
Review Article
Adv Res Text Eng. 2022; 7(3): 1077.
Application and Dyeing Process of Substantive Dye
Wolela AD¹* and Yeshanaw DA²
¹Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University; Kombolcha, Ethiopia
²Department of Textile Engineering, Ethiopian Technical University, Addis Abeba, Ethiopia
*Corresponding author: Asaye Dessie Wolela, Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University, Ethiopia
Received: September 26, 2022; Accepted: November 01, 2022; Published: November 08, 2022
Abstract
This paper reports the studies available on the theory of substantive dye, the application properties of substantive dye and dye ability of fibers in relation to dye structure. Dyeing with substantive dyes and comparison with acid dyes; some properties of aqueous solutions of the substantive dyes; adsorption of substantive dyes from aqueous solution; distribution of a colloid between an interface and the distribution medium was discussed. The interaction between dye and fibre under given dyeing conditions was presented. Different researchers studied the process of dyeing with substantive or colloidal dyes and the factors which affect the dispersion and stability of the dye.
Keywords: Substantive dye; Dyebath; Adsorption; Colloid distribution; Dispersion; Suspension
Introduction
A substantive dye or direct dye is a dye that adheres to its substrate, typically a textile, by non-ionic forces. The amount of this attraction is known as “substantivity”: the higher the substantivity the greater the attraction of the dye for the fiber [1]. Substantive dyes work best on textiles with high contents of cellulose, such as cotton. In contrast to direct dyes, wool and leather goods are dyed by the process of ion exchange, exploiting the cationic nature of proteins near neutral pH. The development of substantive dyes helped make mordant dyes obsolete.
Substantive dyes are set in a slightly basic or neutral environment at temperatures close to boiling point. They are set by formation of aggregates of dyes within interstices of the fibres. Aggregation is enhanced by extended aromatic rings [2].
Direct dyes adhere to cloth without the aid of additional chemicals. Wool and silk, which contain many anionic polar sites, readily form ionic bonds with the cationic sites in triphenyl methane dyes such as malachite green [3].
Cotton, linen, and rayon, which are cellulose fibers, are somewhat less polar than wool and silk and are more difficult to dye directly. The first satisfactory direct dye to be developed for cotton was Congo red. Congo red has two azo (-N=N-) groups that are spaced just the right distance from each other to form hydrogen bonds to repeating hydroxyl groups in cotton, thus making the dye less susceptible to removal by washing [3].
Ionic triphenylmethane dyes like malachite green form ionic bonds to polar materials like wool, a polypeptide that contains anionic polar sites [3].
Congo red can hydrogen-bond to the hydroxyl groups in cellulose materials such as cotton.
Direct dyes possess affinity for cellulosic fibres without any pretreatment to dye or to the fiber. Many natural dyes belong to this class. The most common example is turmeric; others are harda, pomegranate rind, and annatto [4].
According to Burkinshaw et al., [5,6] have been reported to, the direct dyes are classified according to many parameters such as chromophore, fastness properties or application characteristics. The major chromophoric types are as follows: azo, stilbene, phthalocyanine, dioxazine and other smaller chemical classes such as formazan, anthraquinone, quinolone and thiazole. Although these dyes are easy to apply and have a wide shade gamut, their wash-fastness performance is only moderate; this has led to their replacement somewhat by reactive dyes which have much higher wet and washing fastness properties on cellulosic substrates [7]. Example of the basic dye is given in (Figure 4).
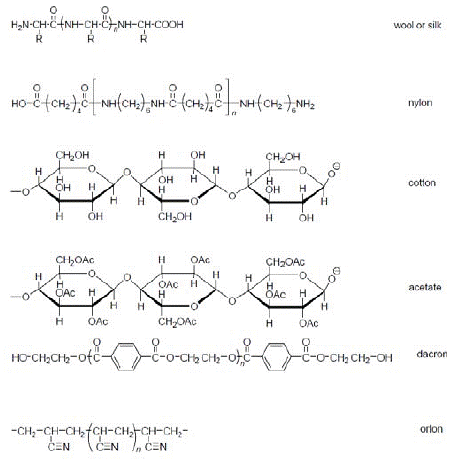
Figure 1: Chemical Structure of Fibers.
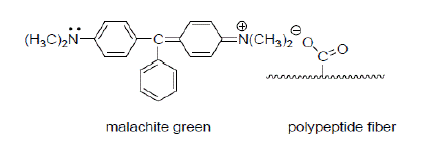
Figure 2: Ionic bond formation between Malachite green and Wool.
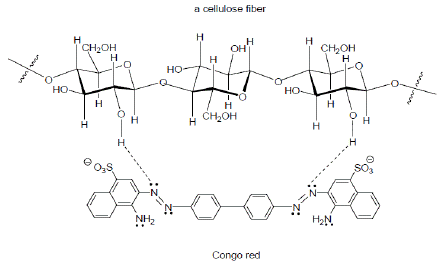
Figure 3: Hydrogen bond formation between Congo Red and Cellulose.
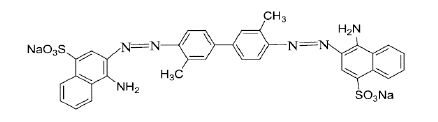
Figure 4: C.I. Direct Red 2.
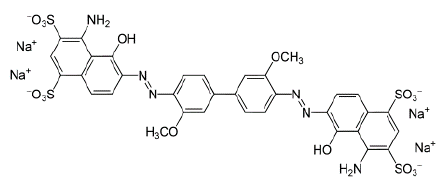
Figure 5: Direct Blue 1.
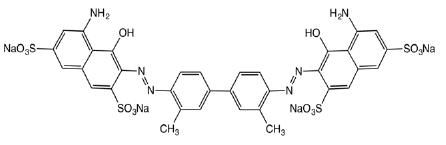
Figure 6: Trypan blue (Direct Blue 14), which also exhibits medicinal
properties.
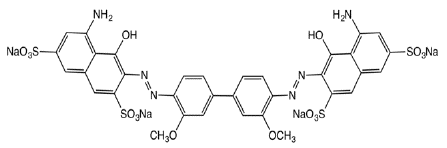
Figure 7: Direct Blue 15.
Direct or substantive dyes are colored compounds that are mainly used to dye materials made from natural or regenerated cellulose (e.g., cotton, jute, viscose, or paper) without employing mordants as auxiliaries. The essential requirement for classification of a dye in this group is its substantivity, i.e., its absorption from an aqueous saltcontaining solution onto cellulosic materials. Absorption onto cotton takes place in a neutral to soda alkaline medium and onto paper in a weakly acid to neutral medium [8].
Substantivity was initially attributed to secondary valence bonding between fiber and dye. The fact that coplanar molecules are always more substantive than nonplanar ones later led to the coplanarity theory with its assumption that coplanar dyes are in contact with the cellulose molecule along their entire length [8,9].
The presence of hydrogen bonds has also been expounded as a possible explanation for high affinity between fiber and dye [10]; however, such bonds are probably prevented by a water layer between fiber and dye [11].
The nature of substantivity has been very convincingly explained [12]. According to this explanation, single dye molecules are adsorbed by the intermicellary cavities of the cellulosic fibers and, unlike nonsubstantive dyes, they form aggregates in these cavities.
Because of their size, these aggregates can no longer be directly washed out with water, but only after further solvation has taken place.
Because direct dyes become aggregated in aqueous solutions at normal temperatures, substantivity often cannot take effect until the temperature has risen. Only then diffusion into the fiber is possible.
The tendency toward aggregation is therefore characteristic of substantive dyes, which also explains why coplanar dyes possess greater substantivity than nonplanar ones.
There is no exact delineation between substantive and nonsubstantive dyes, the boundaries between them are fluid.
Historical Perspective of Substantive Dye
The term ‘‘substantive” as applied to dyes was introduced originally by Edward Bancroft’ in the following words. However, colouring matters seem to fall naturally under two general classes; the first including those matters, which, when placed into a state of solution, may be fixed with all the permanency of which they are susceptible, and made fully to exhibit their colours in or on the dyed substance, without the interposition of any earthy or metallic basis; and the second, comprehending all those matters which are incapable of being so fixed, and made to display their proper colours, without the mediation of some such basis. The colours of the first class I shall denominate substantive; using the term in the same sense as it was employed by the great Lord Verulam, as denoting a thing solid by, or depending only upon, itself; and colours of the second class I shall call adjective, as implying that their lustre and permanency are acquired by their being adjected upon asuitable basis” [13,14].
Having thus defined the term and having pointed out that certain coloring matters, adjective to cotton, might be, and actually were, substantive to wool; Bancroft proceeded to describe the substantive colors which were available at that time. His list included Tyrian Purple (the secretion of a certain shell-fish), Indigo, Turmeric, Safflower, and the various mineral colors [14].
None of the early coal tar colors appears to have been substantive to cotton, and it was not until 1884, when Bottiger discovered Congo Red, that an artificial dye, definitely substantive to cotton, as well as to wool and silk, became available. Since the introduction of Congo Red, many other substantive cotton dyes have been brought into use, notably the Mikado colors and the Primulines. The majority of these dyes are also substantive to wool and silk [14].
Congo red can hydrogen-bond to the hydroxyl groups in cellulose materials such as cotton as presented in (Figure 3).
In the modern classification of dyes, adopted by technical colorists, the term “substantive dye” has taken on a rather specialized and narrow meaning. According to Matthew the so-called substantive dyes “include derivatives of benzidine, tolidine, diamidostilbene, and various azoxydiamines; they also include certain derivatives of stilbene, such as the Mikado colors [15].
Another class of direct cotton or substantive dyes is not included in the azo dyes at all, but is derived from certain bases made from sulphur compounds of paratoluidine or its homologues: these form the primuline group of dyes.” The members of this group of dyes, all more or less ‘soluble’ in water, are similar chemically to the acid dyes, being employed in the dye bath in the form of their sodium salts or as the free dye acid; they differ markedly, however, from the acid dyes in their mode of application to the fiber [15,16].
In the broad sense of Bancroft’s definition, and because of their marked similarity to the substantive dyes, (as defined by Matthews), in respect of their behavior in the dyebath and toward the fiber, one should include among the substantive dyes the Immedial or Sulphur dyes, the Vat dyes (such as the leuco form of indigo), suspensions of the inorganic colors, and certain colloidal developing agents, such as those of the Naphthol AS series, Under certaincircumstances some of the acid and basic dyes appear to behave like substantive dyes [17-20].
Representative Direct Dyes
Dyeing with Substantive Dyes, Comparison with Acid Dyes
In practice the substantive dyes are employed in either neutral, acid, or alkaline dyebaths, usually with the addition of an assistant, the action of which is to effect an increase in the amount of color adsorbed by the fiber and to further the exhaustion of the dye bath. These assistants are stated to be salts, such as sodium chloride or sodium sulphate, though as a matter of fact acids and bases act in the same way. It was found that both hydrochloric acid and sodium hydroxide, in moderate amounts, because an increase in the quantity of Buffalo Direct Red adsorbed by cotton, precisely as is done by sodium chloride or by sodium sulphate. Because of the rather striking assisting action of salts, the substantive colors are often spoken of as ‘salt’ dyes [21].
The substantive dyes differ distinctly from the acid dyes in the effect produced on the dyeing process by bases and salts containing univalent cations, even though the two classes of dyes are chemically similar. Sodium hydroxide acts normally as a restrainer with acid dyes, but as an assistant with substantive dyes, while hydrochloric acid may act as assistant with both. Sodium chloride is without much effect in the case of acid dyes, but is an active assistant with the substantive class. The difference is marked in the case of sodium sulphate; for the latter is a restrainer’ with the acid dyes but an assistant with the substantive dyes; this difference has been strongly emphasized by Matthews [15,22]. I shall therefore take the position that the acid dyes and the substantive dyes do behave very differently, and that the general theory developed by Pelet-Jolivet and by Bancroft for the acid dyes cannot be applied, at least without a great deal of straining, to the substantive colors [22].
While a number of theories have been proposed in explanation of the peculiarities of substantive dyes-notably by Weber, Dreaper, Biltz, Bayliss, and more recently by Haller and by Auerbach-these theories are either incomplete or unsatisfactory and they seem to have made little impression; for Whittaker says that “at the present time it is impossible to offer any definite opinion on the theory of the dyeing of the direct cotton dyestuffs”, and Matthews, with a hopelessly vague group of Fourteen Points, gets nowhere. It is the purpose of this preliminary paper to show that a satisfactory working theory of substantive dyes can be proposed and that this theory is a modification or an amplification of the earlier theories of the four investigators named first in the preceding sentence [21-23].
Some Properties of Aqueous Solutions of the Substantive Dyes
The technical substantive dyes, with which various soluble impurities are invariably found, pass more or less readily into solution in water, more easily when the latter is warm. These solutions are similar in properties to the soap solutions, and, like the latter, they are undoubtedly colloidal, or at least contain ultra-microscopically resolvable ‘colloidalions’. As one might expect, therefore, the strongly flocculated gel, or the dyestuff in the form of crystals, is virtually insoluble in water, as Haller and Nowak have pointed out [20]. The dialyzed solutions, like those of the soaps, are excellent conductors; they exert in a collodion osmometer a surprisingly high apparent osmotic pressure; in the absence of a membrane they possess an easily measurable power of diffusion, though in general they diffuse far less rapidly than the acid and basic dyes; and they invariably contain amicrons or ultramicrons when viewed in the ultramicroscope. It is possible to bring about an increase in the size of the ultramicrons and a corresponding decrease in the degree of dispersion, without causing any visible or actual flocculation of the suspended dye - a property of the substantive dye solutions which is of the utmost significance in the theory of substantive dyeing. Increase in size of aggregates is observed as the concentration of the dye is increased, or as the temperature is lowered; at low temperatures concentrated solutions of the substantive dyes set to a jelly [17,20,24]. Decreased dispersion is also caused by destabilizing electrolytes, while electrolytes in excess bring about flocculation. The growth of aggregates is opposed by stabilizing protecting colloids, such as gelatine.
Practically all of the very complete evidence now available supports the conclusion that in neutral or alkaline dyebaths the substantive dyes are present as electronegative colloids capable of a very high degree of dispersion in the absence of destabilizing agents.
Adsorption of Substantive Dyes from Aqueous Solution
Weber and others have shown that the substantive dyes are taken up by fibers in the form of their undissociated salts and Weber has also remarked that cotton takes up these dyes in exactly the same way that it does tannin in the tannin-mordanting process [13]. Witt therefore assumed that the substantive dyes form solid solutions in the fiber [20]. However, von Georgievies Biltz, and Schaposchnikoff have obtained typical adsorption isotherms for substantive dyes and cotton, and though, in view of the interesting experiments of Reinders and Lely, this may not be regarded as conclusive evidence of adsorption, the majority of recent investigators are agreed that substantive dyes are adsorbed by the fiber and not dissolved [16,17]. As to the chemical theory, it should be observed that there is no evidence whatsoever that chemical compounds between these dyes and fiber exist. It will be assumed in what follows that dyeing with substantive dyes is simply a case of adsorbing from suspension a colloid the degree of dispersion and the stability of which are capable of considerable variation [22].
Distribution of a Colloid between an Interface and the Distribution Medium
The adsorption of a colloid by a solid adsorbent, which for convenience one may consider a fiber to be, can be regarded as a process of distribution of the colloid between the surface of the solid and the bulk of the liquid medium. It will therefore be profitable to consider briefly the general problem presented by the distribution of a colloid between an interface and the dispersion medium. The conclusions drawn from such a consideration of the general problem will, in turn be made the basis of a theory of dyeing with the colloidal dyes, to which class, as we have seen, the substantive dyes belong.
There is therefore good reason to suppose that, since surface and dispersion medium are competing for the suspended material, anything that adds to the stability of the suspension will act against adsorption, while anything that lessens stability will aid adsorption. That is to say: a finely divided solid or colloid in suspension should become decreasingly interfacial in the presence of a peptizing agent, but increasingly interfacial in the presence of a flocculating agent, providing these agents themselves are not strongly interfacial [22,24].
While a flocculating agent will tend to force the suspended colloid into the interface, it should not be present in amounts large enough to produce actual coagulation. The amount of adsorbed colloid in the interface will therefore pass through a maximum as the concentration of the flocculating substance is increased beyond its coagulating value [26].
Flocculating agents are not the only cause of decreased stability and correspondingly increased adsorption within limits, for the stability of a sol may change with the temperature, with age, and with its previous history. The stability and the dispersion decrease with increase in concentration of the sol [16,19].
Substantive Dyeing
It has been proposed that, dyeing with the substantive dyes is simply a case of adsorbing from suspension a colloid, the degree of dispersion and the stability of which are capable of considerable variation. The general process of colloid distribution theory may now be re-stated in terms of the substantive or colloidal dyes as follows: A weak flocculating agent added to the dye bath will produce an increase in the amount of dye taken up by the fiber, providing the dye is not actually thrown out of suspension during the process of dyeing. In the latter case, a flocculating agent active enough to produce actual coagulation will cause the fiber to take up less dye. In consequence of these opposed effects, the amount of dye taken up by the fiber will increase to a maximum and will thereafter diminish, as the concentration of the flocculating agent is made greater. It should be observed that the theory has nothing necessarily to do with the question of fastness to light or to washing.
A peptizing agent added to the dyebath will cause a decrease in the amount of dye taken up by the fiber, unless the peptizing agent is a second colloid which is itself strongly adsorbed by the fiber. In the latter case since by definition the peptizing colloid adsorbs the dye, it may carry dye with itself on to the fiber, and the latter may take up more dye than it otherwise would. A second colloid adsorbed by the fiber would act, in other words, as a mordant [20,22,23].
The question of the electric charge on the fiber and on the particles of dye in suspension has been omitted in this discussion. This question has been considered by Harrison’ who believes that dye particles or ionc: may be held on the fiber by electrical attraction when dye and fiber carry opposite charges, but he admits that other causes of attraction must be sought for when dye and fiber carry like charges, as in the case of the substantive dyes on cotton [24].
Applying the principle of the neutralization of adsorbed ions, Bancroft has worked out a modification of the Pelet-Jolivet theory of dyeing and has discussed its bearing on acid and basic dyes, which, except under unusual conditions of the dyebath are dissolved in the latter in the form of their simple ions. Bancroft did not consider the substantive dyes, except to suggest that his theory was not based on any hard and fast assumption as to the state of the dyes in solution, but should be equally applicable to the colloidal dyes and to the dyes in true solution [19,23,26].
Weber was among the first to discuss the action of salt assistants in the substantive dyebath. He accounted for this action by assuming that salts decreased the solubility of the dyestuff in the bath. It is worthy of note that Weber pointed out that of two direct cotton dyes, the one with the lower coefficient of diffusion possessed the greater affinity for the fiber [25]. Since it is now known from the work of Svedbergs chat the rate of diffusion of a suspended colloid is an inverse function of the size of the particles, Weber’s statement is another way of saying that the affinity between dye and fiber becomes greater the smaller the dispersion and the less the stability of the dye in suspension. Weber, however, had rejected a suggestion made by Schultz that the dyes were not actually dissolved in the bath but were in suspension, so he was forced to assume that salt assistants caused a decrease in the solubility of the dye [22,25].
The same explanation for the action of salts has been used by Dreaperin his “desolution” theory, and by Bayliss in his early work on the adsorption of Congo Red by filter paper, though we have seen that Bayliss probably used the word “solubility” to refer to the degree of dispersion of the dye [24]. Since it has been pointed out previously that Haller and Nowak have shown the substantive dyes to be insoluble in water when crystalline, it is certain that Weber’s “decrease in solubility’’ is equivalent to the decrease in dispersion and stability (destabilization) which we know the dyes undergo when flocculating salts are added to their suspensions [16,25].
The present theory suggests the possibility of a decrease in the amount of substantive dye adsorbed by the fiber when salts are added in quantities sufficient to cause actual flocculation, with precipitation of the dye from the bath. In other words the dye taken up by the fiber should pass through a decided maximum as the concentration of the salt is increased, and the position of that maximum, in respect to the concentration of the salt will be determined by the molar coagulating power’ of the salt [16].
Haller and Nowak infer that such a maximum exists and they account for it essentially in the terms of the present theory when they say [20]: “If the dispersion [of the substantive dyestuff] is too small, precipitation of the dyestuff in the bath takes place, but if it is too large, the dyestuff passes by the surface of the fiber, without being retained by the latter in appreciable amounts’’ Auerbach, however, accounts for the maximum in dyeing obtained with salts in increasing concentrations by postulating that the salt exerts simultaneously two opposite effects, (1) causing a decrease in the amount of dye adsorbed by the fiber by flocculating, at all concentrations, the dyestuff in suspension, and (2) producing an increase in the amount of dye adsorbed by coagulating the dye on the fiber [20,24].
Rath has very recently applied what amounts to the present theory to the case of Naphthol AS, which is used in producing developed dyes on cotton. The naphthols of this series are colloidal and they are apparently adsorbed, like the substantive dyes them selvet, when cotton is brought in contact with their aqueous solutions. Rath points out that the more dispersed the naphthols, the less is their substantivity. He continues as follows [18]: “The alkaline solutions of the naphthols of the AS series show at different temperatures the typical behaviour of colloidal solutions; the higher the temperature, the more highly dispersed is the solution and the less is their substantivity [18].
The present theory requires that a peptizing second colloid in the dyebath should bring about a decrease in the amount of dye taken up by the fiber, providing the second colloid does not act as a mordant. Bayliss, in the experiments mentioned previously, found less Congo Red adsorbed by filter paper from a bath containing both sodium chloride and gelatin’ .than he did when the bath contained the salt alone. Lichtenstein has reported that “solution salt” (sodium benzylsulphanilate), as well as sodium protalbinate and lysalbinate, causes a decrease in the amount of Indigo adsorbed by cotton; these substances are without doubt peptizing agents toward the colloidal leuco compound in the vat dyebath. Starch has been reported to have a similar effect [24,25].
Conclusion
The evidence collected by previous investigators shows conclusively that the process of dyeing with substantive or colloidal dyes is simply a case of adsorbing a colloid from its suspension, in which the dispersion and stability are capable of being greatly varied. The adsorption of a colloid from suspension has been considered as a process of distribution between an interface and the suspending medium. Factors which affect this process have been considered. From a consideration of the general process of colloid distribution, a special theory of substantive dyeing has been formulated and tested by experiment.
A substance which destabilizes the suspension of the dye will act as an assistant in the dyebath up to the point of actual flocculation. A substance which stabilizes the suspension of the dye will act as a restrainer, providing it does not act as a mordant toward fiber and dye. A stabilizing substance and a destabilizing substance may each exert their specific effects in the same dyebath. Transition dyes undoubtedly exist which combine with their properties of acid or basic dyes the characteristics of substantive dyes.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Definition of SUBSTANTIVITY. www.merriam-webster.com. Retrieved 2021- 05-05. https://en.wikipedia.org/wiki/Substantive_dye
- Hunger K, Mischke P, Rieper W. Azo Dyes, 1. General. 2011: 1-24.
- https://chemistry.sites.clemson.edu/organic/Labs/2270Docs/Dyes.pdf. CH 227 Dyes and Dyeing.
- Mansour R. Natural Dyes and Pigments: Extraction and Applications. Handbook of Renewable Materials for Coloration and Finishing. 2018: 75- 102.
- Burkinshaw SM. Nylon. Chemical Principles of Synthetic Fibre Dyeing. 1995: 77-156.
- Burkinshaw SM, Sahilu G. Dyes and Pigments. 2017.
- Benkhaya B, Harfi SE, Harfi AE. Classifications, properties and applications of textile dyes: A review. 2017; 3.
- Hunger K, Mischke P, Rieper W. Azo Dyes, 3. Direct (Substantive) Dyes. 2011: 1-14.
- Burdett BC. The Colour Index: The past, present and future of colorant classification. Journal of the Society of Dyers and Colourists. 1982; 98: 114- 120.
- Boulton J. The application of direct dyes to viscose rayon yarn and staple. Journal of the Society of Dyers and Colourists. 1951; 67: 522-538.
- Rattee ID, Breuer MM. Physical chemistry of dye adsorption. Academic Press. 1974.
- Bach H, Pfeil E, Philippar W, Reich M. Molekülbau und Haftung substantiver Farbstoffe auf Cellulose. Angewandte Chemie. 1963; 75: 407-416.
- BANCROFT E. 1813. The Philosophy of Permanent Colours’(2 vols).
- Bancroft: Ibid. 1813; 1: 313.
- Matthews JM. Application of dyestuffs to textiles, paper, leather and other materials. Wiley. 1920.
- Haller Cf. and Nowak. Kolloidchem. Beihefk. 1921; 13: 86.
- Biltz Cf and Behre. Ber. 1905; 38: 2973.
- RATH J. Colloid Chemical Researches on Naphthol AS. Journal of the Society of Dyers and Colourists. 1923; 39: 334-338.
- Briggs TR. The physical chemistry of dyeing: substantive dyes. The Journal of Physical Chemistry. 2002; 28: 368-386.
- Fischer MH. Die Kolloidchemie der Seifen und der Seifenfabrikation. Kolloidchemische Beihefte. 1921; 15: 1-102.
- WWhittaker CM, Wilcock CC. Dyeing with coal-tar dyestuffs; the principles involved and the methods employed.
- Bancroft WD. The theory of peptization. The Journal of Physical Chemistry. 1914; 18: 118.
- Ganswindt Cf. Thenrie und Praxis der modemenFarberei. 1903; 2.
- Bayliss WM. On some aspects of Adsorption Phenomena, with especial reference to the Action of Electrolytes and to the Ash-Constituents of Proteins. The Biochemical journal. 1906; 1: 175-232.
- Dyer B. XV.—On the analytical determination of probably available “mineral” plant food in soils. Journal of The Chemical Society, Transactions. 1894; 65: 115-167.
- Endler J. Über den Durchtritt von Salzendurch das Protoplasma: Über die Beeinflussung der Farbstoffaufnahme in die lebendeZelledurchSalze. Springer. 1912.
- Endler Josef. Biochem Z. 1912; 42: 440.