
Review Article
Adv Res Text Eng. 2024; 9(3): 1103.
Eco-friendly Polyester Reduction Clearing: Examining Cutting-edge Approaches
Manjiri Paranjape; Ashok Athalye*
Institute of Chemical Technology, Mumbai, India
*Corresponding author: Ashok Athalye. Institute of Chemical Technology, Mumbai, India. Email: ar.athalye@ictmumbai.edu.in
Received: November 28, 2024; Accepted: December 19, 2024; Published: December 26, 2024
Abstract
This study focuses on eco-friendly alternatives and innovations in the reduction-clearing process after polyester coloration. The conventional alkalinereductive method is effective but has environmental drawbacks, including Chemical Oxygen Demand (COD) and the release of harmful by-products such as sulfites and sulfates. This article reviews alternative reducing agents, such as formamidine sulfinate and hydroxy acetone, which show improved stability and biodegradability compared to sodium dithionite. Additionally, natural surfactants, like glucose derivatives, cationic surfactants, and ethoxylated castor oil, are explored to reduce environmental impacts. Furthermore, innovative techniques like ozone and plasma treatments present promising substitutes for the reduction clearing process, showing comparable or superior wash fastness and color properties while significantly lowering water, chemical, and energy consumption. Adopting such alternatives can enhance environmental safety, process efficiency, and the economic viability of polyester dyeing in the textile industry.
Keywords: Natural Surfactants; Enzyme; Polyester coloration; Reduction clearing; Sustainable alternatives
Introduction
Reduction Clearing is a traditional process carried out after the dyeing of polyester. It is an after-treatment carried out to remove the unfixed dye and other impurities from the fabric's surface. The dye molecules move from a higher concentration to a lower concentration. This is accomplished when the dye molecules' rates of entry into the fabric and exit into the solution equal one another. Most dyeing operations are halted commercially before they achieve this thermodynamic equilibrium. Thus, whenever the dyeing process is stopped, there will be a number of dye molecules that will not have penetrated the textile substrate. These molecules may remain on the surface of the textile, or some of these dye molecules may remain trapped in the spaces between fibers or yarns, depending upon the type of substrate being used. These loosely held dye molecules will come off later, especially when the textile is used regulalry. This superficial dye may also adversely affect the brightness of the shade. Therefore, a wash-off process is required after dyeing [1].
Polyester is the most widely consumed man-made fiber. It is generally dyed using Disperse dyes. These dyes have two prominent characteristics: (i) Limited water solubility and (ii) A tendency to form aggregates. This restricts the removal of the superficial dyes, creating a demand for a rigorous wash-off process. Reducing agents like sodium dithionite are used to achieve optimum fastness in dyed polyester [2].
Significance of Reduction Clearing
Traditional reduction clearing has been carried out since the 1950s as an after-treatment for dyed polyester. It is often considered a necessary evil in many dyeing processes, especially for medium and dark shades. The process consists of a treatment for 15 minutes at 70°C to 100°C using sodium hydroxide, a detergent, and sodium dithionite as a reducing agent. The process is observed to have improved fastness properties and is important for removing the formed oligomers. The surface deposits of these oligomers remain undyed by Disperse dyes and remain firmly attached to the fiber, hence appearing as a white dusting powder on the fibers and some parts of the machine [3]. These deposits also function as centres for the nucleation of Disperse dyes in optimum conditions, leading to destabilisation of the dispersion. They also affect the frictional properties of the yarns. Moreover, unlike the simple wash-off process, these oligomer deposits are not water soluble and require an effective method for their removal.
Factors Affecting Reduction Clearing
Many factors affect the reduction-clearing process. The type of substrate used (yarn, fiber or fabric), which subsequently influences the dyeing method, mechanism, and equipment to be used, is one of the initial factors to be considered. The depth of shade of the dye used is also important. As the dyed shade's concentration increases, there is more surface deposition. This makes reduction clearing necessary for shades of medium and higher depths. Another parameter to consider is the production type (lab scale or bulk), which influences the fastness properties.
Mechanism of reduction clearing
The reduction clear procedure eliminates excess dye and dyeing auxiliaries (such as surfactants, carrier residues, and migration inhibitors) without changing the dye's color. To remove the surface deposited dye, carrier, etc., without harming the dye that is adsorbed within the fiber, the procedure depends on polyester's notable hydrophobicity, which stops aqueous agents from accessing the substrate at temperatures lower than the commercial boil [4].
It is believed that when azo dispersion dyes are treated with the reducing agent's aqueous alkaline solution, the chromophore is destroyed, producing the following colorless amino compounds:
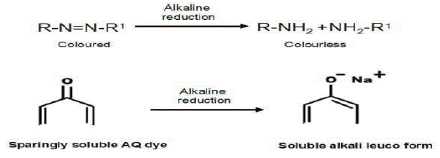
While anthraquininoid dyes are reduced to the dye's corresponding, virtually colorless, low substantivity, water-soluble leuco form.
Reduction Clearing Agent used in Polyester Dyeing
Reduction clearing is a crucial step in polyester dyeing to remove unfixed dye molecules from the fabric surface [5].
The below agents are commonly used for this purpose:
• Sodium Dithionite: This widely used to reduce agent effectively reduces unfixed dye molecules. However, it can be unstable and requires careful handling.
• Thiourea Dioxide: This agent is more stable than sodium dithionite and less prone to oxidation. It is often combined with sodium hydroxide to enhance its reducing power.
Disadvantages of Reduction Clearing
The reduction clearing process has two major disadvantages: one from the perspective of the process and the other is the adverse effects on the environment.
• Change in pH: Disperse dyeing requires an acidic medium, whereas reduction clearing is done in an alkaline medium. Neutralisation is also carried out, and hence, the change in pH takes place twice, adding to the processing time and cost.
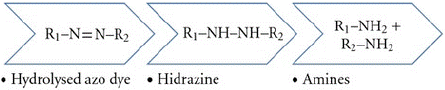
• Environment: Sodium dithionite is an inorganic, nonbiodegradable compound. When discharged into water, it tends to deplete the oxygen, creating an imbalance in the aquatic life. When azo dyes are used, the dye structure may further undergo structural transformations and generate aromatic amines, which are carcinogenic and harmful to the environment.
When hydrosulphites are used as reducing agents, they create an obnoxious odour, and in addition, they contribute to the effluent load in terms of Chemical Oxygen Demand (COD), biochemical oxygen demand (BOD), Total Dissolved Solids (TDS). It is also sensitive to air; hence, many chemicals are used. The waste by-products of sulphites and sulphates are corrosive and degrade the wastewater pipelines. Therefore, there is a need to find alternative agents for reduction clearing or completely eliminating the process.
Environmental Concerns
Reduction clearing with hydro-caustic presents several challenges in polyester dyeing. One significant issue is the potential for color loss, particularly with certain Disperse dyes that are sensitive to alkaline conditions. This can lead to uneven color and reduced shade depth, compromising the overall aesthetic appeal of the fabric. Additionally, the use of hydro-caustic can cause fiber damage, especially if the process parameters are not carefully controlled. Excessive alkalinity and high temperatures can weaken the fiber structure, reducing strength and durability.
Another challenge associated with reduction clearing using hydro-caustic is the generation of wastewater containing high levels of chemicals, including sodium hydroxide and sodium dithionite. These chemicals can have adverse environmental impacts if improperly treated and dispose off. Furthermore, the process can be energyintensive, requiring high temperatures and extended processing times. This contributes to increased operational costs and a larger carbon footprint. To address these challenges, the textile industry is exploring alternative reduction clearing agents and processes that are more environmentally friendly and less damaging to the fabric.
Cutting-edge Approaches in Reduction Clearing
Enzyme-based Reduction Clearing
Name
Features
Advantages
Disadvantages
Formamidine Sulphinic Acid (FAS)
It exists in two tautomeric forms. If the pH is 5, it can be kept at room temperature as an aqueous paste without risk.
It develops a reduction potential only in alkaline medium. It is used in discharge printing as well.
- Comparable wash fastness properties to that given by sodium dithionite.
- RC of PLA fabric: improved wash fastness in alkaline medium.
- The reducing effect of FAS is twice that of sodium dithionite
- Contributes less Sulphur content to the effluent.
- Limited applications due to high price.
- BOD and COD values are in the same order as sodium dithionite.
- Decomposes rapidly in an alkaline medium.
- Has low solubility.
- Increases the nitrogen content of the effluent.
Hydroxyacetone
It is a low molecular weight species.
In an alkaline media, it has strong reduced characteristics.
It is water soluble.
- Less oxidation-sensitive than FAS and sodium dithionite, and stable under atmospheric conditions.
- The effluent generated is free from sulphate and sulphite salts.
- Inherently biodegradable.
- Rarely used due to high cost.
- Increases the effluent's COD and total organic carbon (TOC) content.
Glucose
Used as an additional reducing agent for applying Sulphur dyes and sodium dithionite.
- Biodegradable nature
- It only produces a useful redox potential in high-alkalinity and high-temperature environments.
Table 1: Comparing the advantages and disadvantages of various organic reducing agents.
Enzymatic treatments have emerged as a promising eco-friendly alternative to chemical reduction clearing. Enzymatic reduction clearing has emerged as a promising eco-friendly alternative to conventional chemical reduction clearing methods. The primary enzymes employed in this process are oxidoreductases, particularly laccases [6] and peroxidases, which can effectively modify the surface properties of polyester fibers. These enzymes work by catalyzing oxidation-reduction reactions that break down surfacedeposited disperse dyes and oligomers without compromising the fiber's structural integrity. The advantage of enzymatic treatment lies in its specificity and ability to operate under milder conditions (temperatures between 40-60°C and neutral pH), significantly reducing energy consumption and environmental impact compared to traditional sodium hydrosulfite-based processes [7].
The effectiveness of enzymatic reduction clearing depends critically on several factors, including enzyme concentration, treatment time, temperature, and pH stability. Cellulases and lipases are sometimes combined with oxidoreductases to enhance the surface modification of polyester fibers and improve the removal of unfixed dyes. Recent research has shown that enzyme immobilization techniques can improve the stability and reusability of these biocatalysts, making the process more economically viable for industrial applications. However, challenges remain in scaling up enzymatic processes, including the relatively high cost of enzymes, potential limitations in treatment uniformity, and the need for precise process control to maintain enzyme activity and stability throughout the treatment.
Plasma Treatment
Plasma technology offers a novel approach to surface modification and to reduction clearing in polyester dyeing, providing significant advantages over traditional chemical methods. Exposing the dyed fabric to a high-energy plasma generates reactive species interacting with its surface, modifying its chemical and physical properties.
This treatment enhances the fabric's wettability, facilitating the removal of unfixed dye molecules. Additionally, plasma treatment can improve the color fastness of the dyed fabric by cross-linking dye molecules within the fiber structure. This results in enhanced resistance to fading and rubbing, ultimately improving the overall quality and durability of the dyed textile. While the technology is still evolving, plasma treatment holds immense potential to revolutionize the polyester dyeing industry, offering a more sustainable and efficient approach to reduction clearing [8].
Supercritical Carbon Dioxide Dyeing and Clearing
This innovative method uses supercritical carbon dioxide as both a dyeing medium and a clearing agent. Supercritical carbon dioxide (ScCO2) has emerged as a promising alternative to traditional chemical-based reduction clearing in polyester dyeing. When carbon dioxide is subjected to specific temperature and pressure conditions, it transitions into a supercritical state, exhibiting unique properties that make it ideal for various applications. ScCO2 can effectively remove unfixed dye from the fabric surface in textile dyeing. Its high diffusivity and solvency power allow it to penetrate the fiber structure and dissolve the unfixed dye molecules. Additionally, ScCO2 is nontoxic, non-flammable, and environmentally friendly, making it an attractive option for sustainable textile processing.
However, the high pressure and specialized equipment required for ScCO2 processes can present certain challenges [9].
Advanced Oxidation Processes (AOPs)
Advanced Oxidation Processes (AOPs) offer a sustainable alternative to traditional chemical reduction clearing in polyester dyeing. These processes leverage highly reactive hydroxyl radicals (•OH) to oxidize and degrade unfixed dye molecules, ensuring their effective removal from the fabric surface [10].
AOP techniques such as ozonation, ultraviolet/Hydrogen Peroxide (UV.H2O2), and Fenton's reagent have demonstrated potential in this application. Ozone can directly oxidize dyes or generate hydroxyl radicals through reactions with water. UV/H2O2 systems utilize UV radiation to trigger the formation of hydroxyl radicals from hydrogen peroxide. Fenton's reagent, a combination of ferrous ions and hydrogen peroxide, produces hydroxyl radicals through a complex reaction mechanism. While AOPs offer advantages like reduced chemical usage and improved environmental impact, challenges remain. Precise control of reaction conditions is essential to optimize hydroxyl radical generation and reactivity. Additionally, careful consideration must be given to potential fiber damage and color loss. Ongoing research and development are necessary to fully harness the potential of AOPs in the reduction clearing of polyester dyeing.
Ultrasonic-assisted Reduction Clearing
Ultrasound-assisted reduction clearing offers a promising approach to enhance the efficiency and effectiveness of polyester dyeing [11]. By applying ultrasonic waves to the dyeing process, several benefits can be achieved:
Improved Dye Penetration: Ultrasonic cavitation generates localized high pressures and temperatures, disrupting the fabric's structure and facilitating the penetration of reducing agents and the removal of unfixed dye molecules.
Enhanced Dye Removal: The intense agitation caused by ultrasonic waves promotes the diffusion of unfixed dye from the fiber surface to the dye bath. This leads to improved color fastness and reduced staining.
• Reduced Processing Time: Ultrasonic treatment can accelerate the reduction clearing process, leading to shorter cycle times and increased productivity.
• Lower Energy Consumption: By improving the efficiency of the dyeing process, ultrasound can help reduce energy consumption and lower operational costs.
Microwave-Assisted Processing
Microwave irradiation heats the fabric directly and uniformly, leading to rapid penetration of the reducing agent into the fiber. This accelerates the removal of unfixed dye, resulting in shorter processing times and improved efficiency. Additionally, microwave heating can enhance the effectiveness of the reducing agent, potentially leading to a more thorough removal of unfixed dye. While microwave-assisted reduction clearing shows promise, further research is needed to optimize the process parameters and evaluate its long-term impact on fabric quality and environmental sustainability [12].
Electrochemical Methods
Electrochemical methods for the reduction clearing of polyester offer an environmentally friendly and efficient alternative to traditional chemical methods. These methods involve using electric current to reduce and remove surface dyes and other impurities from polyester fabrics [13].
Direct Electrochemical Reduction
Mechanism: In this method, the dye molecules are directly reduced at the cathode of an electrochemical cell. The electrons from the cathode cause the dye molecules to lose their color and become soluble, allowing them to be easily removed from the fabric [14].
Advantages:
• Environmentally friendly: No harmful chemicals are used
• Energy efficient: The process requires only electricity
• Simple and easy to implement
Disadvantages:
• May require specific electrode materials and operating conditions for optimal performance
• Slower than some chemical methods
Indirect Electrochemical Reduction
Mechanism: This method generates an intermediate reducing agent at the cathode, reducing the dye molecules. This approach can be used with a broader range of electrode materials and operating conditions.
Advantages:
• More flexible than direct electrochemical reduction
• Can be used with a broader range of electrode materials
Disadvantages
• Requires the use of an intermediate reducing agent, which may add to the cost and complexity of the process.
Alternative Sustainable Reducing Agents
1. Hydroxyacetone, a versatile organic compound, undergoes oxidation to form pyruvic acid. This process generates reducing agents through a mechanism known as ketol-enol tautomerism. These reducing agents, produced from hydroxyacetone, form intermediate complexes with Disperse dyes. These complexes facilitate the conversion of surface-bound dye molecules into water-soluble forms, making them easily removable from the fabric [15].
2. Glucose-based reducing agents offer a promising and environmentally friendly alternative to traditional hydro-caustic in polyester dyeing. This approach leverages the reducing properties of glucose and its derivatives to effectively remove unfixed dye from the fabric surface [16].
3. Formamidine Sulfinic Acid (FSA), also known as thiourea dioxide, is a versatile reducing agent that has gained significant attention in the textile industry for its potential to improve the efficiency and sustainability of polyester dyeing. It offers several advantages over traditional reducing agents like sodium dithionite:
FSA acts as a strong reducing agent, reducing the unfixed dye molecules on the fabric surface. This reduction process converts the insoluble dye molecules into water-soluble forms, facilitating their removal during rinsing.
4. Acid reduction clearing agents:
These are a newer type of clearing agents that work at a lower pH (around 4-5). They are becoming increasingly popular because they offer several advantages over alkaline clearing agents, such as:
• Reduced water and energy consumption: Acid reduction clearing can be done in the same bath as the dyeing process, eliminating the need for an additional washing.
• Improved dye yield: Acid reduction clearing is less likely to remove well-dyed fibers, resulting in a deeper and more even color.
• Reduced environmental impact: Acid reduction clearing agents are less hazardous than alkaline ones.
5. Combining multiple sustainable reducing agents in polyester dyeing also offers several advantages. Firstly, it allows for a synergistic effect, where different agents can work together to enhance the overall reduction efficiency and minimize side effects. This can lead to improved color fastness and reduced fiber damage. Secondly, combining agents can help to reduce overall chemical usage, leading to lower environmental impact and cost savings. Additionally, it can broaden the range of dyes that can be effectively removed, improving the versatility of the process [17].
Key takeaways about combined reducing agent systems:
• They offer better stability and efficiency compared to single agents
• Process control is critical for optimal results
• Environmental benefits are significant
• Cost-effectiveness depends on proper optimization
• Continuous innovation is driving improvements
6. Stabilized Reducing agents
Stabilized reducing agents with pre-activated systems and readyto- use preparations offer numerous benefits for polyester dyeing. These formulations provide consistent and reliable performance, eliminating the need for complex mixing and activation procedures. They often have extended shelf lives, reduced waste and improved supply chain efficiency. Pre-activated systems ensure rapid and efficient reduction, leading to shorter processing times and improved productivity. Additionally, stabilized formulations minimize the risk of unintended side reactions, such as oxidation or decomposition, ensuring optimal performance and fabric quality. Metal hydrides like Sodium Borohydride (NaBH4), Lithium Aluminum Hydride (LiAlH4) can be used.
Green Chemical Alternatives
Alkyl polyglucosides (APGs) are popular in the market, as they are made from natural raw materials and exhibit good biodegradability and ecotoxicity. Due to their high solubility, stability to electrolytes and hard water, they are ideal for the textile wash-off process [18]. Work was done to analyse the characteristics and behaviour of APG and linear alkyl sulfonate, both individually and in mixtures of different proportions, to observe and determine the behaviour of dyed polyester fabric, changing its color during washing. The process of dye transfers to white textile items (diacetate, polyamide, cotton, acrylic and wool) was also studied. Before this process, polyester was dyed with Disperse dyes. It is well known that various mechanical, chemical, electrical, and adsorption-related factors can result in the deposition of dispersion dyes on white polyester fabric after washing. The total potential energy between the dye and polyester was evaluated to understand the phenomenon of dye deposition during the washing process. The results were mainly based on the theory of heterocoagulation. It was observed that the zeta potentials of the insoluble Disperse dyes and the fabrics were related to the results obtained by staining white fabric during washing. Lastly, the color fastness properties of the samples washed using APGs were comparable to that of the traditional reduction-cleared samples. Even Soap nut-based washing agents are emerging as a promising alternative. These natural agents, derived from the fruit of the soap nut tree, offer a compelling blend of performance and environmental benefits [19].
Soapnut washing agents have demonstrated superior performance in enhancing color fastness and overall color quality compared to sodium dithionite. They effectively remove impurities and improve color uniformity, producing vibrant and long-lasting colors. Additionally, soap nut agents offer the advantage of sulfate-free effluent, significantly reducing the environmental impact of textile processing.
Summary
Polyester dyeing and reduction clearing processes have evolved significantly, driven by the increasing demand for sustainable and efficient textile production. Traditional methods often relied on harsh chemicals and energy-intensive processes. However, recent advancements have focused on developing more eco-friendly chemical alternatives, such as enzyme-based, glucose-based, biobased, and green reducing agents. Other innovative techniques like plasma, microwave-assisted, supercritical CO2 dyeing, and AOP offer novel approaches. These alternatives minimize chemical usage, reduce wastewater generation, and improve efficiency. However, the shift towards sustainable techniques faces several challenges. These include high initial costs for new technologies, a lack of awareness about eco-friendly options, and the inability to meet consumer requirements in terms of performance and price. Addressing these challenges requires cooperation between industry, government, and researchers to promote and incentivize sustainable practices.
To meet future sustainability requirements, the industry must continue to prioritize innovation and adopt environmentally friendly practices. This includes developing sustainable dyes, optimizing dyeing processes, and implementing advanced wastewater treatment technologies. Exploring alternative fibers, such as recycled polyester and bio-based polyesters, can contribute to a more sustainable textile supply chain.
References
- SM Burkinshaw, N Kumar. The reduction clearing of dyed polyester, color strength. Dyes and Pigments. 2008; 76: 799-809.
- JR Aspland. Chapter 8: Disperse dyes and their application to Polyester. 2014.
- AU Aleem, RM Christie. The clearing of dyed polyester. A comparison of traditional reduction clearing with treatments using organic reducing agents. Coloration Technology. 2016; 132: 280-296.
- U Aleem. An investigation of alternatives to reductive clearing in the dyeing of Polyester. 2013.
- M Waqas, S Zameer Ul Hassan, S Siddiqui, A Asghar. Effective reduction clearing parameters involving alternative reducing agent,” Journal of Pakistan Institute of Chemical Engineers. 2021; 49: 51–61.
- J Jeyabalan, A Veluchamy, Priyan V, A Kumar, R Chandrasekar, S Narayanasamy. A review on the laccase assisted decolorization of dyes: recent trends and research progress. Journal of Taiwan Chemical Engineering. 2023; 151: 105081.
- PK Singh, RL Singh. Bio-removal of azo dyes: a review. International Journal of Applied Science and Biotechnology. 2017; 5: 108-126.
- MT Abate, Seipel S, Yu J, Vikova M, Vik M, Ferri A, et al. Supercritical CO2 dyeing of polyester fabric with photochromic dyes to fabricate UV sensing smart textiles. Dyes and Pigments. 2020; 183: 108671.
- I Yigit, S Eren, H Özcan, O Avinc, HA Eren. An investigation of process parameters on color during the dyeing of polyester in supercritical carbon dioxide media. Coloration Technology. 2021; 137: 625-644.
- HA Eren, I Yigit, S Eren, O Avinc. Ozone: an alternative oxidant for textile applications. 2020: 81–98.
- MM Hassan, K Saifullah. Ultrasound-assisted sustainable and energy efficient pre-treatments, dyeing, and finishing of textiles – A comprehensive review. Sustainable Chem-Pharm. 2023; 33: 101109.
- Y Dulek, I Yildiran, B Sevinc, E Mert, B Yilmaz, D Kut. A comparative study on microwave Assisted dyeing properties of conventional and recycled polyester fabrics. The Eurasia Proceedings of Science Technology Engineering and Mathematics. 2023; 23: 300-306.
- SS Beulah, K Muthukumaran. Methodologies of removal of dyes from wastewater. International Research Journal of Pure and Applied Chemistry. 2020; 21: 68-78.
- Kamenická B, G Kuchtová. Critical review on electrooxidation and chemical reduction of azo dyes: economic approach. Chemosphere. 2024; 363- 142799.
- Md K Islam, Z Uddin, Md R Repon. Enhancement of polyester dyeing performance integrating ecological and cost-effective auxiliaries. Textile & Leather Review. 2024; 7: 1216-1233.
- A Khalique, S Ali, RA Khera, M Asgher. Greener approach to substitute chemical reduction clearing process for fabric dyed using indigenous resources. Zeitschrift für Physikalische Chemie. 2021; 235: 1689-1700.
- MT Ahmed, S Farjana, Md Rasel. Modification of reduction clearing process of polyester blend cotton knitted fabric dyeing. International Journal of Science and Engineering Research. 2018; 9: 101-106.
- FJ Carrion-Fite. Color change of polyester dyed fabric during washing by ecological surfactants. The Journal of The Textile Institute. 2015; 106: 320- 326.
- HR Kumar. Environment friendly approach to remove unbound disperse dyes from polyester fabric. International Journal of Advanced Research in Biological Sciences. 2023; 10: 181-195.