
Research Article
Adv Res Text Eng. 2020; 5(3): 1056..
Identifying the Color Strength, Color Intensity, Chromophore Extent and Colorfastness Properties of Cellulosic Fabrics
Alam SMM1 and Islam S2*
1Department of Textile Machinery Design and Maintenance, Bangladesh University of Textiles, Bangladesh
2Department of Fabric Engineering, Bangladesh University of Textiles, Bangladesh
*Corresponding author: Shariful Islam, Department of Fabric Engineering, Bangladesh University of Textiles, Tegjaon, Dhaka, 1208, Bangladesh
Received: July 01, 2020; Accepted: July 20, 2020; Published: July 27, 2020
Abstract
The aim of this paper is to identify the color strength properties, color intensity properties, chromophore extent properties and colorfastness properties of cellulosic fabrics. The findings of this research established that cellulosic fiber like cotton reacted with natural sustainable dyes and produced permanent covalent bonds in between the fiber cellulose and the terminal reactive group and exhibited improved above stated color properties. The investigation was conducted on gabardine twill fabric using several sustainable dyes namely blackberry, cherry, orange and carrot. With the facility of required chemicals and auxiliaries, cellulosic fiber of cotton fabric responded against the reactive group of blackberry dye, formed stable covalent bonding and ensured enhanced color properties. The requisite experiments were initiated using the standard instructed by ASTM and AATCC as stated underneath the paper. Above stated color properties were experimented using Reflectance Spectrophotometer and FTIR Instrument by the scattering appearances and spectral reflectance of the samples as detailed beneath the paper. Infrared Radiation was ejected with the sustenance of FTIR instrument throughout the samples to provide spectral reflectance with peak values that certifies the existence of colorant or chromophore in the dyed cellulosic fabric with the above stated enhanced color properties. This research revealed potential ways for the scholars to further study in this field.
Keywords: Color strength; Color intensity; Chromophore extent; Transmittance; Cellulosic fabrics; Infrared radiation; Scattering characteristics
Introduction
This paper has great importance on textile coloration and printing technology, as controlling colorfastness properties are must in this area. Different intellectuals worked regarding to this experiment at different times where literature review showed different results. Some of them were parallel and some were extensively contradictory. Due to the range of variables convoluted, if any dyes, chemicals or auxiliaries were changed during experiments, then the other color related properties of the fabrics were also changed [1].
Dyeing is the coloration process of textile materials such as fibers, yarns or fabrics. Dyeing is basically done in a solution that contains dye particles. After the coloration process is done, a strong bonding is happened in between the fibers and the dyestuffs [2]. Temperature, time, pH are the main parameters of dyeing. Dyeing particles are mainly two types such as natural and synthetic [3].
The dye diffusion is outstanding in fiber dyeing, hence the extent of dye applied to dye at this phase is also greater. Fiber dyeing is moderately more expensive than yarn, fabric, and garments dyeing [4]. The choice concerning the assortment of colors has to be made initial in the processing stages. Fiber dyeing is naturally used to dye wool and other fibers those are applied to make yarns with two or more colors [5].
Acid dyes are water solvable anionic dyes those are used to dye the fibers of wool, nylon, silk and reformed acrylic fibers exhausting neutral to acid dye solutions. It is basic dye, which is water solvable cationic dye that is basically used to dye acrylic fibers [6]. Direct dyes are generally applied in neutral or somewhat alkaline solutions around the temperature of boiling point, with the adding of either sodium sulfate or sodium carbonate and these dyes are applied to dye cellulosic fibers [7].
Mordant dye needs a mordant that develops the colorfastness properties of dyes against laundry, sun and sweat. Vat dyes are basically non-soluble in water and unable to color the fibers straightly [8]. The prime use of such dyes is to dye polyester fibers but they are also applied to dye nylon and acrylic fibers. To dye the fabrics with disperse dyes high temperature like 130°C is essential. The dyeing medium is always acidic [9].
Reactive dyes have chromophore groups those are attached with the substances, which are proficient enough to respond quickly with the substituent fibers [10]. The covalent bonds those attribute reactive dyes to sustainable fibers showed permanent colored shades. Disperse dyes are mainly formed to dye the cellulose acetate, which are not solvable in water [11]. Azoic dyeing is the process where insoluble azo dye is formed unswervingly inside the fibers, yarns or fabrics. The Sulfur dyes are produced to dye the cellulosic fibers, yarns or fabrics of darker shades [12].
Scouring is a laundry procedure on cotton fabric to eliminate natural polish and non-fibrous scums from the fabrics and any additional muddying or dust. Fabric is simmered in an alkali solution that creates soaps with free fatty acids [13]. Main chemical substance applied in the cotton scouring is sodium hydroxide that changes saponifiable fats and oils to detergents, liquefies inorganic substances and changes pectose and pectin into their solvable salts [14]. Additional scouring chemical is cleanser that is an emulsifying substance and eliminates soil and dust elements from fabric [15].
Harm can be caused to the cotton fabrics by sodium hydroxide, so decrease alkali materials in the discharge are better. It is noted that bio-scouring is familiarized in the scouring procedure in which organic agents are applied like as enzyme [16].
Bleaching improves the lightness values of fabrics by eradicating natural coloring and residual trace contamination from the cotton substances [17]. If the fabric needs to be colored a dark shade, then light bleaching is recommended and vice versa [18]. On contrary, for dyeing the light shades or bright white color, heavy bleaching is recommended. After completion of scouring and bleaching process, optical whitening agents are applied to increase the brightness of the fabrics [19]. Optical whitening agents or optical whitening agents are of three types like blue, violet and red [20].
Yarn dyeing is the process by which colors are added to the yarns at cone or package form. In skein dyeing process, the yarns are slackly coiled into skeins and then dyeing process is carried out [21]. The yarns have better dye diffusion, but the technique is leisurelier and relatively more costly. The technique of package yarn dyeing is relatively quicker, but the dye consistency may not be as decent as that of skein dyeing process [22].
In beam dyeing process, a punctured warp beam is used in its place of the spools used in package dyeing process. Space dyeing is applied to yield yarns with multiple colors and shades [23]. Basically, yarn dyeing gives satisfactory color fascination and diffusion for most textile substances [24]. Dense and extremely twisted yarns may not have good dye diffusion. This procedure is characteristically used when dissimilar colored yarns are applied in the construction of fabrics [25].
The process of fabric dyeing is known as piece dyeing, where fabrics are dyed after the fabrics have been made. It is popular, cheap and common method of dyeing fabrics of solid colors [26]. The decision concerning coloration can be made after the fabric has been made. Therefore, it is appropriate for rapid response orders [27]. Dye diffusion may not be good in denser fabrics, so yarn dyeing is occasionally used to dye dense fabrics in solid colors. Different types of dyeing machines are applied for piece dyeing [28]. The assortment of the apparatus is based on issues such as dye and fabric physiognomies, price, and the proposed end use [29].
Basically, printing is a process of localized dyeing. This is the system by which dyes or colors in a thick paste form are applied on a surface of fabrics [30]. In printing process, fibers are bonded with the printing paste [31]. Printing is also one type of dyeing but the main difference is, dyeing is done on the whole fiber, yarn or fabric, on contrary, printing is done on the localized or limited area [32].
Materials and Methods
Required fabric
Medium weight gabardine cotton fabrics were applied in this research for experimentation. Twill woven fabrics were used in this research as mentioned in Table 1.
S.N
Composition
Construction
Weave
Weight (g/m2)
Width (²)
1
100% Cotton Twill
16´12/ 115´62
3/1 LHT
298
57
Table 1: Required Fabric for the Research.
It is clear from table 1 that, 100% cotton twill woven fabric was used whose weight and width was 298g/m2 and 57''. Warp yarn count was 16Ne and weft yarn count was 12 Ne. Ends per inch measurement was 115 and picks per inch measurement was 62. Commercially, this type of fabric is called gabardine.
Required dye stuffs
Orange dye: Orange is a wonderful fruit with very good odor, which can be applied to color cotton twill fabric with light orange color. It offers very light shade of orange color to yarns or fabrics. Figure 1 shows the chemical structure of orange dye [33].
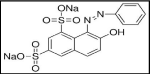
Figure 1: Chemical Structure of Orange Dye.
Carrot dye: Carrot is a common vegetables and used in everyday cocking. Carrot grows plenty in field and it was collected to liquefy for making dye solution. Mild yellow shade can be produced using carrot dyes. This dye is mainly used to dye fibers, yarns and fabrics. Figure 2 shows carrot dye chemical structure [34].
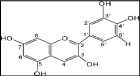
Figure 2: Chemical Structure of Carrot Dye.
Black berry dye: Blackberry is a popular fruit that is easily obtainable in nature. This fruit is economical and can be applied for dyeing the cotton fabric with dark purple shade. Figure 3 shows the chemical structure of blackberry dye [35].
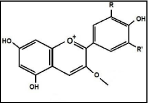
Figure 3: Chemical Structure of Blackberry Dye.
Cherry dye: Cherry is a popular fruit available in nature in each country. It is good for health and also it is delicious to eat. This fruit is used to dye textile materials like fiber, yarn and fabirc. Figure 4 shows the cherry dye chemical structure [36].
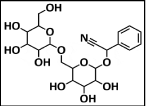
Figure 4: Cherry Dye Chemical Structure.
Required liquid substances
Required liquid substances such as surfactants, sequestering agents, leveling agents and fixing agents all were required in this research for fabric dyeing and experimentation of the different color properties. All the chemicals and auxiliaries were required to dye the fabrics evenly and to achieve the desired fastness properties. Commercial name, chemical formula and functions are mentioned in Table 2. Figure 5 showed the surfactants [37], Figure 6 showed the sequestering agent [38], Figure 7 showed the leveling agent [39]; and figure 8 showed the fixing agent [40].
S.N
Chemical Formula
Commercial Name
Function
1
C44H84NO8P (1-Stearoyl-2-linoleoylphosphatidylcholine)
Surfactants
Eliminating surface active agents
2
C10H16N2O8 (Ethylene-di-amine-tetra-acetic acid)
Sequestering Agent
Diminishing water hardness
3
Na2H2P2O7 (Sodium Acid Pyrophosphate)
Leveling Agent
Leveling of dyes
4
(NH4)2S2O3(Ammonium thiosulfate)
Fixing Agent
Fixation of dyes
5
CuSO4.5H2O (Copper sulphate)
Mordant
Fixative
Table 2: Required Liquid Substancesfor this Research.
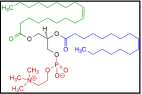
Figure 5: 1-Oleoyl-2-palmitoyl-phosphatidylcholine (C44H84NO8P, Surfactants).
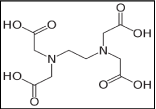
Figure 6: C10H16N2O8 (Ethylene-di-amine-tetra-acetic Acid, Sequestering
Agent).
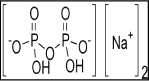
Figure 7: Sodium Acid Pyrophosphate, (Na2H2P2O7 Levelling Agent).
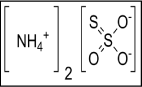
Figure 8: (NH4)2S2O3 (Ammonium Thiosulfate) Fixing Agent.
Required method for the experimentation
Required test method: Experiments such as light fastness, wash fastness, perspiration fastness and crocking fastness of the samples were tested in agreement with the test method provided by mentioned AATCC and ASTM Standard. Light fastness test of the dyed cotton sample was conducted in AATCCTM16.1 Standard. Wash fastness test of the dyed cotton sample was conducted in ASTM D435-42 Standard. Perspiration fastness test of the dyed cotton sample was tested in AATCC TM15 Standard. And crocking fastness test of the dyed cotton sample was tested in AATCC TM8 Standard.
Color intensity and color strength testing method: Kubelka monk developed an equation (K/S) to detect the color strength and intensity outputs by the absorption and reflectance features of the dyed cotton samples. Total color differences were presented by equation 1, on contrary, color measurement values were presented by equation 2. ΔE stated color difference values where, ΔH stated color hue difference values. ΔL stated the differences of the lightness values. All other characteristics expressed their respective values.
ΔE*ab = [(ΔL*)2+(Δa*)2+(Δb*)2]½
Equation 1: Stating the Color Differences with Respect to Lightness Values
ΔH*ab = [(ΔE*ab)2-(ΔL*)2-(ΔC*ab)2]½
Equation 2: Stating the Color Hue Differences with Respect to Lightness and Chroma
Required instruments
Different experiments were carried out with different instruments. All the experiments of this research were conducted in accord to the test method sated above. “Light Fastness Tester TF421” was used to test the light fastness values of the dyed cotton sample. “Launder Meter TF418” was used to test the wash fastness values of the dyed cotton sample. “Perspirometer TF416A” was used to test the perspiration fastness values of the dyed cotton sample. “Crock Meter TF410” was used to test the crocking fastness values of the dyed cotton sample. “Agilent Cary 630 FTIR Instrument” was used to detect the chromophore present in the substances. This instrument also showed the color intensity possessions. FTIR ejected Infrared Radiation to detect the extent of chromophore present in the substances, and also to detect the color fastness properties. Reflectance spectrophotometer detected the color strength and color intensity belongings. A “Pad Dye Pad Steam Machine” manufactured by Benninger was used for dyeing the cotton samples with selected natural dyes.
Dye Extraction and Sample Dyeing
Orange dye extraction and sample dyeing process
Fresh and ripe orange fruits were collected from garden. The fruits were washed off in clean water to take out all the dirt and dust. The fruits were peeling off to get the juice easily and then they were cut into slight pieces using a knife. 500grams amount of orange pieces were taken into a beaker. Using a blending machine, the orange fruit pieces were blended. For the development of the color 10 ml of Na2CO3 solution was poured into the blended juice and mixed again. It was kept for 12 hours and this incident made the solution neutralized to 6-7. The juice established pure orange color and this pure orange color were slowly become to be more developed.
For the protection of the solution, 10ml of tri-chloro-methane or chloroform (CHCl3) was mixed into the solution from being rotten. The mixture of chloroform will also prevent from creating bed smell. 10 ml of chloroform will make evaporation. Sample fabrics were properly mercerized in NaOH solution before starting dyeing process. Mercerized cotton fabrics were washed off in CH3COOH or acetic acid solution for neutralization prior to dyeing. The hardness of water was removed using sequestering agent or ethylene-di-aminetetra- acetic acid. Leveling agent or sodium acid pyrophosphate was mixed into the dye for leveling and mordant such as copper sulphate (CuSO4.5H2O) was used in dyeing process for fixation.
Using a continuous sample dyeing machine “Pad Dye Pad Steam Machine” the sample fabric was dyed. Sodium carbonate solution was mixed in dyeing bath around 7g/l at the liquid ration of 1:10 while dyeing for color fixation. The speed of the sample machine was kept 20 meter per minute. The dyeing temperature was 90oC. Again after dyeing detergent was used for washing the samples for removing unfixed dye chemicals and sample was washed in 5g/l of Na2CO3 solution for fixation of the dyestuffs. Drying the fabric at 60oC the fabric was stored in a safe place.
Carrot dye extraction and sample dyeing process
Carrots are available in nature as a form of vegetables. Fresh carrots were collected from field. The vegetables were washed off in clean water to take out all the dirt and dust and then they were dried. The vegetables were peeling off to get the juice and then they were cut into slight pieces using a knife. 500 grams amount of carrot pieces were taken into a beaker with 500ml of water. Using a blending machine, the carrot pieces were blended. For the development of the color 10ml of Na2CO3 solution was poured into the blended juice and mixed again. It was kept for 12 hours and this incident made the solution neutralized to 6-7.
The juice established pure pink color and this pure pink color were slowly become to be more developed. For the protection of the solution, 10ml of tri-chloro-methane or chloroform (CHCl3) was mixed into the solution from being rotten. The mixture of chloroform will also prevent from creating bed smell. 10 ml of chloroform will make evaporation. Sample fabrics were properly mercerized in NaOH solution before starting dyeing process. Mercerized cotton fabrics were washed off in CH3COOH or acetic acid solution for neutralization prior to dyeing. The hardness of water was removed using sequestering agent or ethylene-di-amine-tetra-acetic acid. Leveling agent or sodium acid pyrophosphate was mixed into the dye for leveling and mordant such as copper sulphate (CuSO4.5H2O) was used in dyeing process for fixation.
Using a continuous sample dyeing machine “Pad Dye Pad Steam Machine” the sample fabric was dyed. Sodium carbonate solution was mixed in dyeing bath around 7g/l at the liquid ration of 1:10 while dyeing for color fixation. The speed of the sample machine was kept 20 meter per minute. The dyeing temperature was 90oC. Again after dyeing detergent was used for washing the samples for removing unfixed dye chemicals and sample was washed in 5g/l of Na2CO3 solution for fixation of the dyestuffs. Drying the fabric at 60oC the fabric was stored in a safe place.
Black berry dye extraction and sample dyeing process
Blackberry fruits are very popular, available plenty in nature. This fruits developed dark violates color shade. Ripe blackberries were collected from trees. The fruits were washed off in clean water to take out all the dirt and dust and then they were dried. 500 grams amount of blackberries were taken into a beaker with 500ml of water. Using a blending machine, the blackberries were blended. For the development of the color 10ml of Na2CO3 solution was poured into the blended juice and mixed again. It was kept for 12 hours and this incident made the solution neutralized to 6-7.
The juice established dark violate colors and this dark violate colors were slowly become to be more developed. For the protection of the solution, 10 ml of tri-chloro-methane or chloroform (CHCl3) was mixed into the solution from being rotten. The mixture of chloroform will also prevent from creating bed smell. 10 ml of chloroform will make evaporation. Sample fabrics were properly mercerized in NaOH solution before starting dyeing process. Mercerized cotton fabrics were washed off in CH3COOH or acetic acid solution for neutralization prior to dyeing. The hardness of water was removed using sequestering agent or ethylene-di-amine-tetra-acetic acid. Leveling agent or sodium acid pyrophosphate was mixed into the dye for leveling and mordant such as copper sulphate (CuSO4.5H2O) was used in dyeing process for fixation.
Using a continuous sample dyeing machine “Pad Dye Pad Steam Machine” the sample fabric was dyed. Sodium carbonate solution was mixed in dyeing bath around 7g/l at the liquid ration of 1:10 while dyeing for color fixation. The speed of the sample machine was kept 20 meter per minute. The dyeing temperature was 90oC. Again after dyeing detergent was used for washing the samples for removing unfixed dye chemicals and sample was washed in 5g/l of Na2CO3 solution for fixation of the dyestuffs. Drying the fabric at 60oC the fabric was stored in a safe place.
Cherry dye extraction and sample dyeing process
Cherry is available in nature as a form of fruits. This fruits developed pink color shade. Ripe cherry fruits were collected from trees. The fruits were washed off in clean water to take out all the oil, dirt and dust and then they were dried. 500 grams amount of cherry fruits were taken into a beaker with 500ml of water. Using a blending machine, the cherries were blended. For the development of the color 10 ml of Na2CO3 solution was poured into the blended juice and mixed again. It was kept for 12 hours and this incident made the solution neutralized to 6-7.
The juice established pink colors and this pink color were slowly become to be more developed by dark pink color. For the protection of the solution, 10ml of tri-chloro-methane or chloroform (CHCl3) was mixed into the solution from being rotten. The mixture of chloroform will also prevent from creating bed smell. 10ml of chloroform will make evaporation. Sample fabrics were properly mercerized in NaOH solution before starting dyeing process. Mercerized cotton fabrics were washed off in CH3COOH or acetic acid solution for neutralization prior to dyeing. The hardness of water was removed using sequestering agent or ethylene-di-amine-tetra-acetic acid. Leveling agent or sodium acid pyrophosphate was mixed into the dye for leveling and mordant such as copper sulphate (CuSO4.5H2O) was used in dyeing process for fixation.
Using a continuous sample dyeing machine “Pad Dye Pad Steam Machine” the sample fabric was dyed. Sodium carbonate solution was mixed in dyeing bath around 7g/l at the liquid ration of 1:10 while dyeing for color fixation. The speed of the sample machine was kept 20 meter per minute. The dyeing temperature was 90oC. Again after dyeing detergent was used for washing the samples for removing unfixed dye chemicals and sample was washed in 5g/l of Na2CO3 solution for fixation of the dyestuffs. Drying the fabric at 60oC the fabric was stored in a safe place.
The Experiment
Color fastness properties test
ASTM (American Society for Testing and Materials) and AATCC (American Association of Textile Chemists and Colorists) developed some standard for testing the color fastness properties those were applied in this research for testing the samples. Experimentation was carried out for detecting the light fastness properties of the fabric samples in accord to the test method developed by “AATCC TM16.1 Standard” with the facility of “Light Fastness Tester TF421”. Experimentation was carried out for detecting the wash fastness properties of the fabric samples in accord to the test method developed by “ASTM D435-42 Standard” with the facility of “Launder Meter TF418”.
Experimentation was carried out for detecting the perspiration fastness properties of the fabric samples in accord to the test method developed by “AATCC TM15 Standard” with the facility of “Perspirometer TF416A”. Experimentation was carried out for detecting the crocking fastness properties of the fabric samples in accord to the test method developed by “AATCC TM8 Standard” with the facility of “Crock Meter TF410”.
Testing of the color intensity and strength
With a view to detecting the color strength and intensity properties of the sample fabrics a Reflectance Spectrophotometer was used in this experiment. The fabric samples were prepared by cutting with a scissors in the size of 4''×2'' to input in this instrument. Reflectance Spectrophotometer contains an area where a clamp is found to set the samples by unplugging them. After opening the clamp, the fabric samples were positioned one after another to test the color strength and intensity values.
After assigning the samples to the right place of spectrophotometer at beneath point of the clamp, it ejected a tungsten flashlight. Refracting the flashlight throughout the samples, it detected the color strength and intensity values. Color strength (K/S) and intensity values of the samples were detected in the perceptible wave range of 330nm to 900nm. Some other color properties of the samples like Lightness values, Hue values and Chroma values were also attained and sited in Table 3.
Fabric Colored with
Wash Fastness
Light Fastness
Rubbing
Fastness
Perspiration Fastness
Dry
Wet
Acidic
Alkaline
Black berry dye
2-3
3
2-3
2
2-3
2-3
Cherry Dye
2
3
2
2
2-3
2
Carrot dye
2
2-3
1-2
1-2
2
1-2
Orange dye
1-2
2
1-2
1
2
1
Table 3: Different Color Fastness Values of Cotton Dyed Samples.
Testing of FTIR
In order to identifying the chromophore present in the dye stuffs of the sample fabrics “Agilent Cary 630 FTIR Instrument” was used in this experiment. The fabric samples were arranged by cutting with a scissors in the size of 3.5''×1'' to set in this apparatus. FTIR (Fourier transform infrared spectroscopy) Instrument has a clamp where the samples were set to achieve the scan results. Fabric samples were positioned one after another to obtain the spectral scan reports. As assigning the samples to the right place of FTIR at beneath point of the clamp, it ejected Infrared Radiation, which passed though the samples and required spectral scan were obtained.
Transmission spectroscopy ensures the passage of infrared radiation throughout samples and detecting the degree of absorption and presence of chromophore. While transmitting infrared radiation, some radiations are absorbed by the sample it covers. Infrared Radiation produced reflected peak values on the basis of chromophore present in the dyed cellulosic fabrics. Highest peaks confirm most intense colors and vice versa. Lower peak values were obtained at less saturated points.
Result and Discussion
Color fastness properties
Using a grey scale color fastness properties were identified of the dyed cellulosic fabrics. Table 3 exposed the values of light fastness, wash fastness, perspiration fastness and crocking or rubbing fastness properties. Cotton fabrics dyed with natural dyes exhibited moderately poor color fastness properties compared to manmade dyes.
Wash Fastness Properties: Table 3 exposed the uppermost value of wash fastness for the cotton sample colored with blackberry dye that is 2.5. This table also expressed the lowermost value of wash fastness for the cotton sample colored with orange dye that is 1.5. Remaining two dyed samples cherry and carrot exposed the wash fastness values of 2.
Perspiration Fastness Properties: Table 3 exposed the uppermost value of perspiration fastness for the cotton sample colored with blackberry dye those were dry 2.5 and wet 2. This table also expressed the lowermost value of perspiration fastness for the cotton sample colored with orange dye those were dry 1.5 and wet 1. Remaining two dyed samples exposed the perspiration fastness values of cherry dye dry 2 wet 2 and carrot dye dry 1.5 and wet 1.5.
Light fastness properties: Table 3 exposed the uppermost value of light fastness for the cotton sample colored with blackberry dye that is 3. This table also expressed the lowermost value of light fastness for the cotton sample colored with orange dye that is 2. Remaining two dyed samples cherry and carrot exposed the light fastness values of 3 and 2.5.
Rubbing fastness properties: Table 3 exposed the uppermost value of rubbing fastness for the cotton sample colored with blackberry dye those were acidic 2.5 and alkaline 2.5. This table also expressed the lowermost value of rubbing fastness for the cotton sample colored with orange dye those were acidic 2 and alkaline 1. Remaining two dyed samples exposed the rubbing fastness values of cherry dye acidic 2.5 and alkaline 2 and carrot dye acidic 2 and alkaline 1.5.
Color strength and intensity values
Using Kubelka Monk equation color strength (K/S) and color intensity values were attained. Reflectance Spectrophotometer exposed the values of color strength and intensity or saturation or Chroma values by the scattering of tungsten light source all the way through the dyed cotton samples. Spectrophotometer also exposed all these values along with color lightness and color hues those are sited in Table 4. This table also certified that the best value of K/S specified the most intense color with the lowermost lightness value (L). On contrary, the lowermost value of K/S specified the most intense color with the uppermost lightness value (L).
Dyed Cotton Samples
K/S
L
a*
b*
c*
h*
Black berry dye
3.12
30.21
0.21
1.32
2.38
78.88
Cherry Dye
2.98
42.32
2.33
3.58
3.98
67.22
Carrot dye
2.62
62.34
3.42
18.64
23.38
76.34
Orange dye
2.48
74.68
3.98
24.96
25.76
79.68
Table 4: Color Strenght, Color Intensity and Lightness Values.
Blackberry dyed cotton sample exposed the best K/S values of 3.12 with the lowermost lightness value of 30.21. On contrary, orange dyed cotton sample exposed the lowermost K/S values of 2.48 with the uppermost lightness value of 74.68. Blackberry dyed cotton sample showed the darkest shade from these dyes with the best color intensity properties. Oppositely, orange dyed cotton sample showed the lightest shade from these dyes with the lowermost color intensity properties.
The lightest color had the least K/S values with highest lightness value; conversely, the darkest color had the best K/S values with lowermost lightness values. Table 4 had additional values of L*, a*, b*, C*, h*. It is known that, a* and b* may possibly have positive and negative values. The negative value of a* and b* expressed green and blue colored shade. Inversely, the positive values of a* and b* expressed red and yellow colored shade.
FTIR transmittance
“Agilent Cary 630 FTIR Instrument” detected the color absorbency properties and the Chromophore present in the dyed samples within the wave range it covers. Fourier Transform Infrared (FTIR) spectral scan detected the color intensity properties and the color fastness properties within the spectral range it covers. IR rays could transmit a few microns through the samples, which created peak values.
FTIR instrument ejected Infrared Radiation (IR) of around 400 to 4800 wave numbers throughout a sample, from which some radiations were transferred and some were absorbed. Radiations those were absorbed in the samples were converted into rotating or vibrating energy by the molecules. Subsequent signal at the sensor stated a spectrum, normally from 650cm-1 to 4500cm-1, that represents the molecular scans of the samples.
IR reflection or absorption generates a distinctive spectral scan based on the frequencies it covers. Molecules generated unique spectral scans, those confirms the color absorbency or the chromophore present in the dyed samples. FTIR spectral scan of the samples were taken using infrared release within the apparent spectral region with the highest IR peak values it produced. It is noticed from the Fourier Transform Infrared (FTIR) spectral scan that the highest peak value was acquired for the strawberry dyed samples.
It is seen from the Table 5 that the highest peak value of 4672 cm-1was obtained for the sample of dyed with blackberry dyes. On contrary, the lowest peak value of 3820cm-1 was obtained for the sample of dyed with orange dyes. Cotton sample dyed with cherry dyes exposed the peak value of 4422cm-1. Besides, cotton sample dyed with carrot dyes exposed the peak value of 3992cm-1.
S.N
Dyed Cotton
Highest Peak Values
01
Black berry dye
4672 cm-1
02
Cherry Dye
4422 cm-1
03
Carrot dye
3992 cm-1
04
Orange dye
3820 cm-1
Table 5: FTIR Spectral Scan (highest peak values).
These peaks clarified the existence of the chromophore and colorant persisted in the dyed cellulosic fabrics. Blackberry dyed cotton sample exposed highest peak values of 4672cm-1 that guaranteed that it had enriched chromophore or colorant existed in the dyestuff with upgraded color fastness properties. On contrary, orange dyed cotton sample exposed lowest peak values of 3820cm-1 that guaranteed that it had a lesser amount of chromophore or colorant existed in the dyestuff with weakened color fastness properties.
Analyzing all, the best color fastness properties were attained in that order of Black berry dye (4672cm-1), then and there in Cherry Dye (4422cm-1), then and there in Carrot dye (3992cm-1) and at end in Orange dye (3820cm-1).
Conclusion
It has been seen from the research that, the terminal reactive group of sustainable dyes responded against the cellulosic part of cotton in alkali condition to construct strong covalent bonds among the dyes and the cellulosic part for presenting fantastic color properties of the dyed cellulosic fabrics. Since, sustainable dyes are economical, decomposable and easily obtainable in nature, therefore, they can be used in industrial production. Besides, these natural dyes are friendly to the environment. Blackberry dyes exposed the best color properties amongst the above stated selected dyes. Reflectance spectrophotometer certified the color strength and intensity properties by scattering of tungsten light through the samples. Cellulosic fabrics exposed these values with the absorption characteristics of the dyed samples. Infrared Radiation was emitted through the samples to detect the chromophore existence in the dyed cellulosic fabrics. Color fastness properties were evaluated with gray scale. This research is cooperative to the individuals who are responsible for dyeing the cellulosic fabrics with sustainable dyes and to adjusting of their color properties.
References
- Liman MLR, Islam MT, Hossain MM, Sarker P, Dabnath S. Coloration of cotton fabric using watermelon extract: mechanism of dye-fiber bonding and chromophore absorption. The Journal of the Textile Institute. 2020; 1-12.
- Rezaie AB, Montazer M. A cleaner and one-step approach for robust coloration of polyester fibers via hydrophobic magnetically recoverable photocatalyst fatty acids/nano iron oxide coating. Journal of Cleaner Production. 2020; 244: 118673.
- Malliga P, Bela RB, Shanmugapriya N. Conversion of textile effluent wastewater into fertilizer using marine cyanobacteria along with different agricultural waste. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels. 2020; 87-111.
- Islam S, Haque Md, Arifuzzaman Md, Ahmed S, Sarker B, Hossain Md. Identifying the Causes of the Spandex Breakage of Woven Garments and its Solutions. Advance Research in Textile Engineering. 2020; 1: 1-24.
- Islam S, Parvin F, Urmy Z, Ahmed S, Arifuzzaman M, Yasmin J, Islam F. A Study on the Human Health Benefits, Human Comfort Properties and Ecological Influences OF Natural Sustainable Textile Fibers. European Journal of Physiotherapy and Rehabilitation Studies. 2020; 1: 1-24.
- Islam S. Attaining Optimum Strength of Cotton-Spandex Woven Fabric by Apposite Heat-Setting Temperature. Journal of the Institution of Engineers (India): Series C. 2019; 100: 601-606.
- Islam S, Alam SMM, Akter S. Identifying the values of whiteness index, strength and weight of cotton spandex woven fabric in peroxide bleaching of different concentration. Fibers and Textiles. 2019; 26: 96-109.
- Parvin F, Islam S, Urmy Z, Ahmed S. A Study On The Textile Materials Applied In Human Medical Treatment. European Journal of Physiotherapy and Rehabilitation Studies. 2020; 1: 26-51.
- Islam S, Alam SMM, Akter S. The Consequences of Temperature on the Shrinkage Properties of Cotton Spandex Woven Fabric. Journal of Textiles and Polymers. 2019; 7: 25-29.
- Islam S, Alam SMM, Akter S. Identifying a suitable heat setting temperature to optimize the elastic performances of cotton spandex woven fabric. Research Journal of Textile and Apparel. 2018.
- Islam S, Alam SMM. Investigation of the acoustic properties of needle punched nonwoven produced of blend with sustainable fibers. International Journal of Clothing Science and Technology. 2018.
- Yavuz G, Zille A, Seventekin N, Souto AP. Structural coloration of chitosan coated cellulose fabrics by electrostatic self-assembled poly (styrene-methyl methacrylate-acrylic acid) photonic crystals. Carbohydrate polymers. 2018; 193; 343-352.
- Islam S, Ahmed S. Attaining optimum values of the colorfastness properties of sustainable dyes on cotton fabrics. FIBRES & TEXTILES in Eastern Europe. 2020; 144: 1-8.
- Sinha K, Aikat K, Das P, Datta S. Dyeing of modified cotton fiber with natural T erminaliaarjuna dye: Optimization of dyeing parameters using response surface methodology. Environmental Progress & Sustainable Energy. 2016; 35: 719-728.
- Islam S, Alam SMM, Akter S. Achieving Optimal Shrinkage of Cotton Spandex Woven Fabrics by Apposite Heat Setting Temperature. Advance Research in Textile Engineering. 2020.
- Mao H, Yang F, Wang C, Wang Y, Yao D, Yin Y. Anthraquinone chromophore covalently bonded blocked waterborne polyurethanes: synthesis and application. RSC Advances. 2015; 5: 30631-30639.
- Islam S, Alam SMM, Akter S. Reviewing the sustainability of natural dyes. Advance Research in Textile Engineering. 2020.
- Sobczyk-Guzenda A, Szymanowski H, Jakubowski W, Blasinska A, Kowalski J, Gazicki-Lipman M. Morphology, photocleaning and water wetting properties of cotton fabrics, modified with titanium dioxide coatings synthesized with plasma enhanced chemical vapor deposition technique. Surface and Coatings Technology. 2013; 217: 51-57.
- Islam S, Alam SMM, Akter S. Investigation of the Colorfastness Properties of Natural Dyes on Cotton Fabrics. Fibers and Textiles. 2020; 27; 96-109.
- Rehman FU, Adeel S, Qaiser S, Bhatti IA, Shahid M, Zuber M. Dyeing behaviour of gamma irradiated cotton fabric using Lawson dye extracted from henna leaves (Lawsoniainermis). Radiation Physics and Chemistry. 2012; 81: 1752-1756.
- Islam S, Alam SMM, Akter S. Identifying the values of whiteness index, strength and weight of cotton spandex woven fabric in peroxide bleaching of different concentration. Fibers and Textiles. 2019; 26: 96-109.
- Islam S, Ahmed S. Investigation of the Mechanical Properties of Thermal Bonded Nonwoven Composite Produced of Blends with Sustainable Fibers. Advance Research in Textile Engineering. 2019; 4: 1039.
- Samanta AK, Agarwal P, Datta S. Studies on color interaction parameters and color fastness properties for dyeing of cotton fabrics with binary mixtures of jackfruit wood and other natural dyes. Journal of Natural Fibers. 2009; 6: 171-190.
- Park Y, Koo K, Kim S, Choe J. Improving the colorfastness of poly (ethylene terephthalate) fabrics with the natural dye of Caesalpiniasappan L. Wood extract and the effect of chitosan and low-temperature plasma. Journal of applied polymer science. 2008; 109: 160-166.
- Islam S, Nasif Chowdhury JY, Arifuzzaman FI, Hasan M, Khatun M. Detecting the Physical Properties of Thermal Bonded Nonwoven Fabrics. Trends in Textile Engineering and Fashion Technology. 2019; 5: 1-18.
- Islam S, Tasnim N, Islam T. Investigation of the change of the shrinkage properties in contradiction to the change of the composition of cotton polyester spandex denim fabrics. Journal of Textile Engineering and Fashion Technology. 2019; 5; 163-168.
- Islam S, Akter S, Chowdhury S. The Experiential Analysis of Woven Fabric for Reproduction. Journal of Textile Science and Technology. 2018.
- Islam S, Yasmin J, Kanon R. Detecting the spandex injuries and therapies of stretched garments. Journal of Textile Engineering and Fashion Technology. 2019; 5: 170-175.
- Islam S, Ahmed S, Arifuzzaman MAKM, Islam S, Akter S. Relationship in between Strength and Polyester Content Percentage of Cotton Polyester Blended Woven Fabrics. International Journal of Clothing Science. 2019; 6: 1-6.
- Mokhtar SM, Mostapha TB, Sabaa MW. γ-Radiation induced graft copolymerization of N-phenyl-and N-p-hydroxyphenyl maleimide onto cotton fabrics. Polymer-Plastics Technology and Engineering. 2002; 41: 183-197.
- Shariful I, Jarin Y, Toufiqul AS, Faridul I, Rowshanuzzaman K. Identifying the strength properties of cotton polyester blended woven fabrics of different fiber content. Research Journal of Material Sciences. 2019; 7: 1-6.
- Islam S, Chowdhury S, Akter S. The experiential analysis of woven fabric for reproduction. Journal of Textile Science and Technology. 2018; 4: 18.
- Zellbi. Free Shipping In Germany On Orders Over. Orange G. ZellBio GmbH.
- Claire Lower. Savor The Science: The Science Of Colorful Carrots. Render. The Magazine. 2015.
- The chemistry of blackberry color. Compound interest. Explorations of everyday chemical compounds. CI. blackberry, foraging, fruit.
- Gwenda. Cherry Plum (Prunuscerasifera). Cherry plum, the remedy for fear of losing control, is make by the boiling method. Bach Flower Remedies - Cambridge. Secrets of the Bach Flower Remedy Plants #8 Cherry Plum. 2015.
- Phosphatidylcholine. Wikipedia, the free encyclopedia.
- Ethylenediaminetetraacetic acid. Wikipedia, the free encyclopedia.
- Disodium Pyrophosphate. Wikipedia, the free encyclopedia.
- Ammonium Thiosulfate. Wikipedia, the free encyclopedia.