
Review Article
Adv Res Text Eng. 2021; 6(1): 1061.
Effect and Role of Salt in Cellulosic Fabric Dyeing
Wolela AD*
Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University, Kombolcha, Ethiopia
*Corresponding author: Asaye Dessie Wolela, Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University, Kombolcha, Ethiopia
Received: February 16, 2021; Accepted: March 11, 2021; Published: March 18, 2021
Abstract
The purpose of this paper was to present the role and function of salt in dyeing of cellulosic fabrics. In aqueous medium, cellulosic fabric such as cotton, viscose or linen acquires negative charge and thus repels negative charged dye anion during dyeing. Such repulsion in the dye bath between fibre and dye is offset by salt. Salts play the role of glue holding the dye molecules in the cloth, and a certain percentage of dyestuff fixed with textiles is added to the alkali. Because of this, salt is used as an exhausting agent in the textile dyeing process with various colorants (direct dye, reactive dye). As an exhausting agent, inorganic salts such as glauber salt, sodium chloride, zinc sulphate, aluminum sulfate, ammonium chloride and copper sulfate. The dyeing efficiency of the various types of salt is determined by the percentage exhaustion, color intensity and color fastness.
Keywords: Salt; Cellulosic fabric; Dyeing mechanism; Exhaustion; Role of salt; Effect
Introduction
For dyeing cotton, reactive dyes are commonly used because they have bright shade, high intensity, and excellent properties of fastness. It is predicted that the amount of reactive dyes consumed will increase faster than that of many other cotton dyes. There are, however, several limitations to their implementation. The dyeing of cotton with reactive dyes is achieved in an alkaline environment. Due to the ionization of hydroxyl in cellulose in the dyeing bath, cotton acquires a negative charge. Between the cotton fiber and the anionic reactive dyes, there is an extremely static repulsion, which can be minimized by adding an electrolyte such as sodium chloride or sodium sulfate. Depending on the color depth and dye composition, the salt consumption ranges from 30g/l to 150g/l [1]. Enough salt accelerates the transfer of dyes to the surface of the cotton fiber from the dyeing solution and thereby increases the exhaustion of the dye. Reactive cotton dye fixations are based on the formation of a covalent bond at a high pH (>10.5) between the dye molecule and the cotton fiber hydroxyl. In addition, in the alkaline bath, some parts of dyestuff molecules may react with OH-, resulting in low dye fixation [2]. High salt use and low reactive dye fixation have therefore drawn considerable interest from the dyeing chemists. Significant environmental issues have been caused by strongly colored effluents with great chemical complexity in the dyeing process [3].
After the completion of the three adsorption, sorption, and desorption procedures, the cloth is saturated with dye molecules and the overall procedure is known as exhaustion. In the fibers, the presence of dye alone in the dye bath does not dissipate entirely. For this purpose, salt is used in the textile dyeing process as an exhausting agent with different dyes (direct dye, reactive dye) [4].
During the cellulosic fabric dyeing process such as cotton, viscose or linen, the surface of the textile substrate is covered by negative ions after soaking into dye liquor and, on the other hand, some dyestuffs such as direct dye or reactive dye have formed a negative charge that functions as a zeta potential. As a result, the dye molecules are unable to demonstrate a chemical reaction to the textile substrate and roll off the surface of the cloth, hampering the ability of the substrate to change color. Salts play the role of glue that retains the dye molecules in the fabric and, with the addition of alkali, a certain percentage of textile-fixed dyes [5].
In reactive coloring, the physical-chemical requirement of using salt and soda is common for any or all technicians. In the nursing exhausting agent, the salt is associated with moving the color towards polysaccharide molecules and thus the alkali (soda ash) is a reactive color hydrolyzing/fixing agent [6].
In two steps, which are fixation and fixation, reactive dyes are added to cotton. Using salt, preferably Glauber’s salt (Na2SO4) or common salt (NaCl), exhaustion is achieved to resolve the negative zeta potential of cotton and facilitate increased dye uptake [7,8]. In fact, when cotton fiber is immersed in water, its surface often becomes anionic due to hydroxyl ions, so the particles of dye and cellulosic fiber appear to repel each other. Adding salt generates an electrically positive double layer that hides the cotton surface’s negative electrostatic charge. This helps the dye to approach the fibre so that attraction forces of H-bonding and other short-range dyefibre can work. The molecules of organic dye would have a greater affinity than the aqueous solution for the fabric. The amount of salt needed is higher than that required for direct dye adsorption due to the low fibre affinity of the reactive dyes [9]. Depending on the appropriate color depth, the structure of the dyes or the dyeing formula, the quantities of current electrolyte can vary up to 100g/l [9,10]. Using alkalis such as Sodium Hydroxide (NaOH) and/or sodium carbonate, the depleted dyes are fixed to the cotton fabric (Na2CO3). Reactive dyes react with hydroxyl cellulose groups, often by neucleophilic substitution or addition, to form covalent bonds under alkaline conditions [10].
It would be hoped that these powerful bonds would lead to excellent laundering of color fastness. The dyes can also, however, react with water hydroxyl groups so that they can no longer react with cellulose. The salt and alkali added depend on the depth of the shade to be produced [11]. In reactive dyeing, due to the heat regulation of the dye bath and to the portion wise addition of salt and alkali to prevent uneven dyeing and optimize fatigue and fixation, the process is too long.
Salt can be a mineral consisting mainly of the metallic element and chloride, the two components. Common salt is the NaCl material consisting of the metallic element and chloride of the weather. There are solid forms of common salt crystals. Via ionic bonding of the metallic element and chloride ions, Salt consists of small cubes that are tightly secure. In the nursing example of crystalline structure, the salt crystal is widely used as an associate in order to know the depth of the subject; the basics behind the word “salt” should be viewed in relation to the textile process [6].
In the textile dyeing process, a colorless crystalline solid NaCl inorganic compound of sodium and chlorine salt in which ionic bonds keep the two components soluble in water in the familiar white crystals plays a key role in preserving the electrolyte balance in the textile dyeing process. Glauber salt is the generic name for Na2SO4.10H2O dehydrates of sodium sulfate; it occurs when white or colorless monoclinic crystals are often used for the purpose of dyeing. By recrystallization of the distilled brine solution, vacuum salt is formed. In the method of vacuum crystallization, raw salt is dissolved in water to create a saturated solution and to clarify the bottom impurities. The vacuum is created by means of the appropriate vacuum pump [12].
All reactive dyes are generated from 3 basic units, a chormophore, a bridge associate in a reactive group/team nursing (either a haloheteocycle or an activated double bond) in the simplest terms [13]. Once these are applied to a plastic fiber in an alkaline dye bath, these dyes are used for coloring plastic fibers associated in nursing, they form a bond with fiber hydroxyl by reacting with fiber chemicals [14]. The bond formed between the dye molecule and the dye molecule of the fiber constructs a fiber molecule position [6,15].
In general, the affinity of reactive colorants is poor. In water, reactive colorants have a negative charge, and in water, cellulose fibers are also electronegative. Electrostatic repulsion between cellulose fibers and anionic dyes can, therefore, prevent cellulose fibers from being dyed by reactive dyes. During reactive-dye dyeing, a dyeing promoter is currently widely used. The dyeing promoters are the most widely applied inorganic salts (anhydrous sodium sulfate or sodium chloride). The inorganic salt cations adsorb to the surface of the cellulose fiber, and the cellulose fiber’s negative charge is weakened. Thus, with anionic reactive dyes, cellulose fibers may be colored. Inorganic salts, however, are also bound, resulting in significant salt contamination of the body of water [16].
Industrial salt is used in the textile industry to treat fabrics and products, for example for the dyeing of cotton and other cellulose materials. When used in a dye bath, salt allows the dye to penetrate fully into the fabric, thereby making the dyeing process uniform and simpler [17].
Types of Salt
For textile dyeing, two kinds of industrial salt named Vacuum and rock salt are available. Both types of salts have a very high sodium chloride content and are available in different grain sizes. The dye results would be superior if the salt used is pure. Usually, because of its high degree of purity, vacuum salt is favored in the textile industry. Because of its high sodium chloride content, rock salt can also be used for dyeing [17].
Why Salt is Used in Dyeing?
To combine with each other, the textile substrate and dye molecule should not necessarily have homogeneous characteristics. In such a scenario, to promote dyeing action on cloth, we need some catalyst. This critical role of the catalyst is played by Salt. Salt, for water, has an exceptionally high affinity. Broadly speaking, during the dyeing process in textiles, salt is needed in three ways, firstly, to push dye into textiles. Secondly, salt use contributes to the complete exhaustion of dye molecules in textiles during the dyeing process. Third, it is used for migration, adsorption and fixation of the dyestuff to the cellulose material as an electrolyte.
In reactive dyeing, salts play an important role by improving the affinity of the dyestuff towards the fibre and accelerating the interaction of the dyestuff and reducing its solubility. For this reason, Glauber’s salt or common salt/ vacuum salt are typically used. Corrosion of the instruments can be caused by the presence of chlorine ions in the common salt. Therefore, Glauber’s salt is preferred over common salt at all times. A common name for sodium sulfate decahydrate, Na2SO4.10H2O, is Glauber’s salt; it appears as monoclinic white or colorless crystals. It effloresces upon exposure to reasonably dry air, creating powdery anhydrous sodium sulphate. The salt was first developed by Johann Glauber’s (from Hungarian spring waters). Glauber’s salt is soluble in water, has a salty, bitter taste and is often used as a mild laxative in medicine; it is also commonly used for dyeing. The generic name of Sodium Chloride (NaCl) is vacuum salt [12,18,19].
Role of Salt in Reactive Dyeing
Inorganic salts have two key roles in the exhaustion dyeing with reactive dyes:
• Enhancement of the dyestuff affinity.
• Acceleration of the interaction of the dyestuff and lowering of its solubility.
Generally, reactive dyes comprise a group of sulphonic acid (-SO3H) that is insoluble in water. These sulphonic acid groups are converted into water-soluble sodium salts of sulphonic acid (-SO3Na) during the manufacture of reactive dyes [19-22] (Figure 1).
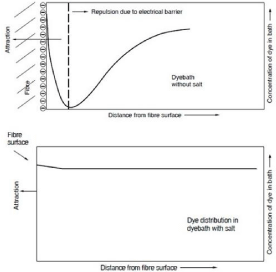
Figure 1: Salt Reaction.
Reactive dye – SO3H + Na+ → Reactive dye SO3Na
In general, it is solubilized when the reactive dye goes into the bath, providing dye anions and sodium cations.
Reactive dye – SO3Na + Water -- → Reactive dye – SO3 - + Na+
(Dye anion) (Sodium cation)
Dyeing Mechanism
Vegetable fibres contain cellulose that ionizes in water in the real dyeing mechanism.
Cell – OH -- → Cell – O- + H+
While the reactive dye goes into the water, the dye anions and sodium cations are soluble.
Reactive dye – SO3Na + Water -- → Reactive dye – SO3 - + Na+
(Dye anion) (Sodium cation)
In the absence of salt, both the negative dye and cellulose ions repel each other during dyeing and thus no exhaustion or very little exhaustion is carried out, but in the presence of salt, it will ionize as follows.
NaCl -- → Na+ + Cl- (Common Salt) or
Na2SO4 -- → 2Na+ + SO4 - (Glauber’s Salt)
The salt thus neutralizes the cellulose negative ion and promotes exhaustion,
(Cell – O- + H+) + (Na+ + Cl-) -- → Cell – Ona
Cell – ONa + SO3 - – Reactive dye -- → Cell – O – Reactive dye
(Exhausted dye on the substrate)
PThe presence of salt in the reactive dyeing thus increases the colorant’s affinity to the cellulosic substrate. Since reactive dyes have low cellulose affinity, it is possible to increase the fixation by exhausting the dye bath by adding Glauber’s salt before fixation. With a decreasing liquor ratio, the quantity of salts needed to produce sufficient exhaustion decreases [19,21].In water, reactive colorants have a negative charge, and in water, cellulose fibers are electronegative. Electrostatic repulsion between the cellulose fibers and the anionic reactive dyes can also avoid the dyeing of cellulose fibers by reactive dyes. During reactive-dye dyeing, a dyeing promoter is currently widely used.
In cotton fabric dyes with reactive dyes, NaCl serves as a dyeing promoter. Owing to the electrostatic attraction between Na+ and cellulose fibers, the Na+ ions of NaCl are adsorbed by cellulose fibers, and the negative charge of the cellulose fibers is weakened. The electrostatic repulsion between cellulose fibers and anionic reactive dyes is thus reduced, enabling anionic reactive dyes to dye cellulose fibers. The use of a significant volume of NaCl, however, results in serious water body salt contamination.
As for the intermolecular force between two molecules or ions, the force of Coulomb (electrostatic force) is much greater than the force of van der Waals. The large Coulomb repulsion between dyes and fibers renders the dyes unable to approach the fiber surface when cellulose fibers with negative charges are dyed with reactive dyes [16].
Function of Salt in the Dyeing Process
• In reactive dyeing, the salt increases the affinity of the colorant to the cellulosic substrate.
• Salt increases reactive dyestuffs’ exhaustion rate.
• As reactive dyestuffs have a lower affinity, more inorganic salt is required in order to accelerate absorption when using reactive dyestuffs.
• While the amount of inorganic salt used differs according to the form of dyestuff used, the amount of inorganic salt can be decreased by recently produced high-fixation dyestuffs with enhanced affinity.
Both Glauber’s salt and common salt (sodium chloride) are used in dyeing due to considerations of efficacy and expense. These two are essentially the same in terms of their position as inorganic salts because of the sodium cation active in both [19,21,22].
Since this alkali imposes load on the treatment plant for effluent, alkaline agents explicitly applied during the dyeing help to change the bath pH and to obtain level dyeing. At the same time, by reducing TDS, the load on the effluent treatment plant is decreased [20].
The salt effect can vary according to the salt ratio in the solution. The effect of salt on the cotton dyeing process is very important. The exact and desirable shade of the object cannot be achieved without salt. The value of salt in dyeing is thus, therefore, uncountable.
Some of the Electrolytes Used in Dyeing
Some of the inorganic salts used to effectively apply colorant as an exhausting agent:
Glauber Salt (Na2SO4.10 H2O)
Sodium Chloride (NaCl)
Zinc Sulphate (ZnSO4)
Aluminum Sulphate [Al2(SO4)3]
Ammonium Chloride (NH4Cl)
Copper Sulphate (CuSO4.5H2O)
Kamrunnahar (2018) examined the use of various inorganic salts as an exhausting agent for reactive dyeing of cotton knitted fabrics. In this study it was revealed the sequence of salt regarding color intensity - Glauber Salt > Sodium Chloride > Ammonium Chloride > Zinc Sulfate > Aluminum Sulfate > Copper Sulfate.
Experimental Observations
Dyeing properties
The cellulose-OH groups are encouraged to deprotonate to give cellulose attack electron-poor regions of the reactive hydroxy group and either conduct aromatic nucleophile to aromatics or nucleophilic addition to alkenes. A cellulose polymer has primary and secondary hydroxyl groups. One issue is that the fiber reactive group can react with the HO two reactions, instead of reacting with cellulose, and this is unfavorable because the hydrolyzed dye may not react further.
Effect of salt in dyeing properties
Shekh Md. et al (2014) explored an examination of a suitable electrolyte in cotton good for the reactive dyeing process. A comparison of the various electrolytes of Glauber salt, common salt and vacuum salt for the dyeing of cotton fibers in a closed dyeing process is recorded in this review. This study showed that the type of electrolytes used in the reactive dyeing process had a major impact on the dyeing rate, the value of K/S and the value of L* of the dyed samples. However, no substantial difference was observed between the three electrolytes in the level of the dyed samples and in the colour fastness of the reactive dyed fabrics [12].
Figure 2 shows the dyeing rates of cotton fabric with reactive dye in the presence of various electrolytes represented in terms of dye bath exhaustion. It can be seen from this statistic that 98% exhaustion that is influenced by Glauber salt is seen in the first 60 minutes of dyeing the dye baths. 95% exhaustion is seen in the case of common salt and vacuum salt. It can therefore be certain that in the reactive dyeing phase, Glauber salt shows greater satisfactory results as an electrolyte (Figure 3).
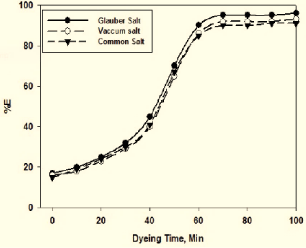
Figure 2: Effect of electrolyte types in terms of percentage of dye bath
exhaustion on rate of dyeing.
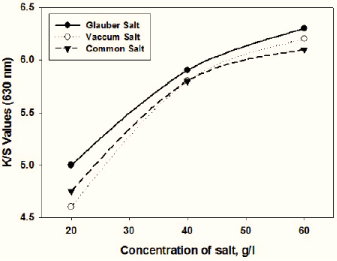
Figure 3: Effect of various salt concentrations on K/S values of dyed fabrics.
It was observed in Figure 3 that the variations in the different Glauber salt concentrations showed substantial changes in the K/S values relative to the other electrolytes. Since Glauber salt (Na2SO4) produces an ion that is twice as much as the typical salt and vacuum salt (Na2+) (NaCl). So, further dye uptake can be affected by Glauber salt [12].
Figure 4 indicates that the L* values have also been affected by salt concentrations. As the salt concentration increases, the depth of shade becomes darker, but 40g/l is the most important point for both electrolytes. From an industrial point of view, 40g/l of salt is ideal for the depth of shade and also for the minimization of costs [12].
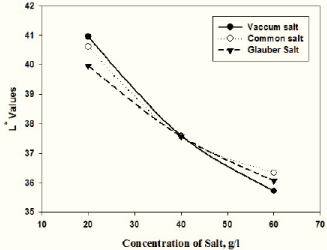
Figure 4: Effect of various salt concentrations on L* values of dyed fabrics.
The depth of shade is also greatly altered by the effect of electrolytes as the salt concentration increases. Visually, Glauber salt was calculated to show the best efficiency for reactive cotton fabric dyeing.
Effect of different salt concentration on K/S value
The color intensity of the sample dyed with sodium chloride and Glauber salt is almost the same for 50gm/l of salt, which is 9.1 and 9.2 respectively, as shown in Figure 5. The k/s value of the sample using 20gm/l salt, however, is 5.2 and 10gm/l salt is 4.7 for sodium chloride; while the k/s value of sample dyes with 20gm/l is 6.5 for glauber salt, and 10gm/l is 5.
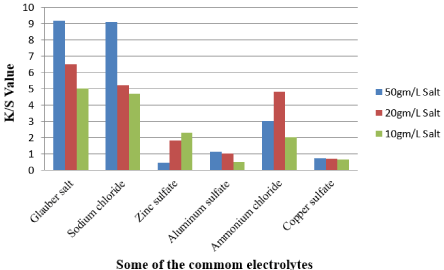
Figure 5: Effect of different salt concentration on K/S value [5].
The color strength of the ammonium chloride color sample is not so high; it is, however, greater than that of copper sulfate and zinc sulfate. So the salt sequence with regard to color intensity-Glauber salt > Sodium chloride > Ammonium Chloride > Zinc sulfate > Aluminum Sulfate > Copper sulphate [5].
Advantage of Glauber Salt
• Glauber salt provides greater depth than common salt.
• The fabric handle becomes smoother with Glauber salt and the fabric handle becomes rough with common salt.
• By using Glauber salt, the dye exhaustion is higher than common salt and thus results in a darker hue. By using Glauber salt, since dye exhaustion is greater than common salt and so it results in darker colour.
• The solubility of the aggregation of dye stuff increases as Glauber salt, while common salt reduces it.
• Sodium chloride adds TDS to the dye bath and adds effluent to the load.
• The hardness of water increases as a result of common salt chloride ions.
• Glauber salt does not cause any dyeing machine corrosion, but common salt induces dyeing machine corrosion due to the presence of chloride ions in it [19].
Conclusion
This study attempted to present the role and functions of salt in the process of textile dyeing. The dye molecules are unable to demonstrate a chemical reaction to the textile substrate and roll off the fabric surface while dyeing cellulosic fibres with anionic dye, which hinders the color-changing ability of the substrate. In such a scenario, to promote dyeing action on cloth, we need some catalyst. This critical role of the catalyst is played by Salt. So, in the dyeing process, salt is used as an exhausting agent. Firstly, during the dyeing process in textiles, salt is needed in three ways to push dye into textiles. Secondly, salt use contributes to the complete exhaustion of dye molecules in textiles during the dyeing process. Third, it is used for migration, adsorption and fixation of the dyestuff to the cellulose material as an electrolyte. Because of this, in dyeing, various forms of inorganic salt have been used. The efficiency of the various salts is determined by measuring the percentage of exhaustion, the value of color strength and the properties of color fastness.
References
- Zhang F, Chen Y, Lin H & Lu Y. “Synthesis of an amino-terminated hyperbranched polymer and its application in reactive dyeing on cotton as a salt-free dyeing auxiliary”. Coloration Technology. 2007; 123: 351-357.
- Terinte N, Manda BMK, Taylor J, Schuster KC & Patel MK. “Environmental assessment of coloured fabrics and opportunities for value creation: spindyeing versus conventional dyeing of modal fabrics”. Journal of cleaner production. 2014; 72: 127-138.
- Sun D, Zhang X, Du H, Fang L & Jiang P. “Application of liquid organic salt to cotton dyeing process with reactive dyes”. Fibers and Polymers. 2017; 18: 1969-1974.
- Cook LF. “Salt requirements put pressure on wet processing plants”. Textile World. 1994; 83.
- Kamrun Nahar. “Application of Different Inorganic Salts as Exhausting Agents for Dyeing of Cotton Knitted Fabric with Reactive Dye”. Global Journal of Research in Engineering. 2018.
- Talukder ME, Kamruzzaman M, Majumder M, Rony MSH, Hossain M & Das S. “Effects of salt concentration on the dyeing of various cotton fabrics with reactive dyes”. International Journal of Textile Science. 2017; 6: 7-14.
- Trotman ER. “Dyeing and chemical technology of textile fibres”. Wiley. 1984.
- Ristic N & Ristic I. “Cationic modification of cotton fabrics and reactive dyeing characteristics”. Journal of Engineered fibers and fabrics. 2012; 7.
- Baffoun A. “Comparative study between two types of electrolyte used in the reactive dyeing of cotton”. Industria Textila. 2019; 70: 30-36.
- Madaras GW, Parish GJ & Shore J. “Batchwise Dyeing of Woven Cellulosic Fabrics: A Practical Guide”. North Carolina State University. 1993.
- Chinta SK & Vijay Kumar S. “Technical facts & figures of reactive dyes used in textiles”. Int J Eng Manag Sci. 2013; 4: 308-312.
- Kabir SMM, Koh J & Momotaz F. “Analyzing the Suitable Electrolyte for Reactive Dyeing Process in Cotton Goods”. Journal of Engineering. 2014; 5: 75-80.
- Broadbent AD. ‘Basic Principle of Textile Coloration”. A.S.T.M. 2001; 1: 337.
- Cotton Morphology and Chemistry.
- dward Noah Abrahart. Dye.
- Ru J, Qian X & Wang Y. “Low-Salt or Salt-Free Dyeing of Cotton Fibers with Reactive Dyes using Liposomes as Dyeing/Level-Dyeing Promotors”. Scientific reports. 2018; 8: 1-9.
- Saravanan K. Role of Salt in Textile Chemical Processing Treatment. 2020.
- Salt as a Dye Enhancer for Textile: Textile Industry (What Is Salt as a Dye Enhancer for Textile?). 2018.
- Sushil Kumar Hada. Benefits of Glauber’s salt in Textile Wet processing. 2011.
- Tex Rej. Assignment Title: Effect of salt in Cotton Dyeing. 2007.
- Abdul Azeem. Function of Salt in the Textile Wet Processing.
- Salt free dyeing of cotton fabric with reactive dyes.