
Review Article
Adv Res Text Eng. 2024; 9(2): 1101.
Study The Reduction Mechanism of Salts and Alkalis in Reactive Dyeing of Cotton Fabric: Review
Ahmed Mohammed Nuru*
Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University, Kombolcha, Ethiopia
*Corresponding author: Ahmed Mohammed Nuru Department of Textile Engineering, Kombolcha Institute of Technology, Wollo University, Kombolcha, Ethiopia. Email: ahme2005a@gmail.com
Received: June 26, 2024 Accepted: July 19, 2024 Published: July 26, 2024
Abstract
Traditional dyeing procedures for cotton fabrics using reactive dyes are the most resource-intensive and environmentally harmful since they require significant amounts of water, salt, and alkali during the dying process. Even with substantial use of alkali and salts, the efficiency of fixation remains restricted. As a result, there is an excessive amount of hydrolyzed dye in the dyeing effluent, necessitating a large amount of water for removal through washing, and the wastewater is harmful to aquatic life, plants, and humans. To colour cotton without utilizing salt or alkali, researchers and industry must now seek new technological advancements.
This study examines the advanced dyeing technique for improving dye process sustainability by employing quaternary organic compounds in the dye bath, reusing the salt-contaminated dye bath, switching from exhaust to pad batch dyeing, and altering cotton fabric before dyeing. This review also includes a more detailed analysis of the numerous alterations performed to cotton fabric with organic and artificial cat-ionizing agents. The study investigated the benefits and cons of several cat-ionizing agents for improving reactive dyeing sustainability. Using these strategies, chemical prices, effluent loads, and customer needs can all be addressed in a sustainable manner.
Keywords: Traditional dyeing procedures; Hydrolyzed; Effluent loads; Customer needs
Introduction
Because of it is used to make the majority of clothing in the world, cotton remains the "king" of fibers [2,8]. Cotton fabric has many wonderful qualities, including good strength, increased moisture and water absorption, comfort in clothing, and ease of dyeing. The clothing industry mostly uses cotton-based products because of these factors. The use of cellulose-based goods extends beyond clothing and includes a wide range of applications such as technical textiles, functional textiles, and many more. Applications based on cellulosic materials account for half of the overall textile manufacturing [2]. Colorant can be used to cotton products, such as clothing and home textiles, to enhance look (value add), add a functional component, and convey a sense of condition, all of which can raise consumer happiness. As a result, cellulose coloring is a crucial step in the process for both functional and aesthetic reasons. Nowadays, the majority of the textile industry primarily uses cellulosic fibers for clothing, which are mostly colored using reactive dyes in the presence of a significant amount of salt and fixed in an alkaline environment. Cellulosic fibers were previously dyed using vat and direct dyes, but with the advent of reactive dyes, their use became restricted [1]. Reactive dyes differ from other dyes in that they can form covalent bonds between the oxygenatoms of cotton hydroxyl groups and the carbon atoms of the dye reactive group in an alkaline environment [8]. Reactive dyes are better than vat and direct dyes in the following areas, in turn: they can process a wide range of bright shades [3]; they have high leveling quality; they are better at washing and holding up to light exposure; they have a simple one-stage dyeing process; they can be dyed at low temperatures (below 100oC); and they are less expensive than vat dyes [4]. Because of this, reactive dyes have maintained the highest yearly consumption of any dye in the world in recent years, solidifying their significant position in the dye manufacturing sector [5]. However, dyeing of cotton fabric with reactive dye is considered as pollution generating process as use huge amount of electrolyte,low dye utilization and unfixed reactive dyes are discharged in the textile effluent [6]. With growing popularity of reactive dyes for dyeing of cotton, environmental problems associated with their use have received attention [7]. When cellulosic fibers are immersed in water, it develops negative charge due to the ionization of hydroxyl groups [2,8]. The anionic nature of the reactive dyes leads to electrostatic repulsion and make the exhaustion of the dye difficult [9] and also, dye fixation potency on cellulosic fiber is low due to reactive dyes can conjointly react with water (dye hydrolysis) in an exceedingly kind that cannot bond to cotton [8,3]. Hence, this static repulsion has been overcome by using the enormous of the electrolytes like sodium chloride (sodium sulphate) and alkali into the dye bath [8]. The role of the alkali is to cause acidic dissociation of some of the hydroxyl groups in the cellulose and it is the cellulose ion (Cell–O–) that reacts with the dye and forms a covalent bond. However, dye fixation efficiency on cellulosic fiber is low [13,11]. Unfortunately, under alkaline conditions hydroxide ions of water also react with the reactive group of the dye in much the same manner as the cellulose’s ion. This produces the hydrolyzed dye, which is incapable of reacting with the fiber. Therefore salt-based dyeing and alkali-based fixation are considered pollution-generating processes due to only 50-70% of the dye utilization being attained and the salt added to the dyebath and alkalis are neither destroyed nor exhausted after dyeing [10].
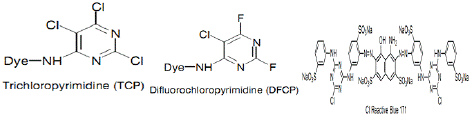
Figure 1: BIS (Aminochlorotriazinyl).
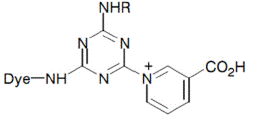
Figure 2: NT reactive dyes [34].
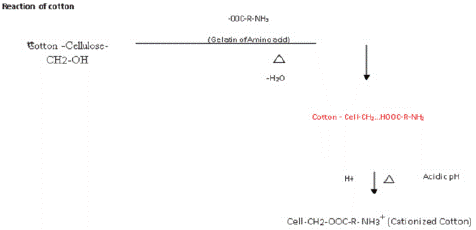
Figure 3: Cat ionization process of cotton fabric.
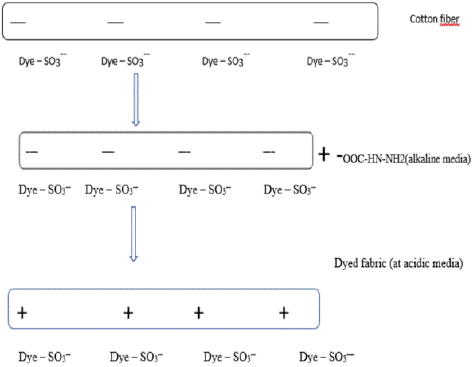
Figure 4: Dyeing of Cat - ionized cotton fabric [8].
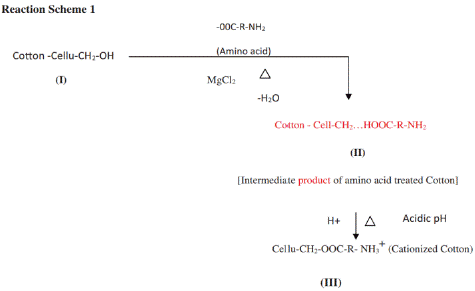
Figure 5: Cationization of cotton cellulose with soya extracted amino acids [29].
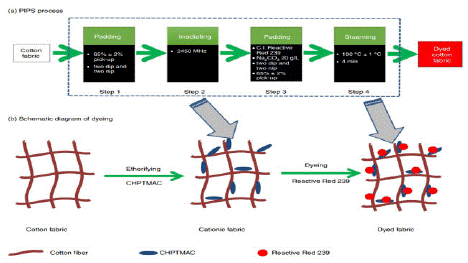
Figure 6: Schematic diagram of the PIPS process and the dyeing mechanism [10,45].
As a result, the residual dyes, alkalis, and salts have led to serious environmental pollution [13]. This results in a loss of dyes and the dyeing effluent consist of an outsized quantity of hydrolyzed dye that consumes time, energy, and a high volume of water to get rid of the hydrolyzed dye with washing and the wastewater are carcinogenic and harmful to human beings as well as plants and aquatic animals [12]. To overcome this problem, the waste must be treated before being discharged into the environment this is also an additional cost for the process [21]. Therefore, finding a way to improve dye utilization and avoid the use of inorganic salts and alkalis are important issues in the field of cotton coloration.
At an academic level, many researchers and industries have investigated and addressed several findings and alternative technological advancements for the coloration of cotton with anionic dyes without salt and alkali. This paper reviews the advanced dyeing approach and the suitable options for reduction of salts and alkalis to improve the sustainability of the dyeing process by using different options (quaternary organic compounds in the dye bath, recycling the salt-contaminated dye bath, shifting from exhaust to pad batch dyeing, and modifying cotton fabric before dyeing).
Options for Reduction of Salts and Alkalis
1. With the need for cleaner, cost-effective, and colorfast textile products, innovative technologies and improved processes have been developed for cotton coloration. The innovations technologies are mainly focused on the following aspects [31]:Development of new dyes and auxiliaries;
2. Developments of Lower liquor-to-fiber ratio dyeing machinery and processes
3. Shifting from exhaust dyeing method to Pad batch dyeing methods
4. Chemical modifications of cotton fiber prior to dyeing. Most of the options taken for reductions of salt and alkali-free dyeing of cotton with reactive dyes are discussed in detail. The first options are developments of bi-functional and Poly-functional reactive dyes which give a higher degree of fixation and lead to lower colors in the dye-house effluent. The second option is the development of Lower liquor-to-fiber ratio dyeing machinery and processes thus reducing the total consumption of chemicals that can be determined by reducing the total volume of dyeing liquor. The other one is a chemical modification of cotton fiber before the dyeing process which is called cat ionization of the fabric [14,32]. The main stride that should be done in the textile industry is modifying dyeing machinery and modifying the hydroxyl groups of the cotton substrate to overcome the above problems.
Developing Reactive Dyes
Developing of reactive dyes containing two or more reactive groups rather than one reactive group per dye molecule fixation can be increase from 60% to 80% [31]. With the unique dye structure and application mechanism, much attention from both industry and researchers has been focused on the development of reactive dyes. To increase the fixation efficiency and reduce chemical waste, reactive dyes with two or more reactive groups have been developed and commercialized. The development of bi-functional and Poly-functional reactive dyes which give a higher degree of fixation and lead to lower colors in the dye-house effluent. The bi-functional reactive dyes, including homo-functional dyes (containing two identical reactive groups, e.g. the Procion HE dyes) and hetero-bifunctional dyes (containing two different reactive groups, e.g. the Everzol dyes), were already widely used in industry [15]. There are also successful commercial trifunctional reactive dyes such as the Avitera SE dyes form Huntsman which are claimed to save energy and time and reduce water consumption [16]. Researchers also came up with some poly functional reactive dyes with more than three reactive groups, but none of them have been commercially applied yet [18]. The drawbacks with more reactive groups include higher cost and lower color strength. The extra reactive groups “dilute” the color value of a dye by increasing the molecular weight of the dye but do not enhance chromogenic strength [33].
Besides the increase in reactive groups, other innovations on reactive dyes include high strength economic chromophores, high light fastness chromophores [26], neutral-fixing [19], or acid-fixing reactive dyes [20], and cationic reactive dyes.
Reactive Dye Fixable at Neutral pH
Reactive systems containing a nicotinic acid residue can allow dye-fiber reaction at a neutral PH. This form of a fiber reactive system was introduced by Nippon Kayacu with the name of Kayacelon React. The requirement of using inorganic alkali for dyeing cotton at alkaline pH [21], becomes unnecessary and the amount of the alkali in dyeing effluent is substantially reduced. The Kyacelon reactive dyes are relatively expensive.
• Benefits of Developing of Reactive DLow amount of salt required.
• Dyeing time is less.
• Less effluent problem
• Eco-friendly in nature
Developing of Acid fixing reactive dyes
Acid fixing reactive dyes are developed by incorporating a reactive group into the dye molecule that can react with the fiber under acidic conditions. The reactive group typically contains a vinyl sulfone or a chlorotriazine group, which can react with the hydroxyl groups on the cellulose fiber to form a covalent bond. This covalent bond ensures that the dye is firmly attached to the fiber and is resistant to washing and rubbing [15].
Acid-fixing reactive dyes were developed for reducing the salt requirement. Burlington Industries (USA) developed reactive dyes containing phosphonic acid and carboxylic acid reactive groups for dyeing without salt. However, the use of these dyes has been reported to cause tendering of cotton. Overall, the development of acid fixing reactive dyes involves a combination of organic chemistry, dye chemistry, and textile testing to produce dyes that are both effective and environmentally friendly [36].
Developing Low Salt Reactive Dyes
The development of low salt reactive dyes aims to reduce the environmental impact of the dyeing process. High salt concentrations in wastewater can be detrimental to aquatic ecosystems and can also increase the energy consumption required for water treatment [31].
One approach to developing low salt reactive dyes is to modify the dye molecule structure to enhance its affinity for the fiber, thereby reducing the need for salt. This can be achieved by introducing functional groups that increase the dye-fiber interaction, such as hydrophobic or cationic groups. These modifications improve the dye's ability to attach to the fiber surface, reducing the reliance on salt for fixation. Another approach is to develop alternative dyeing techniques that minimize the use of salt. For example, researchers have explored the use of ultrasonic or microwave-assisted dyeing methods, which can enhance dye diffusion and fixation without the need for high salt concentrations. It is important to note that while developing low salt reactive dyes is a step in the right direction, it is not the only factor to consider for sustainable textile dyeing. Other aspects, such as water and energy consumption, waste management, and the use of non-toxic chemicals, should also be taken into account to achieve a more environmentally friendly dyeing process.
The “low-salt” Cibacron LS reactive dyes developed by Ciba have allowed meaningful improvements to the discharge effluent. Dye Star introduced Remazol EF as its low salt reactive dye range. The requirement of reduced amount of electrolyte is due to high attraction of such dyes to cotton [15]. Most of these low salt dyes are bifunctional and have high fixation efficiencies. Therefore, such dyes produce lower amounts of unfixed dye and salt in the dyeing effluent. In summary, developing low salt reactive dyes involves modifying the dye molecule structure or exploring alternative dyeing techniques to reduce the reliance on salt for dye fixation. This research aims to minimize the environmental impact of textile dyeing processes and promote sustainability in the textile industry.
Cationic Reactive Dyes
Cationic reactive dyes are a type of dye used in the textile industry to color synthetic fibers such as polyester and nylon. These dyes are characterized by their ability to form a covalent bond with the fiber, resulting in excellent color fastness and durability [22].
The development of cationic reactive dyes involves several steps:
1. Selection of Chromophore: The first step is to choose a suitable chromophore, which is the part of the dye molecule responsible for its color. The chromophore should have a positive charge to make it cationic.
2. Introduction of Reactive Group: A reactive group is then introduced into the dye molecule. This group allows the dye to react with the fiber and form a covalent bond. Common reactive groups used in cationic reactive dyes include vinyl sulfone, epoxy, and chlorotriazine.
3. Synthesis: The chromophore and reactive group are combined through a series of chemical reactions to synthesize the cationic reactive dye. This process may involve multiple steps and purification techniques to ensure the desired dye structure and purity.
4. Testing and Optimization: Once synthesized, the dye is tested for its color strength, solubility, and compatibility with different fibers. Optimization of the dye formulation may be necessary to achieve the desired dyeing properties.
5. Application: The final step is the application of the cationic reactive dye to the textile material. This can be done through various dyeing techniques such as exhaust dyeing, pad dyeing, or printing. The dye reacts with the fiber under suitable conditions, forming a covalent bond and imparting color to the material.
It is important to note that the development of cationic reactive dyes requires expertise in organic chemistry, dye synthesis, and textile dyeing processes. The goal is to create dyes that provide vibrant and long-lasting colors while maintaining good dyeing properties and environmental sustainability. Conventional reactive dyes, being anionic, require high concentrations of salt to overcome the repulsion effects arising from the anionic charge on the surface of the cotton fiber in the dyeing solution. Cationic reactive dyes eliminate the requirement for an electrolyte. Such dyes offer the potential for dyeing of cotton using zero-salt but they are yet to be commercialized.
Developments in Dyeing Machinery and Processes
Low Liquor to Fiber Ratio Dyeing
The mass per unit volume of dyeing solution determines the amounts of alkali and inorganic salt according to industrial dyeing practices. Reducing the overall volume of dyeing liquor can thereby lower the overall consumption of inorganic compounds. Low liquor to fiber ratio dyeing machines has been commercially introduced based on this methodology. On the basis of this approach industry has been motivated to move from 20:1 ratio dyeing’s to lower liquor. Dyeing at ratios as low as 4:1 is possible with the ultra-low liquor ratio dyeing machines [38]. It is also more likely that the reactive dye will be less rejected by cotton fiber when the liquor ratio is reduced.
This indicates that less electrolyte concentration is required for dye depletion. Reducing the water volume used also lessens the chance of dye bath hydrolysis. The creation of low liquor to fiber ratio dyeing equipment also has the benefit of requiring less water, wasting less dye liquor, and using less steam to heat the dye bath, which reduces air pollution from steam boilers [37].
To reduce the chemical, water, and energy consumption in exhaust dyeing, a wide number of low liquor-to-fiber ratio dyeing machines have been developed such as the Luft-roto Plus dyeing machine from Thies with the minimum liquor ratio as 4:1. While for pad dyeing, new padders and low-capacity padding troughs were designed to reduce liquor waste and eliminate tailing [38].
Low Padding Trough Volume
When using pad dyeing techniques, the concentrated dyeing fluid that remains in the padding trough at the end of the dyeing operation is drained into the effluent. Considerable decreases in this kind of waste were achieved by reducing trough volumes to 10 to 15 liters [39]. The new "padders" also benefit from less "tailing," which wastes cotton fabric. The new ‘padders’ also benefit in reduced wastage of cotton fabric caused by ‘tailing’. Tailing is the result of preferential dye uptake of one color by cotton substrate when dyeing with combination colors.
‘E-Control’ Dyeing
The continuous pad-dry-bake dyeing method uses urea to increase the dye-fiber reaction's yield. When using the pad-dry-chemical pad-steam method to prevent dye bleeding during the second chemical padding process, as well as when pad-steam dyeing to promote dye levelness into the fabric, inorganic salt is employed. Salt and urea are no longer necessary thanks to a dyeing machine called "E-control," which was co-developed by BASF and Monforts and is commercially feasible. The hot fixation chamber is intended to be humidified by the machine. Temperatures between 120°C and 25–25% relative humidity have been observed to be ideal for the fixation of dichlorotriazine dyes. These dyes require very modest amounts of alkali (sodium bicarbonate) [40].
Hot Washing-Off
In order to save water and energy, new techniques for exhaust dyeing, like as the "enzymatic after soaping" procedure, have been introduced. After dyeing, washing off is necessary to get rid of the hydrolyzed and unreacted dye from the cotton fiber. Manufacturers of dyes typically advise five steps for washing off: soaping at the boil, warm rinse, and cold rinse. It has been observed that using a hot-washing technique—starting with hot rinses and finishing with a low-temperature rinse—improves dyeing outcomes and slightly lowers effluent chemical oxygen demand [17].
Other Technologies
Supercritical Carbon Dioxide (scCO2), microwave heating, and ultrasonic energy are some further cutting-edge cellulose dyeing processes. Research on ultrasonic-assisted cotton dyeing has demonstrated that using ultrasound can save energy, water, time, and chemicals while also improving color production. When compared to traditional heating techniques, microwaves provide volumetric heating that can improve color yield and dye fixing efficiency while also reducing coloration heating time. Another option is to dye cotton without using water by dispersing reactive dyes in scCO2. Disperse dyes in scCO2 can also be used to color cotton that has been altered to be less hydrophilic [24].
Modification of Cotton Fiber Prior to Dyeing
The area which has become the topic of research to reduce unfixed dyes and salt concentration in effluents is the modification of cotton and cellulosic fibers to improve dye fixation and fastness. Synthesis of new dyes and modification of cotton dyeing processes, while valid for obtaining qualified black cotton dyeing, are likely to involve significant capital investment and development costs. Thus, much attention has focused on modification of cotton fiber as another route to obtaining the desired dyeing performance and fastness properties with existing dyes and processes [44].
Cat-Ionization of Cotton
The process of modifying cotton by developing cation site on its surface without affecting its bulk property is called ‘’cat-ionization’’.
Cat ionization is one of the most important modifications for cellulose." modifying the cotton fiber to increase dye-fiber interactions is thus, best route to overcome the lack of affinity for cotton to commercial reactive dyes, so that it can be dyed without salt. During cationization of cotton, etherification of primary hydroxyl groups on cellulose takes place. Cationization of cotton is one of the most widely researched modifications in recent years since both direct and reactive dyes carry anionic charges and they exhibit high affinity for positively charged cotton. By introduction of cationic groups into cotton fibers, the affinity of reactive dyes for cotton can be significantly improved. The ionic attractions between cat-ionized cotton and reactive dyes can result in increased dye uptake, reduced or no electrolyte use, less dye washing off and less water and energy consumption. The environmental problems caused by dye and salt in effluent can be potentially mitigated by cationization pretreatment of cotton [41].
In order to decrease the amount of effluent from dyeing and to eliminate the usage of salt while
dyeing, cationization have done to create positive sites on cotton so that, anionic dyes can react with them in near neutral conditions.
The introduction of cationic sites within the cellulose is the most expected technique to increase the dye adsorption. Cationic sites can be introduced either by aminization orcat-ionization [44]. The cationization is mainly carried out to improve affinity toward anionic substances, such as dyes in conventional textile processing and metal ions or unfixed dyes in effluent treatment. Cationic modification is the method that has been employed in order to change the surface charge of cellulosic fibers. Cellulosic materials are commonly cat-ionized in three ways: firstly, a direct cationization of cellulose using a chemical compound with suitable functional groups that react with cellulose hydroxyl groups. The second approach involves the addition of binding agent, such as dimethylol dihydroxyethylene urea (DMDHEU), which reacts both with cellulose hydroxyl and the functional group of cationic agents. This process is mainly used for textile application since the common textile pad-dry-cure process can be employed. The third approach utilizes graft polymerization to introduce monomeric or polymeric cat-ionizing agents within the cellulose, but it is not commercially applicable. Numerous chemicals and methods have been used to introduce cationic groups into cotton fiber. Each process has advantages and disadvantages, but none of these processes has been commercially adopted yet [42].
Why and How Cat-Ionization is Performed?
Cotton fibers, being negatively charged and sulfonated when its main component, cellulose, comes into contact with water, exhibit electrostatic resistance towards reactive and direct dyes. Before anionic dyes can be absorbed on cotton fibers, they must first pass through a sizable adverse charge barrier. In the traditional reactive dyeing process for cotton, high quantities of electrolytes, such as sodium chloride and sodium sulfate, are employed to inhibit negative charge accumulation and decrease dye solubility. However, reactive dye fixing rates remain low even with the addition of electrolytes, particularly when large dye concentrations are used. Because of this, the wastewater from the dye bath usually has high amounts of salt and unexhausted dye, which is extremely harmful to the environment [43].
Reactive dye affinity for cotton can be markedly increased by adding cationic groups to cotton fibers. Reactive dyes and cat-ionized cotton have ionic attractions that can lead to reduced or no electrolyte use, less dye washing off, and lower water and energy use in addition to greater dye uptake. A cotton cat-ionization pretreatment may be able to lessen the environmental issues brought on by salt and dye in effluent. Cationic groups have been added to cotton fiber using a variety of substances and techniques [35].
Cationic Reagents
Various cationic reagents have been studied for the purpose of cationizing cotton. Compounds such as monomers and polymers were used to modify cotton both chemically and physically. Commonly employed monomers in cotton fiber modification include epoxy compounds, quaternary compounds of the chlorotriazine type, N methylolacrylamide, choline chlorides, etc. Commonly employed compounds in cotton modification include synthetic polymers, polyepi-chlorohydrin dimethylamine, polyamide epichlorohydrin type polymers, poly-(4-vinylpyridine) quaternary ammonium compounds, dendritic polymers, and biopolymers, which include chitosan, starch, and their derivatives [43].
Modification of Cellulose or Cationization of Cotton: Using Chitosan
This procedure retreals cotton fiber using a new fiber modification technique based on cationic acrylic copolymer. The rationale behind this technique is that pre-treating cellulosic fiber with polymer is thought to present a chance to enhance the fibers' substantivity and reactivity towards reactive dyes in neutral environments. Reactive polymer resins have the ability to react with nucleophilic sites in both the polymer and the cellulosic fibers, securing the polymer to the substrate. Further interactions between the polymer and the dye, the fiber and the dye, and the fiber and the polymer are anticipated to occur during repeated dyeing, creating cross-links within the fibers [27].
Numerous investigators from various nations endeavored to reduce or eradicate the application of electrolyte concentration in reactive dye liquor. The most likely method to improve dye adsorption is to introduce cationic sites into the cellulose. Aminization or Cat-ionization are two ways to introduce cationic sites. Chitosan treatment is an aminization process used to insert a cationic site into the fiber polymer structure of cotton [9,20].
Chitosan, also known as β-(1-4) -2-amio-2-deoxy-D-glucopyranose, is a naturally occurring biopolymer that is both non-toxic and biodegradable. It is particularly common in the exoskeletons of mollusks, arthropods, and crustaceans, as well as in the chitin-containing cell walls of several fungi. To create chitosan, chitin is deacetylated in an alkaline media. Cotton fiber can be treated with chitosan using a variety of techniques. Cotton fibers form cross linking with chitosan resulting positive dye sites on the fiber surface. As a result, anionic dyes such as direct, acid and reactive dyes can easily be adsorbed by electrostatic attraction due to the created cationic nature of the fiber surface [9]. Chitosan causes cotton fibers to cross-link, creating positive dye sites on the fiber surface. Because the fiber surface has become cationic, anionic dyes like direct, acid, and reactive dyes can be readily absorbed by electrostatic attraction.
Modification of Cotton with Low Molecular Weight Amino compounds
Wool, in contrast to cellulose, is naturally substantive to anionic dyes, particularly in acidic environments. A fiber that can be dyed with reactive dyes at pH 5-7 without the need for an electrolyte yields very efficient reactive dyeing when amine groups are introduced into the cellulose structure. This fiber may be thought of as having dyeing properties similar to those of wool [23,25].
Modification of Cotton through Quaternary Amino compounds
By providing new cationic sites, the pre-treatments boost the neutral substantivity of anionic dyes for cotton. Protex marketed glycidyltrimethylammonium chloride, a quaternary amino-epoxy derivative, under the brand name Glytac A. Rupin and colleagues have described the compound's application chemistry, highlighting its use as a compound that offered enhanced dyeability of cotton with reactive and direct dyes. Glytac A was especially helpful when used with direct dyes since it could be added to the dye bath without causing the dye to precipitate, resulting in dyeing that were more wash-fast and had great ertictorial strength than traditional direct dyeing [25].
Modification of Cotton with Amino Polymers
The method of treating cotton cloth with polymers that contain amino groups is known as "amino polymer modification." This alteration can add new functions and improve the qualities of cotton fabric. The addition of amino polymers to cotton improves the fabric's affinity for dyes, facilitating better dye fixing and absorption. There are several ways to accomplish this alteration procedure, including pad-dry-cure and pad-steam methods. It is possible to greatly minimize or completely do away with the need for salt as a dye auxiliary by improving cotton's dye ability.
Chemically treating cotton fibers with polymers that include amino functions is known as "amino polymer modification." This is often referred to as functionalization or amino polymer finishing. All things considered, modifying cotton with amino polymers provides a sustainable and eco-friendly method of dying without the use of salt. It not only lessens the impact of textile dyeing on the environment but also enhances cotton fabrics' color fastness and dying efficiency [23,25].
Modification of Cotton Fabric with Natural Source Based Protein
In order to convert cotton's hydroxyl group into an amide group, which causes the cellulose to behave like wool fiber and allow dyeing at a moderately acidic pH in the absence of an electrolyte and an alkali, Ahmed, M., et al. worked on the modification of cotton for reactive dyeing utilizing protein-based gelatin [8]. Using a laboratory padding mangle machine, the well-prepared bleached cotton fabric was treated with the ideal concentration of 20 g/l of extracted gelatin at 80% wet pickup and a PH of 8. It is well known that amino acids and cellulose both include –COOH and –CH2OH groups. When these groups are joined at a certain temperature during the curing process, an ester linkage is formed in modified cotton that incorporates the free –NH2 group. In the presence of acid, this ester linkage can generate NH3+ in cat ionized cotton.
Through the esterification reaction process, an ester link is created between the hydroxyl (-CH2-OH) groups of the cotton cellulose chain and the carboxylic acid groups produced in the extracted gelatin. When drying at 100oC for 4 minutes in a hot air oven and then curing at a low temperature of 140oC for 4 minutes, there is a chance that the -CH2OH group of cotton cellulose and the -COOH group of mixed amino acids from gelatin protein will undergo an esterification reaction, forming a -CH2-OOC - ester group with an amino side group incorporated in cotton cellulose. This reaction will form an NH3+ side group in the modified cotton (referred to as cationized gelatin cotton forming structure).
This paper's conclusion was to increase the use of reactive dyes by completing the dye's exhaustion process in slightly acidic environments. The reduction of TDS in the effluent—which is difficult to remove from the effluent by allowing salt-and alkali-free dyeing—as well as increased dye exhaustion from the dye bath to the fabric—as well as increased amide group and carboxylic acid cross-linkage—are the most advantageous aspects of dying cotton fabric in acidic media. These factors also contributed to the fabric's increased softness and resistance to creases.
Using cattle horn and hoof, L.M. Wangatia and G.B. Tseghai modified cotton for reactive dyeing. This caused the hydroxyl group of cotton to become an amide group, which caused the cellulose to behave like wool fiber and allowed for dyeing at a moderately acidic pH without the need for an electrolyte or an alkali.
Using bull horn and hoof, a more environmentally friendly method of salt-free reactive dyeing cotton was the aim of this project. The cationic site created by the treatment of cotton fabric in this work with cattle hoof and horn keratin hydrolysate allowed for the dyeing of reactive dyes without the need for electrolytes, yielding outstanding results in terms of color strength, dye exhaustion and fixation, and environmental considerations [32].
In order to dye cotton fabric salt-free and with reactive dyes in an acid bath, Samanta et al. treated the cotton fabric using soya bean seed waste amino acid [29]. This resulted in much greater dye uptake and color strength value (K/S) than unmodified cotton fabric. These studies provide an alternate technique for salt-free dyeing using HE brand reactive dyes under acid bath conditions. The soya bean seed waste amino acid was produced by acid hydrolysis (6 N HCL) with the addition of MgCl2 as an acid donor.This rise in shade depth following dye fixing agent treatment is more pronounced and higher for reactive dyes under the HE brand that contain two homo- or hetero-bifunctional reactive groups. Amino acids and cellulose both include –COOH and –CH2OH groups, which can be coupled with heat and an acidic catalyst to create an ester linkage that incorporates the free –NH2 group in modified cotton and, in the presence of acid, can form NH3+ in cat-ionized cotton.
There is the possibility of esterification reaction between the –CH2OHgroup of cotton cellulose and the –COOH group of mixed amino acids from soya protein, forming a –CH2-OOC – ester group having an amino side group incorporated in cotton cellulose, which in the presence of MgCl2 and mild heat at 110oC for 8 minutes by calendaring (ironing) may form a NH3 +side group in the modified cotton (termed as cationized soya cotton forming structure III, shown below in Scheme 1).
In addition to reporting on the Pad-Irradiate-Pad-Steam (PIPS) process, the introduction to the cat-ionization of cotton fabrics, and the factors influencing the nitrogen content and grafting effectiveness of cotton fiber, Yu et al. pioneered a novel continuous dyeing route. This article examines the effects of cat-ionizing conditions (such as CHPTMAC concentration, microwave irradiation power, treatment time, and the mole ratio of sodium hydroxide to CHPTMAC) in the pad-irradiate sub-process on the reactive dyeing process and the characteristics of the dyed cationic fabric that results. In addition, a thorough explanation of the process of cat-ionization of cotton fiber was covered, and measurements and comparisons of the colored cotton fabric's fastness to light, washing, and rubbing were made [10].
In order to create cationized fabric that is amenable to a newer method of salt-free reactive dyeing in an acid bath, A.K. Samanta et al. worked on modifying cotton with glycine to incorporate new functional groups producing –NH3 or –C=NH -ion (cationic groups) in an acid bath [10]. This results in a much higher dye uptake and color strength value than unmodified cotton fabric.
In order to modify cotton for reactive dyeing with methylamine, Minghua Wuet al. [30] focused on changing the hydroxyl group of cotton into an amide group. This causes the cellulose to behave like wool fiber and allows for dyeing at a moderately acidic pH without the need for an electrolyte or an alkali.The amount of chitosan present in the fabric had a discernible impact on dyeability. In this study, increasing the chitosan content results in fabric with dye absorption that is either similar to or greater than the fabric colored with salt, as measured by the fabric's K/S value. Additionally, the chitosan-treated materials' fastness values are nearly identical to those of the untreated cloth. Therefore, the application of chitosan to cotton to enhance its reactive dyeability offers ample opportunity for more research to meet the current demand for environmentally friendly dyeing methods [30].
However, not many of these have been commercialized because they may require large investments, and in some cases increased processing costs.
Different cationic reagent types have been studied by researchers in order to cationize cotton. Cotton was modified chemically and physically using chemicals such as polymers and monomers. N methylolacrylamide, epoxy compounds, quaternary compounds of the chlorotriazine type, choline chlorides, etc. Typical substances utilized in cotton modification include synthetic polymers, polyepi-chloro-hydrindimethylamine, polyamide epichlorohydrin type polymers, poly-(4-vinylpyridine) quaternary ammonium compounds, dendritic polymers, and biopolymers such as chitosan, starch, and their derivatives [14].
Numerous researchers have previously examined cotton chemical modification using a variety of techniques, the results of which are extensively documented in the literature. The majority of the chemicals that are used to give cotton cationic sites are not environmentally safe in and of themselves. Consequently, it is necessary to investigate the viability of the cationization approach with environmentally acceptable chemicals. This inquiry has advanced with the utilization of natural amino acid, a polymer obtained from fleshing waste discharged from leather tannery. In order to lower the amount of electrolyte and alkaline concentration in textile dyeing, the textile industries are currently searching for and developing environmentally safe technologies and procedures.
In order to process textiles in a way that is safer for the environment, there are a number of alternatives to reduce the amount of salt used. These include switching from exhaust to pad batch dyeing, recycling the salt-contaminated dye bath after the hydrolyzed dye has been removed, and molecularly altering fibers to make them more attractive to anionic dyes [4]. To completely remove the usage of salt and alkaline in the dye bath, the best way to address the environmental issue is to change cotton fiber in a way that promotes dye-fiber interaction. Based on this, numerous non-biodegradable synthetic monomers and polymers have been used to catalyze the ionization of cotton.
However, the cotton cloth was altered or treated beforehand before dying in alkaline media, which requires a specific quantity of electrolytes. However, this study will modify cotton fabric in mildly acidic conditions while extracting a biodegradable byproduct from fleshing waste that is discarded from the leather industry. To solve these issues, this study will use a biodegradable gelatin product made from the fleshing waste of the leather industry to catalyze the ionization of cotton fabric. Therefore, minimizing environmental issues could greatly benefit from a dyeing process those results in excellent dye fixation. Various strategies include changing the structure to give it more substance to the substrate.
Significance of Different Methods of Cotton Modification
The significance of different methods of cotton modification for salt-free dyeing with reactive dyes lies in the improvement of dyeing efficiency, reduced environmental impact, and enhanced color fastness. One of the commonly used methods involves pretreating cotton fibers with chemicals that modify the fiber structure, making it more receptive to reactive dyes without the need for salt in the dyeing process. This modification enhances the dye-fiber interaction, leading to better color uptake and fixation.
1. The importance of these methods includes:Improved Dyeing Efficiency: Cotton fibers treated with specific modifications become more reactive to reactive dyes, resulting in better penetration and fixation of the dye molecules. This leads to higher dyeing efficiency, as a larger percentage of dye is utilized in the process.
2. Environmentally Friendly: Salt-free dyeing methods contribute to reducing the environmental impact of textile processing. The traditional dyeing process with salt generates large amounts of effluent, which can be harmful to the environment. Salt-free dyeing minimizes the discharge of salt-containing effluents, making it a more sustainable and eco-friendly option.
3. Enhanced Color Fastness: Modified cotton fibers can exhibit improved color fastness properties, such as better wash fastness and light fastness. This is crucial for maintaining the vibrancy and longevity of the dyed fabrics.
4. Energy and Cost Savings: Salt-free dyeing processes may require lower temperatures and shorter dyeing times compared to traditional methods, leading to energy savings. Additionally, the elimination of salt reduces the overall cost of the dyeing process.
Conclusion
Cotton modification, which gives cotton a permanent positive charge, is a different and more appealing environmentally friendly method of dyeing cotton. Cotton fabrics may now be dyed without the need of salt and with up to 100% reactive dye utilization thanks to the introduction of cationic groups into cotton fibers, which also greatly improved the affinity of anionic dyes for cotton. With long-term economic and environmental savings, the persistent positive charge enables the absorption of appropriate anionic dyestuffs without the need for salt. The method of dyeing cat-ionized cotton has the potential to be environmentally friendly because it uses less energy, water, and chemicals. In addition, the cationic dyeing method significantly decreased the time and water needed to thoroughly rinse and remove hydrolyzed reactive dye. So, the cat ionized cotton dyeing procedure also resulted in a sizable reduction in process costs. To achieve the necessary dyeing performance and colorfastness features with current dyes, a lot of research has concentrated on molecularly altering cotton rather than synthesizing new dyes and altering the cotton dyeing method. Cat-ionization has been demonstrated to be successful in boosting anionic dye uptake in all well-researched modifications of cotton. Additionally, it permits anionic dyeing without the need for salt. In summary, the significance of different methods of cotton modification for salt-free dyeing with reactive dyes lies in the positive environmental impact, improved dyeing efficiency, enhanced color fastness, and potential cost savings in the textile industry.
References
- Dong Y, Wang J, Liu P. Dyeing and finishing of cotton fabric in a single bath with reactive dyes and citric acid. Coloration Technology. 2001; 117: 262–265.
- Ahmed M, Sukumar N, Gideon RK. Crease resistance finishing optimization of citric acid and fibroin solution for cotton fabrics. Journal of Natural Fibers. 2019; 18: 297–307.
- Ojstršek A, Doliška A, Fakin D. Analysis of reactive dyestuffs and their hydrolysis by capillary electrophoresis. Analytical Sciences. 2008; 24: 1581–1587.
- Bhuiyan MAR, Shahid A, Hannan A, Kafi A. Influence of Mixed Alkali on Fixation of Deep Shade on Single Jersey Cotton Fabrics with Reactive Dyes. Journal of Chemical Engineering. 2013; 27: 58–63.
- Uddin MG, Ghosh NC, Reza MS. Study on the Performance of Eco-Alkali in Dyeing of Cotton Fabric with Reactive Dyes. International Journal of Textile Science. 2014; 3: 51–58.
- Ramasamy M, PV Kandasaamy. Effect of cationization of cotton on it’s dyeability. Indian Journal of Fiber &Textile Research. 2005; 30: 315–23.
- Yang Y, Xu L. Reusing hydrolyzed reactive dyebath for nylon and wool dyeing. American Dyestuff Reporter. 1996; 85: 27–34.
- Ahmed M, Sukumar N, Yusuf A, Awol Y. Cationisation of Cotton with Natural Source Based Gelatin for Salt-Free Reactive Dyeing of Cationised Cotton. Journal of Natural Fibers. 2022; 19: 15353–15366.
- Rehman A. Chemical Modification of Cotton to Enhance its Dyeability. PhD diss., University of Leeds. 2007.
- Yu C, Liu Y, Lu Y, Tao K, Zhong Y. A salt-free pad-irradiate-pad- steam reactive dyeing process for cotton fabric and the influence of cationising conditions on its coloration. Coloration Technology. 2021; 137: 399–406.
- Aysha T, Ahmed NSE, El-Sedik M, Youssef YA, El-Shishtawy RM. Eco-friendly salt/alkali-free exhaustion dyeing of cotton fabric with reactive dyes. Scientific Reports. 2022; 12: 22339.
- Ristic N, I Ristic. Cationic modification of cotton fabrics and reactive dyeing characteristics. Journal of Engineered Fibers and Fabrics. 2012; 7: 113–21.
- Samanta AK, TR Kar, A Mukhopadhyay, D Shome, A Konar. Eco-Friendly salt-free reactive dyeing ofcotton (muslin) fabric after cationization with amino acid from soya. Textile Research Journal. 2016; 86: 2179–92.
- Niu T, Wang X, Wu, C., Sun, D., Zhang, X., Chen, Z., & Fang, L. (2020). Chemical modification of cotton fabrics by a bifunctional cationic polymer for Salt-Free reactive dyeing. ACS Omega, 5(25), 15409–15416.
- Taylor J. Recent developments in reactive dyes. Review of Progress in Coloration and related Topics. 2000; 30: 93-108.
- Lewis DM. Developments in the chemistry of reactive dyes and their application processes. Coloration Technology. 2014; 130: 382-412.
- Hauser PJ, Tabba AH. Improving the environmental and economic aspects of cotton dyeing using a cationised cotton†. Coloration Technology. 2001; 117: 282–288.
- He WD, Broadbent PJ, Lewis DM. Reactive dye compounds. PatentWO2002096995 (Procter & Gamble). 2002.
- Wittig I, Karas M, Schägger H. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Molecular & Cellular Proteomics. 2007; 6: 1215–1225.
- Gillingham EL, Lewis DM, Nabi A, Srikulkit K. Triazinylamino-alkylphosphonate reactive dyes for cellulosic fibres. Coloration Technology. 2007; 123: 178-83.
- Lewis DM, Broadbent PJ, Vo LTT. Covalent fixation of reactive dyes on cotton under neutral conditions. AATCC, Review. 2008; 8: 35-41.
- Shahid MH, Islam SU, Mohammad F. Recent advancements in natural dye applications: a review. Journal of Cleaner Production. 2013; 53: 310–331.
- Rupp J. Recent developments in dyeing. Textile World. 2010; 160: 36-7.
- Rosa JM, Tambourgi EB, Vanalle RM, Gamarra FMC, Santana JCC, Araújo MC. Application of continuous H2O2/UV advanced oxidative process as an option to reduce the consumption of inputs, costs and environmental impacts of textile effluents. Journal of Cleaner Production. 2020; 246: 119012.
- Rehman A. Chemical modification of cotton to enhance its dyeability. 2007.
- Bhaumik NS, A Konar, AN Roy, AK Samanta. Comparative studies on dyeability with direct, acid andreactive dyes after chemical modification of jute with mixed amino acids obtained from extract of waste soya beanseeds. Journal of the Institution of Engineers (India): Series E. 2017; 98: 121–33.
- Chattopadhyay DP. Cationization of cotton for low-salt or salt-free dyeing. Indian Journal of Fibre & Textile Research. 2001; 26: 108–15.
- Chen W, S Zhao, X Wang. Improving the color yield of ink-jet printing on cationized cotton. Textile Research Journal. 2004; 74.
- Samanta A, Kar T. Eco-friendly salt-free reactive dyeing of cotton (muslin) fabric after cationization with amino acid from soya. Textile Research Journal. 2016; 86: 2179-2192.
- Madad, Mohsin. Exhaust Cationization of Cotton. (Under the direction of Dr. Peter J. Hauser).
- Awais Khatri, et al. Developments in reactive dyeing of cotton to improve sustainability. Conference Paper. 2010.
- LM Wangatia, GB Tseghai. Cat-ionization of cotton using cattle hoof and hornfor salt-free reactive dyeing. The Journal of Textile Institute. 2016; 107: 1375-1380.
- Morris KF, Lewis DM, Broadbent PJ. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Coloration Technology. 2008; 124: 186-94.
- Lewis DM, Vo LT. Dyeing cotton with reactive dyes under neutral conditions. Coloration Technology. 2007; 123: 306-11.
- Ma W, Zhang SF, Yang JZ. Development of functional polymers in modification of cotton for improving dyeability of reactive dyes. The Proceedings of the 3rdinternational conference on functional molecules. Citeseer. 2002.
- Renfrew AHM. Acid fixing reactive dyes. In A. H. M. Renfrew (Ed.), Reactive Dyes for Textile Fibres. (First ed., pp. 156-167): Society of Dyers and Colourists. 1999: 156-167.
- Wilber’s L, Seiler G. New concept for dyeing of cellulosic substrates at ultra-low liquorratios. Melliand International. 2002; 8: 66-67.
- Anderson CB. Dyeing reactive dyes using less salt. American Dyestuff Reporter. 1994; 83: 103-105.
- Hunger K. Reactive dyes. In K. Hunger (Ed.), Industrial Dyes. 2003a; 113-133.
- Hyde RF. Review of continuous dyeing of cellulose and its blends by heat fixation processes. Review of Progress in Coloration. 1998; 28: 26-31.
- Blackburn RS, Burkinshaw SM. Treatment of cellulose with cationic, nucleophilicpolymers to enable reactive dyeing at neutral pH without electrolyte addition. Journal of Applied Polymer Science. 2003; 89: 1026-1031.
- Zhang S, Ma W, Ju B, Dang N, Zhang M, Wu S, et al. Continuous dyeing of cat-ionisedcotton with reactive dyes. Coloration Technology. 2005; 121: 183-186.
- Srikulkit K, Santifuengkul P. Salt-free dyeing of cotton cellulose with a model cationicreactive dye. Coloration Technology. 2000; 116: 398-402.
- Chavan RB. Environment-friendly dyeing processes for cotton. Indian Journal of Fiber andTextile Research. 2001; 26: 93-100.
- K Tao, Yu C, Chang Y, Xi Z, Lu Y. Salt-Free Dyeing of Cotton Fabric Using 3-Chloro-2-Hydroxypropyltrimethyl Ammonium Chloride by Pad-Irradiate-Pad-Steam Process, and Prediction of Its K/S Value by LS-SVM, Journal of Natural Fibers. 2019; 18: 674-684.
- Ru J, Qian X, Wang Y. Low-Salt or Salt-Free Dyeing of Cotton Fibers with Reactive Dyes using Liposomes as Dyeing/Level-Dyeing Promotors. Scientific Reports. 2018; 8: 13045.
- Nallathambi A, Venkateshwarapuram Rengaswami GD. Industrial scale salt-free reactive dyeing of cationized cotton fabric with different reactive dye chemistry. Carbohydrate Polymers. 2017; 174: 137–145.
- Javadi MS, Mokhtari J, Nouri M, Mazaheri F. Novel cationic softener containing MCT reactive dyes for cotton: Simultaneous dyeing and functional finishing. Fibers and Polymers. 2013; 14: 920–925.
- Hanbing W, Haase H, Mahltig B. Cationic Pretreatment for Reactive Dyeing of Cotton and its Simultaneous Antibacterial Functionalisation. TEKSTILEC. 2020; 63: 27–37.
- Wu A, Ma W, Yang Z, Zhang S. Efficient Cationization of Cotton for Salt-Free Dyeing by Adjusting Fiber Crystallinity through Alcohol-Water-NaOH Pretreatment. Polymers. 2022; 14: 5546.