 ml container. Then shaked by using coating fast mixer BGD 760 for 2,4,6,8 hours
This experiment done with 4 different chemistries (4 samples)
Different textile inkjet ink formulations were prepared in this study, as listed in Table 1.
30%
40%
50%
60%
Disperse red 60 (Dye)
8
8
8
8
Dispersing agent
A,B,C,D
2.4
3.2
4
4.8
Defoamer
1
1
1
1
NMP
5
5
5
5
DEG
20
20
20
20
Biocide
0.1
0.1
0.1
0.1
Water
63.6
62.8
62
61.2
Table 1: General Formulation of Prepared Inks based on 30-60% of the used dispersing agent.
The amount of dispersing agent was varied from 30%-60% while other components remained constant also the time of milling is varied 4,8 hours for each chemistry.
Characterization
Bruker FT-IR analyzer: ALPHA-Platinum FT-IR Spectrometer with ATR Platinum–Diamond sampling module from 400 to 4000 cm–1
Gel Permeation Chromatography (GPC): Agilent model 1515 pump system equipped with 1260 infinity refractive index detector and using THF as eluent. Operating with a flow rate of 1.00 ml/min at 35 0C. Column PL-gel 3 lm Mixed E 300 7.5 mm covering a molecular weight range of 600–400,000 mg/g was used and was calibrated using polystyrene standards.
Dynamic Surface Tension (DST): SITA pro line t15 is an allround talent among bubble pressure tensiometer. The measuring device has a comprehensive bubble lifetime range from 15 ms to 20 s which covers a wide concentration range from several grams to a few micrograms of surfactant in 1-liter process solution.
Particle Size Distribution (PSD): The Mastersizer 3000 is the latest generation of the world’s most widespread particle sizing instrument, fast measurement times for high sample throughput and a measurement size range from 10nm to 3.5mm. Combined with a range of wet and dry dispersion accessories.
Static Surface Tension (SST): Theta Lite is a compact and robust optical tensiometer for simple and precise operations. Measuring range (°, mN/m) 0…180, 0.01…1000, Accuracy (°, mN/m) ± 0.1, ± 0.01.
Viscosity: DV-E Viscometer Direct display in cP or mPa·s, % Torque, Spindle, and Speed Torque measurement accuracy: 1% of full-scale range Repeatability: 0.2% of full-scale range.
Characterization of the printed textile by using the prepared inkjet inks
Light Fastness: ISO105-B02:1994(160h).
Wash Fastness: ISO105-C06:2010(40h).
Alkali Perspiration Fastness: ISO105-E04:2013.
Crock Fastness: ISO105-X12: 2001.
Results and Discussion
Characterization of used dispersing agents
FTIR
Dispersing D: The FTIR of the prepared dispersing agent D showed that the OH broadband Peak at 3501 cm-1, C-H stretching peaks at 2868-2900 cm-1, also the strong C-O stretching peak at 1095 cm-1. Also, aromatic C-H stretching at 3033cm-1 which corresponding to 1 naphthol aromatic ring as well as C=C aromatic pending at 1638 cm-1. The IR represented in Figure 1.
Figure 1: FTIR of the prepared dispersing agent D.
GPC: Molecular weight is an important factor that affects many polymer characteristics. The M.wt of the used dispersing agents has a direct relationship with particle size, So, based on the obtained results which were showed in the Figures 2-5, Obviously, the dispersing agent A (Mn = 5.9863e2, Mw=9.0266e2) and the dispersing agent B which is alkyleno oxide based (Mn = 6.9852e2, Mw=8.7071e2), on the other hand the other two dispersing agent give lower molecular weight distribution than the first two dispersants
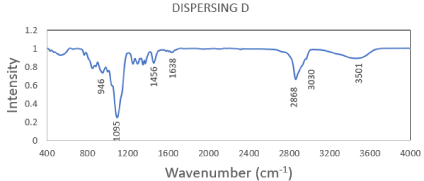
Figure 2: Gpc of dispersing A.
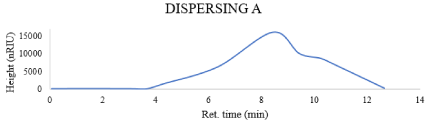
Figure 3: Gpc of dispersing B.
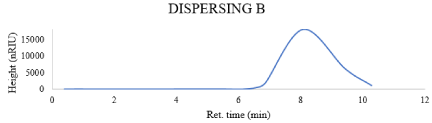
Figure 4: Gpc of dispersing C.
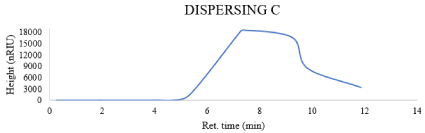
Figure 5: Gpc of dispersing D.
The dispersing agent C which is Modified styrene acrylate based has values of (Mn = 1.5458e2, Mw=3.7757e2) and the dispersing agent D which is Naphthol dye derivative dispersant has values of (Mn = 1.9971e2, Mw=3.2369e2).
Effect of changing chemistry and milling time on viscosity of prepared inks
Inks suitable for inkjet printing have viscosity in the range of 1-15mpa.s [125]. The viscosity values of all prepared inks in this research are shown in Table 2. And the Effect of changing concentration & chemistry of dispersing agent on the viscosity after 4 and 8 hr milling also represented in Figures 6, 7. Results show narrow difference between all chemistries that means that changing chemistry doesn’t affect significantly the rheological behavior of prepared inks.
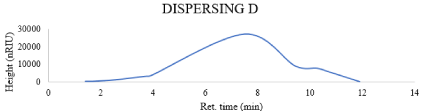
Figure 6: Effect of changing concentration & chemistry of dispersing agent
on the viscosity after 4 hours.
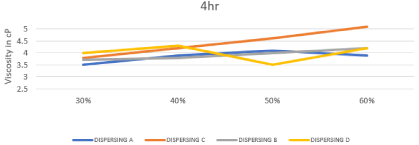
Figure 7: Effect of changing concentration & chemistry of dispersing agent
on the viscosity after 8 hours.
4hr
8hr
30%
40%
50%
60%
30%
40%
50%
60%
DISPERSING A
3.5
3.9
4.1
3.9
3.5
3.7
3.8
4
DISPERSING C
3.8
4.2
4.6
5.1
3.5
4.2
4.5
4.8
DISPERSING B
3.7
3.8
4
4.2
3.7
4.2
3.8
4
DISPERSING D
4
4.3
3.5
4.2
3.7
3.7
3.8
3.9
Table 2: Viscosity obtained results for the prepared inkjet inks after 4 and 8 milling based on 30-60% of dispersing agents.
Effect of changing chemistry and milling time on Static Surface Tension of prepared ink
Also based on the literature survey the ideal inks for inkjet printing should be have a surface tension in the range of 25–60 mN/m [25]. So, the surface tension values of all prepared inks based on different types of the dispersing agents are shown in Table.3 also effect of changing concentration & chemistry of dispersing agent on Static Surface Tension after 4 and 8 hours represented in Figures 8, 9. From this result we can detect the prepared inks with different chemistries reduced the surface tension value within the range of 27-33 mN/m. these values are within the optimum surface tension range for printing water-based ink. The overall result shows that static surface tension decreased as dispersion time and dispersant concentration increased, and agreement with this type of the inkjet printing inks.
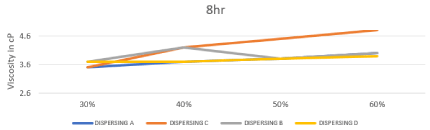
Figure 8: Effect of changing concentration & chemistry of dispersing agent on
Static Surface Tension after 4 hours.
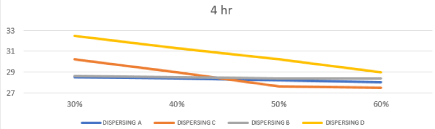
Figure 9: Effect of changing concentration & chemistry of dispersing agent on
Static Surface Tension after 8 hours.
4hr milling
8hr milling
30%
40%
50%
60%
30%
40%
50%
60%
DISPERSING A
28.5
28.4
28.2
28
27.9
27.6
27.6
27.5
DISPERSING C
30.2
29
27.6
27.5
29
28.5
28.1
28
DISPERSING B
28.6
28.5
28.4
28.4
28.6
28.5
28.4
28.3
DISPERSING D
32.5
31.3
30.2
29
29.7
29.4
29.5
29.5
Table 3: Static Surface Tension obtained results for the prepared inkjet inks after 4 and 8 milling based on 30-60% of dispersing agents.
Effect of changing chemistry and milling time on Dynamic Surface Tension of prepared inks
The bubble pressure is monitored during a time interval starting with the time when the bubble first emerges from the orifice until bubble release, and the dynamic surface tension is calculated based on the difference between Pmax and hydrostatic pressure P0 and the capillary radius. Upon increasing the gas flow (bubble rate), the surface age of the bubbles is decreased, and thus, the time for the surfactant molecules to migrate to the bubble surface and to absorb is short. Consequently, the coverage of the surfactant on the surface becomes more and more incomplete and the surface tension increases with decreasing bubble age [26,27]. The results of water based systems have a DST of 72 Dyne/cm were mentioned in Table.4, and Effect of changing concentration & chemistry of dispersing agent on Dynamic surface Tension after 4 and 8 hours represented in Figures 10, 11, this figures showed that DST in all systems decreased due to presence of dispersing agent in the range of 47-51 Dyne/cm. and the dispersing A (polyurethane based) and Dispersing B (oleo alkyleno oxide) show initial DST 48.7 and 48.6 Dyne/cm respectively, which increased with increasing time and concentration to 49.1 and 48.9 Dyne/cm respectively this is due to the lower wetting effect of polyurethane group and aliphatic long chain of oleo alkyleno oxide towards compact aromatic structure of disperse red 60. While Dispersing C (styrene acrylate based) and Dispersing D (naphthol derivative) show initial DST 48.9 and 49.7 respectively, which decreased with increasing time and concentration to 48.3 and 47 respectively due to higher affinity of the aromatic anchor group of styrene in Dispersing C and naphthol group of Dispersing D towards compact aromatic structure of disperse red 60.
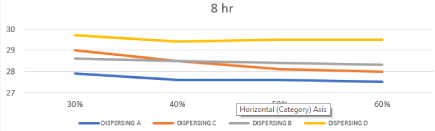
Figure 10: Effect of changing concentration & chemistry of dispersing agent
on Dynamic surface Tension after 4 hours.
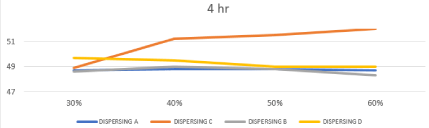
Figure 11: Effect of changing concentration & chemistry of dispersing agent
on Dynamic Surface Tension after 8 hours.
@25°c
4hr
8hr
30%
40%
50%
60%
30%
40%
50%
60%
DISPERSING A
48.7
48.8
48.8
48.7
50.3
50
49.5
49.1
DISPERSING C
48.9
51.2
51.5
52
50
49.7
48.9
48.3
DISPERSING B
48.6
49
48.8
48.3
48.8
49.1
48.7
48.9
DISPERSING D
49.7
49.5
49
49
48
47.6
47.5
47
Table 4: Dynamic Surface Tension obtained results for the prepared inkjet inks after 4 and 8 milling based on 30-60% of dispersing agents.
Effect of changing chemistry and milling time on Particle Size Distribution of prepared ink
The inkjet printing ink should be have a particle size of 0.5 μm or below is required. Smaller particles allow for improved stability, optical density, color gamut, gloss and light fastness [28]. The size of the particles has a major influence on a number of properties of materials and is a valuable indicator for quality and performance, as the less particle size passing through the inkjet printhead easily and also to ejected without causing any kind of blocking to the nozzles of print heads, the particle size analysis results reflect a sharp and clear difference between the different chemistries as shown in table.5, represented in Figures 12, 13. So based on the above survey, the obtained results of the prepared inks based on dispersing B showed that the highest particle size compared to the other dispersants because of the poor compatibility of straight chain alkyl group towards the highly aromatic disperse red 60. And based on the dispersing A the obtained results were significantly better than dispersing B but still too high compared to dispersing C and dispersing D because of the presence of polyurethane chains which consist of hydrophilic (Poor affinity with dye) and hydrophobic sites (Good affinity with dye) on the anchor group this means that the hydrophobic site make incomplete anchoring on the surface of disperse red 60, this mechanism can be represented as in Figure 14. Dispersing C and Dispersing D gave the lowest particle size distribution among the tested dispersants due to the intense presence of styrene and naphthol groups respectively which have high affinity to the highly aromatic disperse red 60 throw p - p interaction (delocalized p electrons).
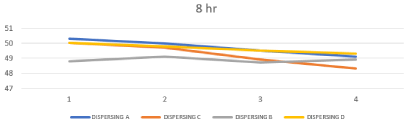
Figure 12: Effect of changing concentration & chemistry of dispersing agent
on Particle Size Distribution after 4 hours.
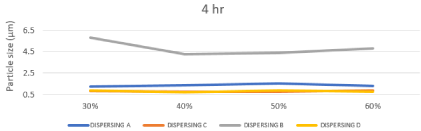
Figure 13: Effect of changing concentration & chemistry of dispersing agent
on Particle Size Distribution after 8 hours.
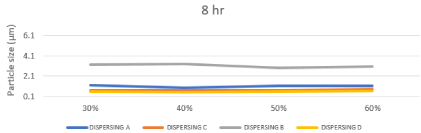
Figure 14: Illustration of a fully networked associative thickening mechanism
based on the using dispersing agent.
Particle Size Distribution
4hr
8hr
30%
40%
50%
60%
30%
40%
50%
60%
DISPERSING A
1.22
1.32
1.53
1.31
1.17
0.915
1.14
1.14
DISPERSING C
0.843
0.765
0.738
0.862
0.71
0.69
0.664
0.835
DISPERSING B
5.8
4.27
4.35
4.8
3.2
3.3
2.9
3
DISPERSING D
0.902
0.674
0.884
0.769
0.583
0.508
0.571
0.6
Table 5: Particle Size Distribution obtained results for the prepared inkjet inks after 4 and 8 milling based on 30-60% of dispersing agents.
Morphology of the prepared inkjet printing inks after 4-8 hours milling
TEM analysis of the prepared inkjet ink based on the prepared dispersing agent D after 4 and 8 milling was carried out and is shown in Figure 15 and 16. Interestingly, in Figure 14, the morphology of the prepared ink is observed. A bright thick shell rings around the seeds for the disperse red 60 (dye) particles were obviously observed. The dark component could be attributed to core while the lighter component could be due to the prepared dispersing agent (D) shell. In some particles, the boundaries between the shell and core were much less distinct which could mean that there is greater mixing ratio of the the disperse red 60 (dye) to prepare dispersing agent (D). A similar variety of structures were found for the red dye encapsulated with dispersing agent ( D) particles are shown in Figure 15, after 8 milling at the same conditions with some particles having a uniform structure and mono-dispersed spherical particles, but after 8 hrs milling the particle size became lower than as in after 4 hrs milling and this is maybe due to the good dispersion of ink components as the represented in Figure 17 which are shown the mechanism of the dispersing agent process. Tem images are shown in Figure 16. Which have an average diameter of (138-571 nm) as in Figure 14. After 8 hrs. milling and average diameter of (387 to 581) as in Figure 15 after 4 hrs. milling.
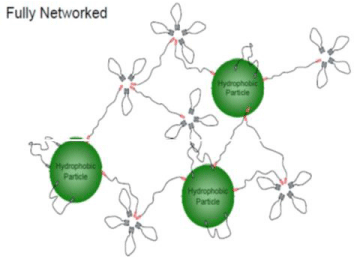
Figure 15: TEM of the prepared inkjet ink beased on (DISPERSING D) after
8 hrs miling.
Figure 16: TEM of the prepared inkjet ink beased on (DISPERSING D) after
4 hrs miling.

Figure 17: The dispersion process.
Characterization of Printed Polyester by Using Prepared Ink Based on the Dispersing Agent
Based on the obtained results which tabulated in the Table 6,
Final Ink
Transfer conditions
Light Fastness
Washing Fastness
Alkali Perspiration Fastness
Crock Fastness
ISO105-B02:1994(160h) Rating 1~7
ISO105-C06:2010(40°C) Rating 1~5
ISO105-E04:2013 Rating 1~5
ISO105-X12: 2001 Rating 1~5
Magenta
200°C/ 30sec
6
4/5
4/5
4/5
Table 6: Fastness obtained results for the printed polyester fabric.
The Light Fastness of the printed polyester equals 6, that means that the light fastness of disperse red 60 doesn’t affected by the dispersant used keeping, the light fastness value of dye as it is.
Wash and alkali perspiration fastness give a very good results (4-5), that means no migration of dispersant over the polyester fabric and deeply absorbed inside polyester fibers and high penetration.
Crock fastness also give a very good result 4-5, that also mean complete absorption of disperse red 60 inside polyester fibers and that successfully done by the good dispersibility of Dispersing D to the disperse red 60 dye. All of this results were showed in Figure 18. The diffierent pictures of printed polyester by using the prepared inkjet printing ink based on the prepared dispersing agent and are showed the results of light, wash, Alkali perspiration, crock fastness which obtained in table 6.
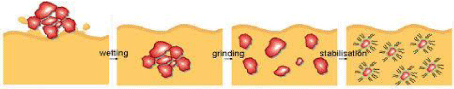
Figure 18: Diffierent pictures of printed polyester by using the prepared inkjet
printing ink based on the prepared dispersing agent and are showed the
results of light, wash, Alkali perspiration, crock fastness are shown in table 6.
Conclusion
Four different chemistries of dispersing agent were used in this study with different concentration and milling time, four physical properties were studied viscosity, surface tension (dynamic-static) and particle size.
Changing chemistry of dispersing agent with different concentration and milling time doesn’t effect significantly the Viscosity, static surface tension properties, but it obviously clear in Dynamic surface tension and the particle size distribution.
Polyether modified 1-Naphthol-glutarldehyde condensate chemistry (dispersing agent D) gave the best distribution among other chemistries with concentration of 40% and 8 hours milling time followed by styrene acrylic based with concentration 50% and 8 hours milling also with the same method of testing unlike the other two chemistries polyurethane and oleo alkyleno oxide which gave high particle sizes in all concentrations also with increasing time of dispersion.
References
- Magdassi.S. The chemistry of inkjet inks, first ed. World scientific, Singapore. 2010.
- Kosolia CT., Varka EM, Tsatsaroni EG. J Surfact Deterg. 2011; 14: 3-7.
- Marie MM, El-Hamaky YH, Maamoun D, Ibrahim DF, Abbas SM. Pigment Ink Formulation for Inkjet Printing of Different Textile Materials, International Journal of Innovation and Applied Studies. 2013; 4: 239-247.
- Ahmed AS, Kandile NG, Negm NA. Preparation and evaluation of (resorcinol – aldehyde) based polymeric dispersants for inkjet printing ink. 2018; 35: 298- 314.
- Yossif NA, Kandile NG, Mohamed AA, Negmc NA. Preparation and characterization of polymeric dispersants based on vegetable oils for printing ink application, Progress in Organic Coatings. 2017; 111: 354-360.
- Sharma M.K. Surface Phenomena and Additives in Water-Based Coating and Printing Technology, New York: Plenum Press. 1995.
- Schweikart KH, Fechner B, Macholdt H-T. “DSTM, A Modern Method for Optimizing Pigment Preparations for Ink Jet”, Proceedings ECC: The Power of Ink Jet. 2004; 15-26.
- Bieleman J, Heilen W, Silber S, Ortelt M, Scholz W. Surface-Active Agents, Additives for Coatings, Willy publication, Weinheim. 2000; 67-99.
- Tadros TF. Dispersion of Powders in Liquids and Stabilization of Suspensions, Wiley Publication. 2012; 1-16.
- Schofield J.D. Extending the boundaries of dispersant technology, Prog in Org. Coat. 2002; 45: 249–257.
- Lokhande GP, Jagtap RN. Design and synthesis of polymeric dispersant for waterborne paint by atom transfer radical polymerization. Des. Monomers Polym. 2016; 19: 256– 270.
- Pirrung F, Auschra, C. Polymeric Dispersants; in Macromolecular Engineering. Precise Synthesis, Materials Properties, Applications. Wiley publishing. 2007; 2135-2180.
- van den Haak HJW. Journal of Coatings Technology. 1997; 69: 137.
- Duivenvoorde FL. Pigment dispersing in powder coatings: synthesis and use of block copolymer dispersing agents Eindhoven: Technische Universiteit Eindhoven. 2000.
- Dr. Joy T. Kunjappu. Polymers in Ink Chemistry, Ink world magazine. 2009.
- Pigment preparations for water- and solvent-based ink jet inks: for details see brochure “The Source of Ink Jet Printing Excellence”, Frankfurt (Germany). 2004.
- Zahran M.K, Mahamoud RI, El-Rafie MH. “Utilization of partially methylated polysaccharide guar gum in pigment printing”, RJTA. 2007; 11: 41-8.
- Fijan R, Sostar-Turk S, Lapasin R. “A study of rheological and molecular weight properties of recycled polysaccharides used as thickeners in textile printing”, Carbohydrate Polymers, 2009; 76: 8-16.
- Kim YD, Kim JP, Kown OS, Cho IH. “The synthesis and application of thermally stable dyes for ink-jet printed LCD colour filters”, Dyes and Pigments, 2009; 81: 45-52.
- Saffour Z, Viallier P, Dupuis D. “Rheology of gel-like materials in textile printing”, Rheologica Acta. 2006; 45: 479-85.
- Crouch E, Cowell DC, Hoskins S, Pittson RW, Hart JP. “Amperometric, screen-printed, glucose biosensor for analysis of human plasma samples using a biocomposite water-based carbon ink incorporating glucose oxidase”, Anal. Biochem. 2005; 347: 17-23.
- Rolly L.J. “Inkjet ink composition”, WIPO Patent Application WO/2008/024592, WIPO, Geneva. Saffour Z, Viallier P, Dupuis D. “Rheology of gel-like materials in textile printing”, Rheologica Acta. 2006; 45: 479-485.
- El-Molla M.M, Schneider. R. “Development of ecofriendly binders for pigment printing of all types of textile fabrics”, Dyes and Pigments. 2006; 71: 130-137.
- Abd El-Wahab H, El-Molla MM, Lin L. “Preparation and characterisation of ink formulations for jet printing on nylon carpet” Pigment & Resin Technology.2010; 39: 163–169.
- Yin H, Zhao XM, Du YG, 2010, Oligochitosan, A plant diseases vaccine—A review, Carbohydrate Polymers. 2010; 82: 1–8.
- Yin H, Du YG, Zhang JZ. Low Molecular Weight and Oligomeric Chitosan-s and Their Bioactivities, Current Topics in Medicinal Chemistry. 2013; 9: 1546- 1559.
- Karl-Heinz S, Björn F, Hans-Tobias M. “Dynamic Surface Tension: A Key Parameter for Excellent Ink Jet Preparations”, IS&T’s International Conference on Digital Production Printing and Industrial Applications. 2005.
- Bugner DE, Bermel AD. “Particle size effects in pigmented inkjet inks”, International Conference on Digital Printing Technologies. Seattle, Washington, 1999; 43: 320-324.