
Review Article
Adv Res Text Eng. 2022; 7(2): 1076.
Major Development in Cotton Coloration: A Review
Wolela AD¹* and Eshete GA²
¹Department of Textile Engineering, Kombolcha Institute of Technology (KIOT), Wollo University, Ethiopia
²Department of Textile Engineering, Ethiopian Institute of Textile and Fashion Technology (EiTEX), Bahir Dar University, Ethiopia
*Corresponding author: Wolela Asaye Dessie, Department of Textile Engineering, Wollo University, Kombolcha, Ethiopia
Received: September 02, 2022; Accepted: October 07, 2022; Published: October 14, 2022
Abstract
Most cotton fabrics are dyed with reactive dyes because they produce a full range of bright fashion colours with a high degree of wash fastness.Reactive dyes are frequently considered as king of dyes for cotton owing to their simple application techniques. However, reactive dyes are anionic and cotton fibers gain anionic surface charge in water, the charge repulsion adversely affects the dye bath exhaustion. Large quantity of electrolyte is added to overcome this problem. Some problems such as low dye utilization, high degree of salt utilization and colored effluent due to unexhausted, unfixed, and hydrolyzed dyestuffs, and high volume of waste water discharged, always exist in the application of reactive dyes.These electrolytes are neither exhausted nor destroyed and hence remain in the discharge dye liquor which leads to enormous environmental problem. Due to these problems this class of dyes is the most unfavorable one from the ecological point of view, these effluents produced gives high values of BOD/COD and increases salinity of the rivers affects the delicate biochemistry of aquatic life.A number of more environmentally sustainable approaches for reactive dyeing of cotton have been proposed to overcome the polluted effluent problem. This paper reviews options to improve the sustainability of the colouration process through development in dye structure, modification of dyeing machinery and processes, chemical modification of cotton fibre prior to dyeing, and use of organic compounds in place of inorganic chemicals.
Keywords: Cotton; Fiber-Reactive dyes; Modification; Environment-friendly Dyeing
Introduction
Cotton is a natural cellulosic fiber and the most popular among all the textile fibers. About 48% cotton fiber is consumed as clothing material all over apparel industry for a number of its unique characteristics such as softness, versatility, absorbance, hydrophilic in nature, comfort permeability, biodegradability, no static electricity, and breathability [1-3].
Cellulose is a macromolecule made up of anhydroglucose units united by 1, 4, oxygen bridges as shown in (Figure 1). The anhydroglucose units are linked together as beta-cellobiose; therefore, anhydro-beta-cellobioseis the repeating unit of the polymer chain. The number of these repeats units that are linked together to form the cellulose polymer is referred to as the degree of polymerization and is between 1000 and 15000 [4, 5].
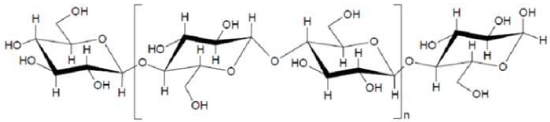
Figure 1: Chemical structure of cellulose.
Dyeing of textile fibers comprehends utilization of various chemicals and auxiliaries for various purposes, such as exhaustion of the dyestuff from the dyeing liquor to the textile substrate, fixation of the dyestuff on the substrate, giving identical level dyeing results etc [2,3,6]. One of the most well-known classes of dyes for cotton dyeing is a class of reactive dyes. They are frequently considered as king of dyes for cotton owing to their simple application techniques. The dyeing of these fiber are generally done with reactive dyes due to its brilliancy, bright shade, variety hue, good color fastness properties, convenient usage, low energy consumption, high applicability, etc [7- 11].
For apparel textiles, the predominant dye-fibre combination is that of reactive dyes and cotton [12,13]. Of all the dye-fibre combinations, cotton dyed with reactive dyes consumes the highest volume of water per kilogram of fibre. Moreover, this combination causes the highest discharge of salts, alkalis and organic matter per unit fibre mass [14]. Per capita demand for apparel textiles is projected to continue to grow. Therefore, it becomes important to find solutions for reducing water use and the discharge of polluting chemicals.
Since ICI introduced the first commercial reactive dyes for cotton in the 1950’s, this dye class has become increasingly popular [15]. This is mainly because of their very good washing fastness, a wide gamut of bright colours, and versatility for different application methods. The high fastness to washing of reactive dyes is due to their unique reactive system(s), which form covalent bonds with the hydroxyl groups of the cotton fibre under alkaline pH conditions [16].
The dyes also react with hydroxyl ions present in the aqueous dyebath under alkaline pH conditions. This produces nonreactive hydrolysed dye which remains in the dyebath as well as in the fibre. In order to obtain the required levels of washing fastness, it is necessary to remove all unreacted and hydrolysed (unfixed) dye from the cotton fibre. This is achieved by ‘washing-off’ which is a series of thorough rinsing and ‘soaping’ steps. The dye fixation efficiency is typically in the range of 50-80% [12,17]; i.e. 20 to 50% of the dye necessary to achieve the desired depth of colour is discharged to the environment.
Reactive dyes are soluble anionic dyes which, in solution, are repelled by the negatively charged surface of the cotton fibre [2,3,7- 11]. An electrolyte such as sodium chloride or sodium sulphate is added to promote the dye transfer (exhaustion) and penetration (diffusion) into the fibre. The amount of the salt can vary up to 2 kilograms per kilogram of the fibre depending on the dye structure, depth of colour and dyeing method. Once sufficient dye is on the fibre, either by exhaustion (exhaust dyeing methods) or padding (pad dyeing methods), the alkaline pH is obtained by using the alkali such as sodium carbonate, sodium bicarbonate or sodium hydroxide to initiate the dye-fibre reaction. The quantities and composition of the alkali system depend on the pH required for the particular type of reactive system of the dye and the dyeing method [12].
Irrespective of the dyeing method and the type of reactive system, almost all of the unfixed dye, inorganic salt and alkali are discharged to the effluent. Such effluents are characterised by high levels of dissolved solids and oxygen demand [14,18]. Inorganic salts are the most non-biodegradable compounds and contribute the major portion of the total dissolved solids content in the dyeing effluent.
Urea, which is often used in pad dyeing methods and in printing to increase dye solubility and yield of the dye-fibre reaction, is another environmentally undesirable chemical [19,20]. Urea, when used in the pad-dry-bake dyeing process, decomposes and increases the nitrogen content of the effluent.
There have been a number of developments for improving the quality of effluent for cotton dyeing systems with reactive dyes. This paper presents a review of such developments.
The aim of this paper review is to discuss about the major development in cotton coloration. This includes development of reactive dyes, modification of dyeing machinery and processes, chemical modification of cotton fibre prior to dyeing, and use of organic compounds in place of inorganic chemicals. The paper highlights the significance and limitations of these ways of improving sustainability in reactive dyeing, and proposes possibilities to further improve the sustainability.
Major Development in Cotton Coloration
With a great variety of dyes available, there are still limitations for cotton coloration. As an example, obtaining ultra-deep shades on cotton with good colorfastness in an environmentally responsible way is difficult. Sulfur blacks, as the most commonly used black dyes for cotton, have advantages on low cost and high wash fastness over other dyes. However, they are not environmentally friendly due to the large amounts of sodium sulfide used in manufacture and application. Other classes of black dyes used for cotton also have their own limitations, such as the poor wash fastness of direct dyes and large amounts of dye and salts in the dye bath effluent of reactive dyes when applied in conventional dyeing processes [21,22].
Major developments in environment-friendly dyeing of cotton with reactive dyes are in terms of innovations in dyes and dyeing processes for high dye bath exhaustion and fixation, use of low-salt reactive dyes, machinery developments for dyeing at low liquor ratio, pad troughs with reduced volumes, replacement of chemicals, econtrol process etc are some of the approaches for eco friendly colouration of cotton with reactive dyes [23].
With the need for cleaner, cost-effective, and colorfast textile products, innovative technologies and improved processes have been developed for cotton coloration. The innovations mainly focused on three aspects:
• Development of new dyes and auxiliaries;
• Developments in dyeing machinery and processes;
• Chemical modifications of cotton fiber prior to dyeing
• Use of organic compounds in place of inorganic chemicals [12,21,24].
Developments in Dye Structure
With the unique dye structure and application mechanism, much attention from both industry and research has been focused on the development of reactive dyes. To increase the fixation efficiency and reduce chemical waste, reactive dyes with two or more reactive groups have been developed and commercialized. The bifunctional reactive dyes, including homobifunctional dyes (containing two identical reactive groups, e.g. the Procion H-E dyes) and heterobifunctional dyes (containing two different reactive groups, e.g. the Everzol dyes), were already widely used in industry [25]. There are also successful commercial trifunctional reactive dyes such as the Avitera SE dyes form Huntsman which are claimed to save energy and time and reduce water consumption [26]. Researchers also came up with some polyfunctional reactive dyes with more than three reactive groups, but none of them have been commercially applied yet [27-29]. A typical example is the reddish-grey to black chromium complex (Figure 2) patented by Hoechst. The drawbacks with more reactive groups include higher cost and lower color strength. The extra reactive groups “dilute” the color value of a dye by increasing the molecular weight of the dye but do not enhance chromogenic strength [30].
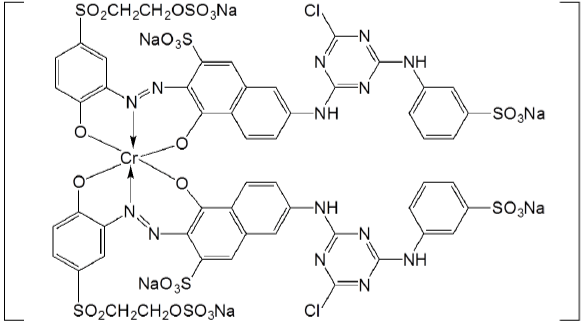
Figure 2: Reddish-grey to black chromium complex.
Besides the increase in reactive groups, other innovations on reactive dyes include high strength economic chromophores, high light fastness chromophores [5], neutral-fixing [31,32], or acid-fixing reactive dyes [33], and cationic reactive dyes [34-36].
Developments in Dyeing Machinery and Processes
To reduce the chemical, water, and energy consumption in exhaust dyeing, a wide number of low liquor-to-fiber ratio dyeing machines have been developed [37-39] such as the Luft-roto Plus dyeing machine from Thies with the minimum liquor ratio as 4:1 [40,41]. While for pad dyeing, new padders and low capacity padding troughs were designed to reduce liquor waste and eliminate tailing [38,39]. Also, the Econtrol dyeing process developed jointly by BASF and Monforts eliminates the use of urea and salt for continuous dyeing of cellulosic fibers [42]. While for washing-off, new approaches such as the “enzymatic after soaping” process for exhaust dyeing were announced for water and energy savings.
Other emerging technologies on cellulosic dyeing include the use of ultrasonic energy, microwave heating, and supercritical carbon dioxide (scCO2). The studies on ultrasonic-assisted cotton coloration shown that the use of ultrasound can result in better color yield and savings in energy, water, time, and chemical [43-47]. Compared with the conventional heating method, microwave is a volumetric heating which can shorten the heating time for coloration at the same time of improving color yield and dye fixation efficiency [48,49]. In another aspect, cotton can be dyed with disperse reactive dyes in scCO2, which eliminates the use of water [50]. Also, cotton modified to be less hydrophilic can be dyed with disperse dyes in scCO2 [51, 52].
Chemical Modification of Cotton Fiber Prior to Dyeing
The chemical modification of cotton to improve dyeing with anionic dyes, such as direct, reactive, sulphur and vat dyes, is an emerging area. This research has focussed on the introduction of cationic groups to the cotton fibre (Lewis & Lei, 1991; Lewis & McIlroy, 1997). Such modifications, usually referred to as Cationization, are achieved by treating cotton with low molecular weight cationic chemicals or with cationic polymers (resins) [12].
Synthesis of new dyes and modification of cotton dyeing processes, while valid for obtaining qualified black cotton dyeing, are likely to involve significant capital investment and development costs [53]. Thus, much attention has focused on modification of cotton fiber as another route to obtaining the desired dyeing performance and fastness properties with existing dyes and processes. Cationization of cotton is one of the most widely researched modifications in recent years since both direct and reactive dyes carry anionic charges and they exhibit high affinity for positively charged cotton. Numerous chemicals and methods have been used to introduce cationic groups into cotton fiber [21,22].
Cationization of Cotton
The process of modifying cotton by developing cation site on its surface without affecting its bulk property is called ‘cationization’. Modifying the cotton fibre to increase dye-fibre interactions is thus, best route to overcome the lack of affinity for cotton to commercial reactive dyes, so that it can be dyed without salt. It was found that during cationization of cotton, etherification of primary hydroxyl groups on cellulose takes place [54,55].
The introduction of cationic sites within the cellulose is the most expected technique to increase the dye adsorption. Cationic sites can be introduced either by aminization or cationization. Cationization is one of the most important modifications for cellulose. The cationization is mainly carried out to improve affinity toward anionic substances, such as dyes in conventional textile processing and metal ions or unfixed dyes in effluent treatment. Cationic modification is the method that has been employed in order to change the surface charge of cellulosic fibers [56,57].
Cellulosic materials are commonly cationized in three ways: Firstly, a direct cationization of cellulose using a chemical compound with suitable functional groups that react with cellulose hydroxyl groups.
The second approach involves the addition of binding agent, such as Dimethylol dihydroxy ethylene urea (DMDHEU), which reacts both with cellulose hydroxyl and the functional group of cationic agent. This process is mainly used for textile application since the common textile pad-dry-cure process can be employed. The third approach utilizes graft polymerization to introduce monomeric or polymeric cationizing agents within the cellulose, but it is not commercially applicable. Each process has advantages and disadvantages, but none of these processes has been commercially adopted yet [56].
Reasons for Cationization
As previously mentioned, cellulose is the major component of cotton. While in contact with water, negative charges build up on the surface of cotton due to the partial ionization of hydroxyl groups on cellulose. Thus, cotton fibers have electrostatic repulsion to reactive and direct dyes, which are sulfonated and negatively charged. Anionic dyes have to overcome a significant adverse charge barrier before they are adsorbed on cotton fibers [58]. High concentrations of electrolytes, such as sodium chloride and sodium sulfate, are used in the conventional dyeing procedure of cotton with reactive dyes to suppress the negative charge build-up and reduce the solubility of dyes. Even with electrolytes added, the fixation rate of reactive dyes is still relatively low, especially when high concentrations of dyes are applied. As a result, the dye bath wastewater typically contains high concentrations of both salt and unexhausted dye, which causes serious environmental problems [21].
By introduction of cationic groups into cotton fibers, the affinity of reactive dyes for cotton can be significantly improved. The ionic attractions between cationized cotton and reactive dyes can result in increased dye uptake, reduced or no electrolyte use, less dye washing off and less water and energy consumption. The environmental problems caused by dye and salt in effluent can be potentially mitigated by cationization pretreatment of cotton [21,22].
Cationic Reagents
Researchers have investigated different kinds of cationic reagents for the cationization of cotton. Based on the molecular weight, the cationic reagents can be divided into two groups: monomeric reagents and polymeric reagents [21].
Many researches worked to use variety of cationic compounds in order to improve the dyeability of cotton fabrics toward reactive dyes.
A number of processes have been proposed from early 1930s till date, to improve the substantivity of anionic dyes towards cellulosic fibres by introducing cationic sites in the fibres. Schlack (1938) was the first to report improved affinity of acid dyes towards cellulose, modified through the introduction of epoxy derivatives [59]. Clampetier et al. and Merle modified cellulose by a reactions epoxy diethylamine-3-propane followed by an ethyl iodide quatemization and reported the dyeing the dyeability of modified cellulose towards acid dyes [60,61]. Rupin (1976) and Rupin et al. (1970) studied the dyeability of cellulose towards direct and reactive dyes after pretreatment with glycidyltrimethyl ammonium chloride (Glytac) and simultaneous modification and dyeing by exhaust, pad-batch and pad-cure processes [62,63].
Glytac-pretreated cellulose showed improved dyeability in exhaust application, towards reactive dyes in the presence of alkali and salt. Pretreatment of cotton with polyamide epichlorohydrintype polymer, offers the opportunity for increasing both the substantivity and reactivity of cotton towards reactive dyes under neutral to acidic condition due to the introduction of cationic azetidinium group [64]. Wu and Chen (1992) treated cotton with Poly Epichlorohydrin (PECH) dimethylamine which was manufactured by initial polymerization of epichlorohydrin, followed by amination with dimethylamine [65].
The epichlorohydrin was polymerized in carbon tetrachloride using boron trifluride etherate as catalyst. The dyeability of treated cotton towards direct dyes was investigated and it was found that PECH-amine could improve the direct dyeability of modified cotton. In another work they have reported (Wu and Chen, 1993) the effect of PECH-amine treatment on the reactive dyeability of cotton. It was found that the modified cotton can be dyed with selected low reactivity dyes under neutral condition using limited salt concentrations or with selected high reactivity dyes without salt [66].
The effect of modification of cotton using various Nethylolacrylamide derivatives, viz. bis (N-methylol-2-cabamoylethyl) butylamine, N-(N,N’-dimethylol- 2-cabamoylethyl) diethylamine and N-(N, N’-dimethylol-2-cabamoylethyl) dimethylamine on acid dyeability has been investigated by [67]. These agents were applied to cotton fabric by pad-dry-cure procedure. Such treatments were found to improve the crease recovery angle and acid dyeability. But these agents release hazardous formaldehyde during curing as well as on storage [68-71].
Researchers have investigated different kinds of cationic reagents for the cationization of cotton. Based on the molecular weight; the cationic reagents can be divided into two groups, monomericreagents and polymeric reagents. Epoxy compounds, chlorotriazine type quaternary compounds, N methylolacrylamide, choline chloride etc are the monomers commonly used in modification of cotton fiber. Synthetic polymers polyepichloro hydrin dimethylamine, polyamide epichlorohydrin type polymers, poly-(4-vinylpyridine) quaternary ammonium compounds and dendritic polymers, and biopolymerschitosan, starch and their derivatives are typical compounds commonly used in modification of cotton [22,72,73].
Researchers have investigated different kinds of cationic reagents for the cationization of cotton. Both chemical and physical modifications of cotton with compounds including monomers and polymers were employed. Epoxy compounds, chlorotriazine type quaternary compounds, N methylolacrylamide, choline chlorides etc. are the monomers commonly used in modification of cotton fiber. Synthetic polymers, polyepi-chloro-hydrindimethylamine, polyamide epichlorohydrin type polymers, poly-(4-vinylpyridine) quaternary ammonium compounds and dendritic polymers, and biopolymers-chitosan, starch and their derivatives are typical compounds used in modification of cotton [21,22,72,73].
All agents used for cationization, both monomeric and polymeric, have their limitations, but generally speaking, the more complicated the reagent, the more synthesis steps are required resulting in high costs for the reagents. Additionally, vinyl reagents must be free radical polymerized in situ with an initiator. Finally, reagents such as choline chloride with no reactive group require a cross linker such as DMDHEU and the complications associated with cross linking such as formaldehyde release and reduced breaking strength of the treated fabric. Moreover, all the above chemicals are non-biodegradable as well as non-renewable [22,74].
Different approaches, such as modification of the dye structure to make it more substantive to cellulose, cationization of cellulose through chemical reaction with compounds containing cationic groups or controlled dosing of dye and salt during the exhaustion process, have been recommended to control the above mentioned effluent problem [21,68].
Most of the techniques developed so far for reducing salt concentration in cotton dyeing are complicated in nature. Some of these methods are successful only for highly reactive dyes. Under this background, efforts were made to find out easier method for salt free reactive dyeing of cotton [69].
Currently, there is a growing interest in the development of biodegradable cationizing agent in keeping with the requests of people for environmental protection. In terms of environmental friendliness, cost, and ease of application, using bio product cationizing agent, is without a doubt the method of best choice for cationization of cellulose being biodegradable and renewable.
Monomeric Cationic Reagents
Introduction of amine groups into the cellulose structure has long been recommended for cationization of cotton. A typical form of the cationic reagent is the quaternary ammonium compound with a reactive group which can form covalent bond with cellulose. The 2, 3-epoxypropyl trimethylammonium chloride (EPTAC, Figure 3) and its epichlorohydrin precursor, 3-chloro-2-hydroxypropyl trimethylammonium chloride (CHPTAC, Figure 4) are very commonly used cationization reagents for cotton to improving dyeability [53,62,63,75]. The epoxy group can react with cellulose under alkaline conditions.
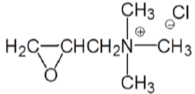
Figure 3: 2,3-Epoxypropyl Trimethyl Ammonium Chloride (EPTAC).
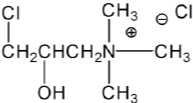
Figure 4: Epichlorohydrin precursor, 3-Chloro-2-Hydroxypropyl
Trimethylammonium Chloride (CHPTAC).
Other aminated epoxy derivatives have also been synthesized and applied on cellulose for cationization. For example, N-Oxiranylmethyl- N-methylmorpholinium chloride (Figure 5) was reported to have higher reactivity with cellulose than EPTAC [76]. Figure 6 shows the structure of the cationic reagents with different alkyl groups attached to ammonium. It was reported that, the cationization efficiency and amount of dye adsorbed decreased as the length of the hydrocarbon chain attached to the ammonium group increased [77]. As shown in Figure 7, there are other cationic reagents with substituted counter ions and hydrocarbon chains [78].
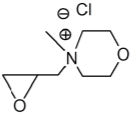
Figure 5: N-Oxiranylmethyl-N-methylmorpholinium chloride.
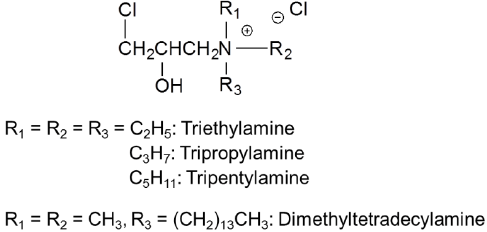
Figure 6: Aminated epoxy based cationic reagents.
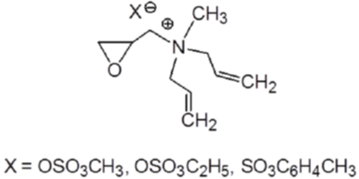
Figure 7: Alkyl di-allyl ammonium salts.
To increase cationization efficiency, aminated epoxy derivatives with multiple functional groups were synthesized and applied on cotton. A bi-functional cationic agent (Figure 8) was reported to have higher cationization efficiency than EPTAC while applied using exhaustion method [79]. Cotton modified with 2,4,6-tri-[(2-hydroxy- 3-trimethyl-ammonium) propyl]-1,3,5-triazine chloride (Tri-HTAC) (Figure 9) showed improved printing performance with reactive dyes compared with untreated cotton [80]. However, reaction efficiency of Tri-HTAC was not compared with the mono-reactive agent EPTAC.
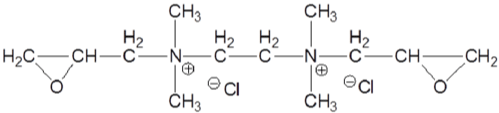
Figure 8: Bi-functional cationic agent.
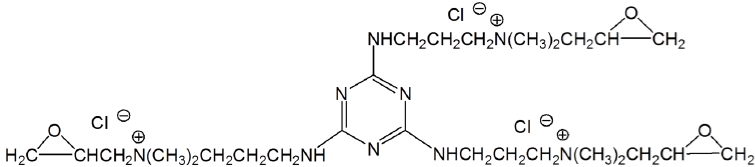
Figure 9: 2,4,6-Tri-[(2-hydroxy-3-trimethyl-ammonium) propyl]-1,3,5-triazine
chloride (Tri-HTAC).
Cotton treated with chlorotriazine type quaternary ammonium compounds was also found to have increased exhaustion with anionic dyes [81,82]. This kind of cationic agent exhibit better thermal stability compared with the epoxy based agents. (Figure 10 & 11) show typical structures of mono-chlorotriazine type cationic reagent with either mono-[83,84] or bis-quaternary ammonium. Since monochlorotriazine type cationic reagent was reported to have relatively low substantivity for cellulose, bis-chlotriazinebis-quaternary agents (Figure 12) were employed for cationization of cotton [85]. With promoted dye exhaustion and fixation, dyed cotton fabric pre-treated with bis-reactive bis cationic quaternary agent may suffer a decrease in light fastness [86].
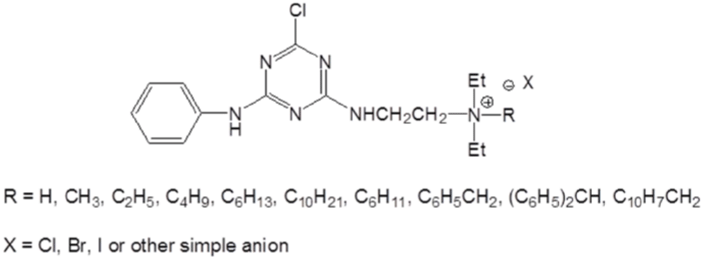
Figure 10: Mono-chlorotriazine mono-quaternary cationic reagents.
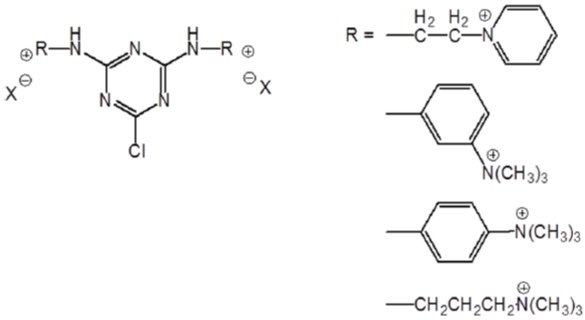
Figure 11: Mono-chlorotriazinebis-quaternary cationic reagents.
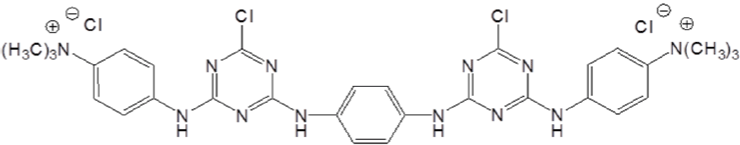
Figure 12: Bis-chlotriazineBis-quaternary agents.
With the acrylamide as the reactive group, 1-Acrylamido-2- Hydroxy-3-Trimethylammonium Propane Chloride (AAHTAPC) can react with cellulose under alkaline conditions (Figure 13). Cotton pad-baked with the agent can be dyed with reactive dyes without salt or alkali [87]. Some other acrylic-based cationic agents can be fixed on cotton by grafting polymerization [88,89].
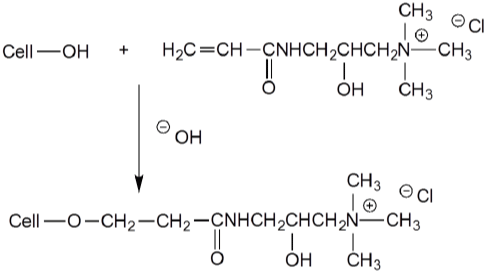
Figure 13: Reaction between AAHTAPC and cellulose under alkaline
conditions.
By using crosslinking agents, the non-reactive cationic agent choline chloride (Figure 14) can also be used for producing cationized cotton with good reactive dye uptake under acidic conditions in absence of salt [90]. Various cross linking agents Trimethylolmelamine (TMM), Trimethylolacetylenediureine (3ACD), Dimethylol Dihydroxy Ethylene Urea (DMDHEU), and dimethylpropylcarbamate (DMPC), which are normally associated with durable-press finishing processes, were used to bind choline chloride to cotton fabric by a pad-dry-cure method [91].
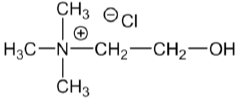
Figure 14: Non-reactive cationic agent choline chloride.
Besides cationization by introducing quaternary ammonium, introduction of amine groups (ammonization) can also improve the dyeability of cotton with anionic dyes under acid to neutral conditions. As show in (Figure 15), 1,1-Dimethyl-3-hydroxy Azetidinium chloride (DMAC) forms covalent bond with cotton while in presence of alkali [58]. Treated cotton showed excellent substantivity for reactive dyes under neutral condition in the absence of electrolyte [68,91].
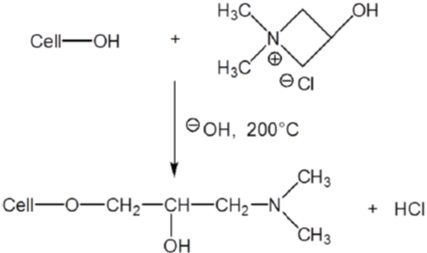
Figure 15: Reaction between DMAC and cellulose under alkaline conditions.
Polymeric Cationic Reagents
Polymeric cationic reagents, which are mainly polymeric amines or amides, have been applied for cationization of cotton due to their good affinity for cellulose. Polyamide-epichlorohydrin resin (Hercosett 125; Hercules Powder Corp.), is a reactive cationic polymer prepared by the initial condensation of adipic acid with diethylenetriamine followed by reaction and partial cross-linking with epichlorohydrin [92, 93]. The main reactive group in the polymer is 3-hydroxy azetidinium chloride which can react with nucleophiles [94]. (Figure 16) presents the reactive and nucleophilic sites that may exist on the surface of the polyamide-epichlorohydrin treated cotton substrate [93]. Under neutral conditions in absence of salt, the treated cotton showed good dyeability with highly reactive Dichloros- Triazine (DCT) and Difluorochloropyrimidine (FCP) type reactive dyes. However, the system did not work well for low reactivity dyes including Monochloro-s-Triazine (MCT) and Dichloroquinoxaline (DCQ) reactive dyes [91]. In another aspect, introduction of thiourea [95] or ethylenediamine [96] into the application process of polyamide-epichlorohydrin has benefical effects on the results obtained.
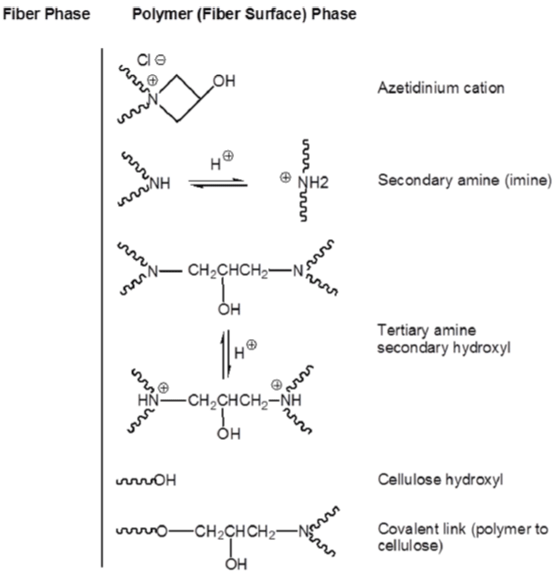
Figure 16: Illustration of reactive surface of Hercosett treated cotton.
Polyepichlorohydrin dimethylamine (PECH-amine) was also applied on cotton for cationization [65,66,97]. It was prepared by initial polymerization of epichlorohydrin with boron trifluorideetherate as catalyst, followed by amination with dimethylamine (Figure 17) [65]. With good affinity, the PECH-amine can be applied to cotton by exhaustion method. Treated cotton was reported to have improved dyeability for direct [65], reactive [66], and acid dyes [98].
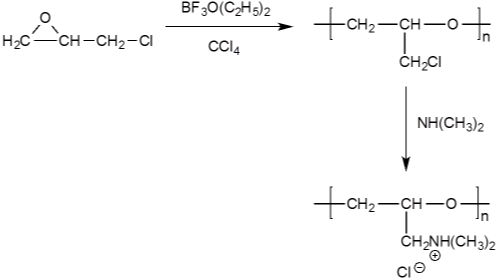
Figure 17: Formation of poly epichlorohydrin-dimethylamine.
Sandene 8425, developed by Courtaulds and Clariant (formally Sandoz), is a polyamino cationic resin with high substantivity to cotton under alkaline conditions [58,91]. It can be applied on cotton by an exhaustion method. The treated cotton not only attracts anionic dyes, but also offers new dyeable groups for fixation that complement the cellulose hydroxyl groups [99]. The major disadvantages of the pretreatment include a reduction in light fastness of some azochromophores and a dulling in shade [58, 100].
Since most synthetic chemicals used for cationization of cotton are not safe environmentally, chitosan, the natural biopolymer, has been used as a substitute for improving cotton dyeability. Chitosan is a long-chain unbranched polymer derived from chitin by deacetylation with hot alkali. It has very similar structure to cellulose except that the hydroxyl group in the C2 position of the glucose ring has been replaced by an amino group. Figure 18 shows the structure of cellulose, chitin and fully deacetylated chitosan. In slightly acidic conditions, the amino group in chitosan can accept a proton and creates positive charge to attract anionic dyes. Rippon improved the dyeability of immature cotton for direct dyes by pretreatment with chitosan [101]. However, decreased wash and rub fastness were observed on treated cotton dyeing. Bandyopadhay et al. applied chitosan on cotton using a pad-dry method. With chitosan-treated cotton, the amount of salt required can be reduced to 50% to produce a comparable shade to that of untreated fabric [102].
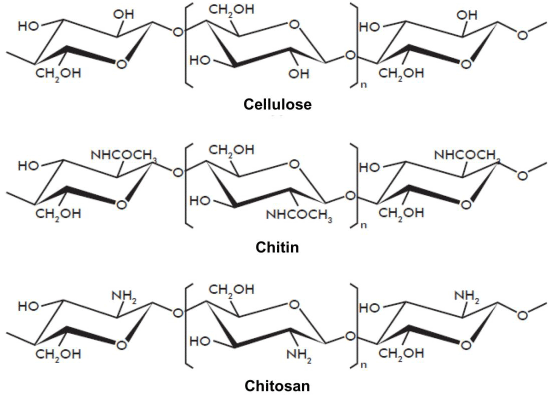
Figure 18: Structure of cellulose, chitin, and chitosan.
To improve cationization efficiency, Lim and Hudson [103] treated cotton with a fiber-reactive chitosan derivative, O-acrylamidomethyl- N-[(2-hydroxy-3-trimethylammonium)propyl] chitosan chloride (NMA-HTCC). The compound was applied to cotton under alkaline conditions using a cold pad-batch method and was able to form a covalent bond with cellulose (Figure 19). Treated cotton dyed with direct and reactive dyes without salt showed higher color yield than conventional dyeing on untreated cotton.
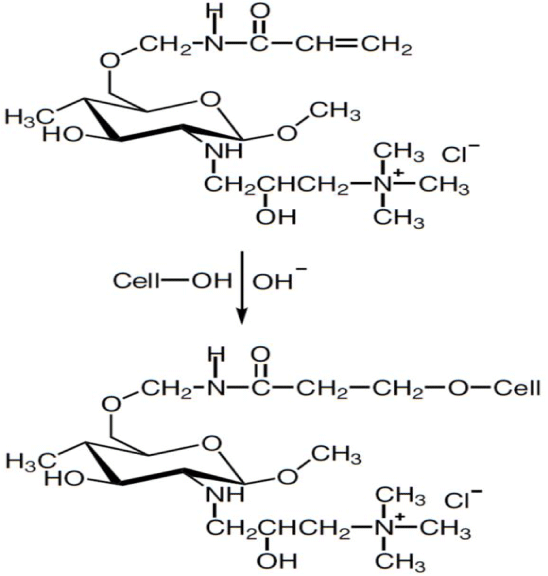
Figure 19: Reaction between NMA-HTCC and cellulose under alkaline
conditions.
There are many other cationic polymers which were successfully applied for cotton cationization. Cai et al. modified cotton with a commercial cationic acrylic copolymer, Polymer PL, using a pad-dry method. Treated cotton was reported to have increased dyeability with reactive dyes and comparable light fastness [104]. Ma et al. investigated the pretreatment with poly (vinylamine chloride) (Figure 20) for salt-free dyeing of cotton with reactive dyes. Higher dye fixation, excellent wash fastness, and good rub fastness was obtained on treated cotton. Zhang et al. improved cotton dyeability with reactive dyes using cationic starch, which was treated with EPTAC [105]. El-Shishtaway and Nassar improved printability of cotton with pigment and anionic dyes by applying Solfix E, which is a polyaminochlorohydrin quaternary ammonium polymer with epoxide functionality [106].
Figure 20: Poly (Vinylamine Chloride).
Besides linear polymers, some innovative hyperbranched polymers with amino groups were also applied on cotton for improving dyeability. Burkinshaw et al. applied a commercial dentrimer product using an exhaustion method. The structure shown in (Figure 21) is only one of the isomers present in the product [107]. Zhang et al. synthesized a water-soluble amino-terminated hyperbranched polymer from methyl acrylate and diethylenetriamine by melt polycondensation and applied on cotton using a pad-drycure method [108]. In both of the researches, treated cotton showed improved dyeability with reactive dyes. However, the treatment efficiency and applicability should be further investigated and compared with regular cationic agents.
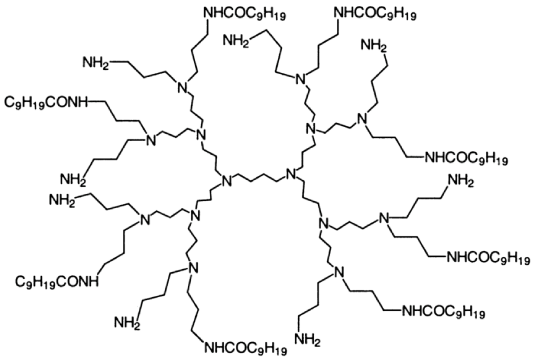
Figure 21: Dentrimer used for cotton modification.
All agents used for cationization, both monomeric and polymeric, have their limitations. Monomeric cationic agents, such as EPTAC, often suffer from relatively low substantivity, poor thermal stability, and unpleasant odor. While for polymeric cationic agents, ring dyeing, poor light fastness, and change of hue are the major issues. Thus, for optimum cationization efficiency and dyeing performance, the cationic agent and its application method should be carefully selected.
Use of Organic Compounds in Place of Inorganic Chemicals
Use of selected organic compounds has been shown to be an effective alternative to inorganic salt. This reduces effluent pollution [25,109], as most of such compounds tend to be biodegradable. ‘Betaine’, an organic compound, has also been reported to reduce the amount of inorganic salt [110]. Organic surfactants have also been studied as salt substitutes [111].
The sodium salts of organic acids have been explored as the alternative to sodium chloride and sodium sulphate. Prabu et al [112] have demonstrated the use of trisodium citrate as an alternative to conventional inorganic salts for exhaust dyeing of cotton with reactive, direct and solubilised vat dyes. Salts of polycarboxylic acids have been shown to be the most effective alternatives to inorganic salts [109,113].
An alkaline polycarboxylic sodium salt, tetrasodium ethylene diaminetetraacetate (sodium edate), has been reported an alternative to inorganic electrolyte, sodium sulphate, and alkali, sodium carbonate in exhaust dyeing of cotton with reactive dyes [114]. Recently, we have found that trisodiumnitrilo triacetate (NTA), another alkaline polycarboxylic sodium salt, can effectively substitute inorganic salt and alkali in continuous pad-steam reactive dyeing of cotton [115]. NTA and sodium edate were shown to produce reduced effluent TDS where NTA gave improved colour yields compared to sodium edate.
In the pad-dry-bake reactive dyeing method, where urea is used, the process conditions result in some decomposition of the urea. That causes an increase in the residual nitrogen content and to some extent reduces the yield of dye-fibre reaction. Using reduced amount of urea with a dicyandiamide in the dyebath has been proposed to reduce the environmental impact [14]. Caprolactum products have been reported as partial or complete substitution of urea in reactive dyeing and printing of cotton fabrics [116].
Conclusion
This paper has explored the range of options available to textile manufacturers to reduce the environmental impact of dyeing cotton textiles with reactive dyes. Some of them have commercially been adopted.
Further developments in reactive dye structures may provide reduced environmental discharge loads and improved dyeing results. Ultra low liquor ratio dyeing machines and padders with low trough volume capacity are commercially successful developments for reducing the amount of unsustainable chemicals in the effluent. Their wider use should be encouraged. Cationisation of cotton has been shown to be an effective way of reactive dyeing of cotton without inorganic salt and alkali. However, most pretreatments identified to date are complicated and expensive. Moreover, most of cationization treatments using synthetic compounds cause increase in effluent pollution. Cationisation using natural polymers may be an environmentally safer approach. Replacement of inorganic chemicals with organic compounds is another way of reducing effluent pollution effectively. Like cationization pretreatments, alternate organic compounds are more expensive.
Most of the environmentally safer options are more costly. Thus industrial dyers are not encouraged to implement them. Throughout the world, governments and water authorities are introducing tougher load based discharge permits, charges and penalties. The higher costs of environmentally safer options may be offset, in whole or part, by the costs of effluent purification or by penalties on more polluted effluent.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
The authors do not receive any grant or financial support from any organization or supporting bodies.
References
- Singha K, Maity S, Singha M. The salt-free dyeing on cotton: An approach to effluent free mechanism; can chitosan be a potential option. International Journal of Textile Science. 2012; 1: 69-77.
- Ashenafi B, Berhane H, Gashawbeza H. Studies on dyeing properties of chitosan modified cellulosic fiber. J Textile Eng Fashion Technol. 2020; 6: 37-42.
- Ashenafi B, Berhane H, Gashawbeza H. Functionalization of Cellulosic Fibers Using Chitosan: a Salt Free Dyeing Approach. Adv Res Text Eng. 2020; 5: 1043.
- Bilgen, M. Wrinkle recovery for cellulosic fabric by means of ionic crosslinking. 2006.
- Sharma R, Sayed U. Surface modification of cellulosic fabric. Int J Adv Chem Eng Biol Sci. 2016; 3: 90-96.
- Agrawal BJ. Eco-friendly Chemical-free free Dyeing of Polyester. Cotton Blended Fabrics. 2016; 5: 20-5.
- Dessiea A, Govindanb N. Eco-friendly, Salt-Free Reactive Dyeing by Cationization of Cotton with Amino Acids Obtained from Soya Bean Hull. Journal of Textile Science & Engineering. 2018; 08.
- Asaye D, Nalankilli GA. Salt-Free Reactive Dyeing Approach for Cotton by Cationization with Amino Acid Derived from Soya Bean Hull. Adv Res Text Eng. 2018; 3: 1032.
- Dessie A, Nalankilli G. Reactive dyeing of cotton without use of salt by cationization with amino acid derived from natural protein of soya bean hull. International Journal of Industrial Engineering. 2018; 2: 233-46.
- Wolela AD. An Overview on Surface Modification of Cotton using Cationic Reagents for Salt-Free or Low Salt Dyeing. Current Trends in Fashion Technology & Textile Engineering. 2019; 5: 37-46.
- Wolela, A. D. Cationic Modification of Cotton for Salt-free Reactive Dyeing: A Review.
- Khatri A, Padhye R, White M. Developments in reactive dyeing of cotton textiles to improve sustainability.
- King D. Dyeing of cotton and cotton products. In S. Gordon & Y.-L. Hsieh (Eds.), Cotton: Science and Technology (First ed., pp. 353-377): Woodhead Publishing Limited. 2007; 353-377.
- Philips D. Environmentally friendly, productive and reliable: priorities for cotton dyes and dyeing processes. Journal of the Society of Dyers and Colourists. 1996; 112: 183-186.
- Holme I. TECHNOLOGY-Dyeing Cellulosic Fibres. Textiles Magazine. 2004; 31: 8-13.
- Broadbent AD. Reactive dyes. In A. D. Broadbent (Ed.), Basic Principles of Textile Coloration (First ed., pp. 332-357): Society of Dyers and Colourists. 2005.
- Smith B. Wastes from textile processing. In A. L. Andrady (Ed.), Plastics and the Environment (First ed., pp. 293 - 295): John Wiley and Sons, Inc. 2003.
- Babu BR, Parande AK, Raghu S, Kumar TP. Cotton textile processing: waste generation and effluent treatment. Journal of cotton science. 2007.
- Chavan RB. Environment-friendly dyeing processes for cotton. 2001.
- Kissa E. Urea in reactive dyeing. Textile Research Journal. 1969; 39: 734- 741.
- Fu S. Studies on dyeing cationized cotton. North Carolina State University. 2016.
- Fu S, Hinks D, Hauser P, Ankeny M. High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose. 2013; 20: 3101–3110.
- Chavan RB. Environment-friendly dyeing processes for cotton. 2001.
- Moore SB, Ausley LW. Systems thinking and green chemistry in the textile industry: concepts, technologies and benefits. Journal of cleaner production. 2004; 12: 585-601.
- Taylor JA. Recent developments in reactive dyes. Review of Progress in Coloration and Related Topics. 2000; 30: 93-108.
- Lewis DM. The chemistry of reactive dyes and their application processes. In Handbook of textile and industrial dyeing (pp. 303-364). Woodhead Publishing. 2011.
- He WD, Broadbent PJ, Lewis DM. Reactive dye compounds. Patent WO2002096995 (Procter & Gamble). 2002.
- Smith B, Berger R, Freeman HS. High affinity, high efficiency fibre-reactive dyes. Coloration technology. 2006; 122: 187-193.
- Morris KF, Lewis DM, Broadbent PJ. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Coloration Technology. 2008; 124: 186-194.
- Renfrew AHM.. Reactive dyes for textile fibres. Society of Dyers and Colourists. 1999; 169.
- Morimura N, Ojima M. New reactive dyes fixable at neutral pH. American Dyestuff Reporter. 1985; 74: 28-36.
- Lewis DM, Broadbent PJ, Vo LT. Covalent fixation of reactive dyes on cotton under neutral conditions. AATCC review. 2008; 8: 35.
- Gillingham EL, Lewis DM, Nabi A, Srikulkit K. Triazinylaminoalkylphosphonate reactive dyes for cellulosic fibres. Coloration Technology. 2007; 123: 178-183.
- Hinks D, Burkinshaw SM, Lewis DM, Renfrew AHM. Cationic Fiber Reactive Dyes for Cellulosic Fibers. AATCC Review. 2001; 1.
- Srikulkit K, Santifuengkul P. Salt-free dyeing of cotton cellulose with a model cationic reactive dye. Coloration Technology. 2000; 116: 398-402.
- Zheng C, Yuan A, Wang H, Sun J. Dyeing properties of novel electrolyte-free reactive dyes on cotton fibre. Coloration Technology. 2012; 128: 204-207.
- Rupp J. Recent developments in dyeing. Textile World. 2010.
- European IPPC Bureau. Reference document on best available techniques for the textiles industry. Institute for Prospective Technological Studies. Spain. 2003.
- Leube H. Textile dyeing. Industrial Dyes: Chemistry, Properties, Applications. 2002; 339-425.
- Wilbers L, Seiler G. New concept for dyeing of cellulosic substrates at ultralow liquor ratios. Melliand International. 2002; 8: 66–67.
- Khatri A, Peerzada MH, Mohsin M, White M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. Journal of Cleaner Production. 2015; 87: 50-57.
- Ali S, Khatri Z, Brohi KM). Econtrol dyeing process: an ecological and economical approach. In Energy, Environment and Sustainable Development (pp. 291-297). Springer, Vienna. 2012.
- Haggag K, Elshemy NS, Nasef NA. Enhancement of cotton and cotton/wool blend dyeability by microwave heating. Research Journal of Textile and Apparel. 2012. 16: 34-45.
- Hashem M, Taleb MA, El-Shall FN, Haggag K. New prospects in pretreatment of cotton fabrics using microwave heating. Carbohydrate polymers. 2014; 103: 385-391.
- Schmidt A, Bach E, Schollmeyer E. The dyeing of natural fibres with reactive disperse dyes in supercritical carbon dioxide. Dyes and Pigments. 2003; 56: 27-35.
- Özcan AS, Clifford AA, Bartle KD, Broadbent PJ, Lewis DM. Dyeing of modified cotton fibres with disperse dyes from supercritical carbon dioxide. Journal of the Society of Dyers and Colourists. 1998; 114: 169-173.
- Özcan AS, Clifford AA, Bartle KD, Lewis DM. Dyeing of cotton fibres with disperse dyes in supercritical carbon dioxide. Dyes and Pigments. 1998; 36: 103-110.
- Thakore KA. Application of Ultrasonics in Dyeing of Cotton Fabrics with Direct Dyes: Part I-Kinetics of Dyeing. 1988.
- Thakore KA. Application of Ultrasonics in Dyeing of Cotton Fabrics with Direct Dyes: Part I-Kinetics of Dyeing. 1988.
- Thakore KA. Ultrasound Treatment in Exhaust and Pad-Batch Dyeing. AATCC Review. 2011; 11.
- Khatri Z, Memon MH, Khatri A, Tanwari A. Cold Pad-Batch dyeing method for cotton fabric dyeing with reactive dyes using ultrasonic energy. Ultrasonics sonochemistry. 2011; 18: 1301-1307.
- Öner E, Baser I, Acar K. Use of ultrasonic energy in reactive dyeing of cellulosic fabrics. Journal of the Society of Dyers and Colourists. 1995; 111: 279-281.
- Hauser PJ, Tabba AH. Improving the environmental and economic aspects of cotton dyeing using a cationised cotton. Coloration Technology. 2001; 117: 282-288.
- Shahidi S, Wiener J, Ghoranneviss M. Surface modification methods for improving the dyeability of textile fabrics. Eco-friendly textile dyeing and finishing. 2013; 10: 53911.
- Tseghai GB, Wangatia LM. Surface modification of cotton using slaughterhouse wastes. International Journal of Materials and Textile Engineering. 2015; 9: 3011-3015.
- Shahin M. The influence of cationization on the dyeing performance of cotton fabrics with direct dyes. International Journal of Engineering Research and Applications, 2015; 5, 62-70.
- Bhuiyan MR, Shaid A, Khan MA. Cationization of cotton fiber by chitosan and its dyeing with reactive dye without salt. Chemical and materials engineering. 2014; 2: 96-100.
- Lewis DM, Lei X. Improved Cellulose Dyeability by Chemical Modification of the Fiber. Textile Chemist & Colorist. 1989; 21.
- Schlack, P.; US Patent: 2,131,120 (Sept. 27, 1938).
- Champetier G, Montegudet G, Petit J. Obtention De Derives Amines De La Cellulose. ComptesRendusHebdomadaires Des Seances De L Academie Des Sciences. 1955; 240: 1896-1898.
- Merle Y. Sur La Preparation De Derives Amines De Lalcool Polyvinylique Et De Lamidon. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences. 1958; 246: 1425-1426.
- Rupin M. Dyeing with Direct and Fiber Reactive Dyes. Textile Chemist & Colorist. 1976; 8.
- Rupin M, Veaute G, Balland J. Utilisation de composes reactifs epoxy ammonium quatinairsdans la cellulose encolorante direct etreactifs. Textilveredlung. 1970; 5: 829-838.
- Earle RH, Saunders RH, Kangas LR. chemistry of the Chlorination/Hercosett resin shrinkproofing process. In Applied polymer symposia. 1971.
- Wu TS, Chen KM. New cationic agents for improving the dyeability of cellulose fibres. Part 1-pretreating cotton with polyepichlorohydrin-amine polymers for improving dyeability with direct dyes. Journal of the Society of Dyers and Colourists. 1992; 108: 388-394.
- Wu TS, Chen KM. New cationic agents for improving the dyeability of cellulose fibres. Part 2-pretreating cotton with polyepichlorohydrin-amine polymers for improving dyeability with reactive dyes. Journal of the Society of Dyers and Colourists. 1992; 109: 153-158.
- El-Kharadly EA, Shakra S, Youssef BM, Youssef YA. Dyeing of chemically modified cotton fabric. 1996.
- Chattopadhyay DP. Cationization of cotton for low-salt or salt-free dyeing. 2001.
- Chattopadhyay DP, Chavan RB, Sharma JK. Salt-free reactive dyeing of cotton. International Journal of Clothing Science and Technology. 2007; 19: 99-108.
- Ramasamy M, Kandasaamy PV. Effect of cationization of cotton on it’s dyeability. 2005.
- Shahin M. The influence of cationization on the dyeing performance of cotton fabrics with direct dyes. International Journal of Engineering Research and Applications. 2015; 5: 62-70.
- Ma W, Jz Y. Development of functional polymers in modification of cotton for improving dyeability of reactive dyes. In The Proceedings of the 3rd international conference on functional molecules. 2002.
- Aktek T, Millat AKMM. Salt free dyeing of cotton fiber—A critical review. Int J Text Sci. 2017; 6: 21-33.
- Farrell MJ. Sustainable cotton dyeing. North Carolina State University. 2012.
- Hashem M, Hauser PJ, Smith B. Reaction Efficiency for Cellulose Cationization Using 3-Chloro-2- Hydroxypropyl Trimethyl Ammonium Chloride. Textile Research Journal. 2003; 73: 1017-1023.
- Hasani M, Westman G, Potthast A, Rosenau T. Cationization of cellulose by using N-oxiranylmethyl-N-methylmorpholinium chloride and 2-oxiranylpyridine as etherification agents. Journal of applied polymer science. 2009; 114: 1449-1456.
- Seong HS, Ko SW. Synthesis, application and evaluation of cationising agents for cellulosic fibres. Journal of the Society of Dyers and Colourists. 1998; 114; 124-129.
- Topfl R. U.S. Patent No. 5,147,411. Washington, DC: U.S. Patent and Trademark Office. 1992.
- Yuan H, Hauser PJ. Synthesis of a bi-functional cationic agent and its application in cotton dyeing. AATCC Journal of Research. 2014; 1: 8-12.
- Xie K, Liu H, Wang X. Surface modification of cellulose with triazine derivative to improve printability with reactive dyes. Carbohydrate Polymers. 2009; 78: 538-542.
- Chavan RB, Chattopadhyay DP. Cationization of cotton for improved dyeability. Colourage. 1998: 127-133.
- Sharif S, Ahmad S, Izhar-ul-Haq MM. Role of quaternary ammonium salts in improving the fastness properties of anionic dyes on cellulose fibres. Coloration Technology. 2007; 123: 8-17.
- Clipson JA, Roberts GAF. Differential dyeing cotton. 1 – Preparation and evaluation of differential dyeing cotton yarn. Journal of The Society of Dyers and Colourists. 2008; 105: 158-162.
- El-Shishtawy RM, Youssef YA, Ahmed NS, Mousa AA. Acid dyeing isotherms of cotton fabrics pretreated with mixtures of reactive cationic agents. Coloration technology. 2004; 120: 195-200.
- Evans GE, Shore J, Stead CV. Dyeing behaviour of cotton after pretreatment with reactive quaternary compounds. Journal of the Society of Dyers and Colourists. 1984; 100: 304-315.
- Youssef YA. Direct dyeing of cotton fabrics pre-treated with cationising agents. Coloration Technology. 2000; 116: 316-322.
- Wang H, Lewis DM. Chemical modification of cotton to improve fibre dyeability. Coloration Technology. 2002; 118: 159-168.
- Liu Y, Liu Y, Ren X, Huang TS. Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Applied Surface Science. 2014; 296: 231-236.
- Jang J, Ko SW, Carr CM. Investigation of the improved dyeability of cationised cotton via photografting with UV active cationic monomers. Coloration Technology. 2001; 117: 139-146.
- Harper Jr RJ, Stone RL. Cationic Cotton Plus Easy Care. Textile Chemist & Colorist, 1986: 18
- Lewis DM, Mcllroy KA. The chemical modification of cellulosic fibres to enhance dyeability. Review of Progress in Coloration and Related Topics. 1997; 27: 5-17.
- Keim GI. U.S. Patent No. 2,926,154. Washington, DC: U.S. Patent and Trademark Office. 1960.
- Burkinshaw SM, Lei XP, Lewis DM. Modification of cotton to improve its dyeability. Part 1 — pretreating cotton with reactive polyamideepichlorohydrin resin. Journal of The Society of Dyers and Colourists. 2008; 105: 391-398.
- Carr ME, Doane WM, Hamerstrand GE, Hofreiter BT. Interpolymer from starch xanthate and polyamide–polyamine–epichlorohydrin resin: structure and papermaking application. Journal of Applied Polymer Science. 1973; 17: 721-735.
- Burkinshaw SM, Lei XP, Lewis DM, Easton JR, Parton B, Phillips DAS. Modification of cotton to improve its dyeability. Part 2 – pretreating cotton with a thiourea derivative of polyamide–epichlorohydrin resins. Journal of The Society of Dyers and Colourists. 2008; 106: 307-315.
- Lei XP, Lewis DM. Modification of cotton to improve its dyeability. Part 3 – polyamide–epichlorohydrin resins and their ethylenediamine reaction products. Journal of The Society of Dyers and Colourists. 2008; 106: 352- 356.
- Wu TS, Chen KM. New cationic agents for improving the dyeability of cellulosic fibres. Part 3 — the interaction between direct dyes and polyepichlorohydrin-dimethylamine polymers. Journal of The Society of Dyers and Colourists. 2008; 109: 365-368.
- Rong L, Feng G. Dyeing properties of PECH-amine cationized cotton with acid dyes. Journal of Applied Polymer Science. 2006; 100: 3302-3306.
- Choudhury AKR. Textile preparation and dyeing(1st ed.). CRC Press. 2006.
- Rong L, Feng G. Dyeing properties of PECH-amine cationized cotton with acid dyes. Journal of Applied Polymer Science. 2006; 100: 3302-3306.
- Rippon JA. Improving the dye coverage of immature cotton fibres by treatment with chitosan. Journal of the Society of Dyers and Colourists. 1984; 100: 298-303.
- Bandyopadhyay BN, Sheth GN, Moni MM. Chitosan can cut salt use in reactive dyeing. International Dyer. 1998; 183: 39-42.
- Lim SH, Hudson SM. Application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carbohydrate Polymers. 2004; 56: 227-234.
- Cai Y, Pailthorpe MT, David SK. A new method for improving the dyeability of cotton with reactive dyes. Textile research journal. 1999; 69: 440-446.
- Zhang S, Ma W, Ju B, Dang N, Zhang M, Wu S, et al. Continuous dyeing of cationised cotton with reactive dyes. Coloration technology. 2005; 121: 183-186.
- El-Shishtawy RM, Nassar SH. Cationic pretreatment of cotton fabric for anionic dye and pigment printing with better fastness properties. Coloration technology. 2002; 118: 115-120.
- Burkinshaw SM, Mignanelli M, Froehling PE, Bide MJ. The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton. Dyes and pigments. 2000; 47: 259-267.
- Zhang F, Chen Y, Lin H, Lu Y. Synthesis of an amino-terminated hyperbranched polymer and its application in reactive dyeing on cotton as a salt-free dyeing auxiliary. Coloration Technology. 2007; 123: 351-357.
- Rucker JW, Guthrie DMI. I Reduction of Salt Requirements in Dyeing Cotton with Fiber Reactive Dyes. 1997.
- Liu LJ, Yao JB. Application of betaine in low-salt dyeing of cotton fabric with reactive dyes. Journal of Tianjin Polytechnic University. 2009; 28: 53-57.
- Rucker JW, Guthrie DM. Salt substitute for dyeing cotton with fiber reactive dyes. Sen’iGakkaishi. 1997; 53: P256-P260.
- Prabu HG, Sundrarajan M. Effect of the bio-salt trisodium citrate in the dyeing of cotton. Coloration technology. 2002; 118: 131-134.
- Guan Y, Zheng QK, Mao,YH, Gui MS, Fu HB. Application of polycarboxylic acid sodium salt in the dyeing of cotton fabric with reactive dyes. Journal of applied polymer science. 2007; 105: 726-732.
- Ahmed NS. The use of sodium edate in the dyeing of cotton with reactive dyes. Dyes and Pigments. 2005; 65: 221-225.
- Khatri A, Padhye R, White M, Cowlishaw K. Biodegradable organic salts for reactive dyeing of cotton. 2010.
- Sheth GN, Musale AA. Substitute products for urea in application of reactive dyes to cotton fabrics. 2004.