
Research Article
Austin Therapeutics. 2014;1(1): 4.
Effects of Different Dialyzed Models on Serum Levels of Nitric Oxide and Endothelin-1 in Patients with End Stage Renal Disease
Pedja Kovacevic1,3*, Saša Dragic3, Zvezdana Rajkovaca1, Slavimir Veljkovic2 and Tijana Kovacevic4
1Department for Physiology, Medical School Banja Luka, Bosnia - Herzegovina
2Institute for Physiology, Medical School Niš, Serbia
3Medical Intensive Care Unit, Clinical centre Banja Luka, Bosnia - Herzegovina
4Department for hospital pharmacy, Clinical centre Banja Luka, Bosnia - Herzegovina
*Corresponding author: Pedja Kovacevic, Department for Physiology, Medical School Banja Luka, Filipa Kljajica Fice 49, Banja Luka 78000, Republika Srpska, Bosnia- Herzegovina
Received: July 04, 2014; Accepted: Aug 02, 2014; Published: Aug 04, 2014
Abstract
Pathophysiological disturbances of vasoactive substances (nitric oxide - NO and endothelin- 1 - ET-1) are often found in uremic patients. End stage renal disease (ESRD) and its treatment modules affect almost all organs and organ systems including vascular endothelium. There is a small number of studies which investigated serum levels of NO and ET-1 in ESRD patients treated with hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD). Therefore our study aimed to measure serum levels of NO and ET-1 in this population.
This study included 51 ESRD patients (28 treated with HD) and (23 treated with CAPD). Mean duration of HD treatment was 4.14±12.9 years and CAPD treatment was 3.4±14.7 years. Besides this groups of patients (HD and CAPD), we included a third group which consisted of 30 healthy controls (14 males, 16 females).
Our results show significantly higher serum levels of NO in HD (x±SD = 19,09±6,4) and CAPD patients (x±SD = 19,09±6,9) in comparison to the control group (x±SD = 9,5±1,9) (p < 0,05). There was significant difference in serum levels of ET-1 between HD patients (x±SD = 10,3±5,3) and the control group (x±SD =6,6±4,2), (p < 0,05), but no significant difference in serum levels of ET-1 between CAPD patients (x±SD = 7,3±5,6) and the control group, (p > 0,05).
We concluded that imbalance in production of vasoactive substances is present in CAPD patients. This imbalance can be one of the reasons for disturbance in local blood flow control. These pathophysiological mechanism can cause significant hemodynamic disturbance (hypertension) and atherosclerosis.
Keywords: HD; CAPD; Nitric oxide; Endothelin-1
Introduction
End Stage Renal Disease (ESRD) requires treatment with one of dialysis models. Physiological and pathophysiological mechanisms in these patients very often can cause damage of endothelium or blood vessel in whole. All these disorders in blood vessels can cause endothelial dysfunction [1-3]. Vascular endothelium is not just a mechanical barrier in blood vessel, but endocrine organ as well which produces many substances out of which some have vasoactive effects. Two most potent vasoactive substances with opposite effects are nitric oxide (NO) vasodilatator nad endothelin-1 (ET-1) one of the most powerful known vasoconstrictor [4,5]. Besides its vasoactive effects these substances (NO and Et-1) express many others metabolic and biochemical effects [6,7]. It is well known that cardiovascular diseases (e.g. hypertension) are leading cause of death in ESRD patients. Endothelial damaging as well as imbalance in production of vasoactive substances can be connected with these facts [8-11]. Moreover all dialysis modules attribute to this cardiovascular and endothelial damaging.
There are a small number of studies which investigated serum levels of NO and ET-1 in ESRD patients treated with haemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD). Therefore our study aimed to measure serum levels of NO and ET-1 in this population [2,12].
Material and Methods
We performed a prospective study which included ESRD patients treated with HD and CAPD. The HD group included 28 patients (15 males, 13 females, mean age 55, 9±16, 2 years) who were treatedwith HD at the Institute for nephrology of University hospital in Niš. The mean duration of haemodialysis was from 180 to 240 minutes (individual approach), three times a week. The dialysers used were produced by Gambro and Fresenius companies with controlled ultrafiltration, and bicarbonate module were applied. Haemodialysis was performed on the following dialysers: E4H, F6, F60, F60s. Heparinisationwas continuous with 4000-5000 i.u. of heparin per patient. No patients had primary pulmonary disease nor had haemodynamic instability during haemodialysis. The average period of haemodialysis duration in these patients was 4, 14±12,9year.
The CAPD group included 23 patients (10 males, 13 females, mean age 55,8 ±15,8 years) who were treated with CAPD at the Institute for nephrology of University hospital in Nis. Dialysis solution was changed three times per day and patients were trained to do it by themselves or it was done at the Institute under the supervision of the medical staff.
Beside HD and CAPD groups of patients, we included a third group which consisted of 30 healthy subjects (14 males, 16 females, mean age 51,8 ±15,6 years) to serve as a control group. We measured the levels of nitric oxide and endothelin-1 in the control group and its mean level (+/- SD) served as a referent value. All studied subjects where non smokers. Table 1 represents basic demographic characteristics of patients and control group. Table 2 shows HD and CAPD patients regarding primary diseases which caused ESRD.
n
males
females
age (x±SD)
Length of dialysis
years (x±SD)
HD
28
15
13
55,9±16,2
4,14±12,9
CAPD
23
10
13
55,8±15,8
3,4±14,7
Control group
30
14
16
51,8±15,6
-
Table 1: Basic demographic characteristics of patients
Primary
disease
Diabetic
nephropathy
Hypertensive
nephropathy
Polycystic
kidney disease
Chronic
pyelonephritis
Unknown
HD
12
3
3
2
8
%
43
11
11
7
28
CAPD
8
6
2
1
6
%
35
26
9
4
26
Table 2: Laboratory values at hospital admission.
Blood samples from all observed patients were taken from cubital vein.
From the patients in HD group (patients treated with hemodialysis) blood samples were collected immediately before of the following hemodialysis since we wanted to exclude the impact of ultrafiltration on instantaneous change in No and ET-1 production through blood pressure dynamics.
From the patients in CAPD group (patients treated with continuous ambulatory peritoneal dialysis) blood samples were collected immediately before emptying of peritoneal cavity so that these patients would have the most similar volume and toxins overload to HD patients in previous group. From the control group blood were collected in basal conditions.
Measurement of NO serum levels
The NO level in whole blood is determined by measuring nitrite and nitrate (NO3 2- u NO2 2-) production using clasical colorimetric reaction (Griess). Blood samples for the determination of NO concentration were diluted 1:1 (vol/vol) with 0.9% saline, proteinprecipitated using 30% ZnSO4, 0.05 ml per ml of blood and centrifuged at 700 g for 10 minutes and frozen at -20°C. Conversion of NO3 2- into NO2 2- was done with nitrate reductase elementary zinc. NO2 2-concentration in serum was determined by classic colorimetric Griess reaction. Briefly, equal volumes of samples and Griess reagent (sulfanilamide and naphthalene-ethylene diamine dihydrochloride) were mixed at room temperature. After 5 min, the absorbance was measured at 546 nm using spectrophotometer. The concentration of nitrite was determined by a standard curve prepared with sodium nitrite.
Measurement of ET-1 serum levels
From the whole blood specimen serum was separated in heated bath on 37 °C. Activity of serum ET-1 was measured with EIA methodology which is based on immunometric assay so called sandwich technique. Measurement was performed using computer ased ELISA reader (ELx 800 Universal Microplate Reader Biotek Instruments, INC) with wavelength 405 nm. We used prepared enzyme kit (Endothelin - 1; EIA kit - IBL Hamburg, Germany). Endothelin test kit used in this study was an enzyme radioimmunoassay designed for direct determination of endothelin-1 in biological fluids.
Statistical analysis
The results were processed using standard statistical method (Student’s t-test) for small independent samples (modification by Cochran & Cox) shown as mean ±standard mean error (X ± SX). We tested significance of the difference in mean values between studied groups with an aim to monitor changes in serum NO levels. We considered the value of p < 0, 05 statistically significant.
Results
Study included 28 HD patients, 23 CAPD patients and 30 healthy volunteers in the control group. Demographic characteristics of all 81 patients are shown in Table 1. Table 2 shows HD and CAPD patients regarding primary diseases which caused ESRD. Figure 1 presents comparison of mean serum levels of NO in HD group of patients (19,09±6,4), CAPD group of patients (19,09±6,9) and control group (9,5±1,9). Statistical analysis with Student’s t-test for small independent samples (Cochran and Cox modification) showed significantly higher serum levels of NO in HD and CAPD patients in comparison to control group (p < 0,05). Figure 2 presents comparison of mean serum levels of NO in HD group of patients (19,09±6,4) and CAPD group of patients (19,09±6,9). Statistical analysis with Student’s t-test for small independent samples (Cochran and Cox modification) demonstrated similar serum levels of NO in HD and in CAPD groups of patients (p > 0,05). Figure 3 presents comparison of mean serum levels of ET-1 in HD group of patients (10,3±5,3), CAPD group of patients (7,3±5,6) and control group (6,6±4,2). Statistical analysis with Student’s t-test for small independent samples (Cochran and Cox modification) showed significantly higher serum levels of ET-1 in HD group of patients in comparison to control group (p < 0,05), while the serum levels of ET-1 in CAPD patients were higher than the levels in control group but without statistical significance (p > 0,05). Figure 4 presents comparison of mean serum levels of ET-1 in HD group of patients (10,3±5,3) and CAPD group of patients (7,3±5,6). Statistical analysis with Student’s t-test for small independent samples (Cochran and Cox modification) showed significantly higher serum levels of ET-1 in HD patients in comparison to CAPD group of patients (p< 0,05).
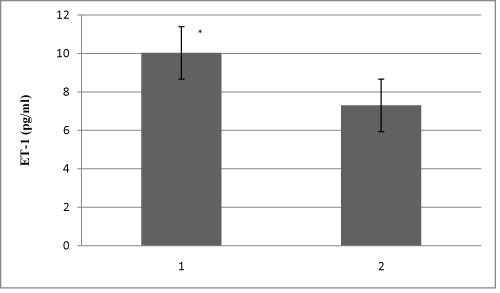
Figure 4: Mean serum levels of ET-1 in 1-HD patients and 2-CAPD patients.* p < 0,05
Discussion and Conclusion
Our study showes significantly higher serum levels of NO in patients treated with HD and CAPD compared to control group (figure 1). Some other authors and literature data regarding physiology and pathophyisiology of NO in ESRD patients treated with one of dialysis modes are contradictory [6]. One group of data suggests that serum levels of NO in ESRD patients treated with some mode of dialysis are significantly lower in comparison to healthy subjects, while other state there no statistically significant difference in NO serum levels between these observed groups [13-15]. One possible explanation for lower serum levels of NO in uremic patients could be decreased concentration of L -arginine due to reduction of renal parenchyma. Besides this some authors found increased levels of endogenous inhibitors of NO synthase (NOS) such as asymmetric dimethylarginine (ADMA). This overload of ADMA is presented in dialyzed patients as well [16-18]. Reduction in renal mass leads to increase in secretion of proinflamatory mediators platelet derived growth factor (PDGF) and transforming growth factor (TGF). Both of these mediators are very potent inhibitors of NOS.
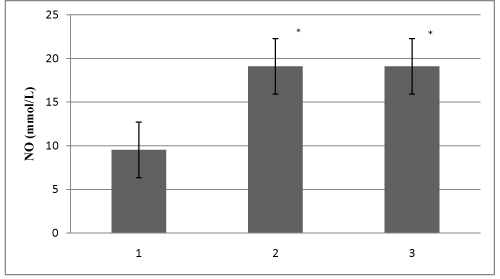
Figure 1: Mean serum levels of NO in all studied subjects (1- Healthy subjects; 2-HD patients; 3-CAPD patients). * p < 0,05.
Recent in vitro and in vivo data on rats have shown that a decreased renal mass leads to an increased synthesis of a potent vasoconstrictor, endothelin-1, which decreases the production of NO [19-21]. In our study serum levels of NO were significantly higher in HD and CAPD patients compared to healthy population (p<0,05) figure 1. Serum levels of NO in two dialyzed group of patients (HDand CAPD) were similar (figure 2). Explanation for these results can be found in increase production of NO from mesothelial cell. Devenport et al. state that mesothelial and endothelial cell originate from the same germ layers [22,23]. Besides this way of NO production tissue macrophages which are involved in inflamatory processes in peritonitis represent significant source of NO [24]. This is likely because CAPD patients develop bacterial peritonitis frequently [25]. By investigating physiology and pathophysiology of these changes in CAPD patients Imai et al. found that patients with chronic hypotension compared to normotensive patients have similar stroke volumes and heart rates, but lower levels of peripheral vascular resistance. There is an assumption that some ESRD patients during progression of their disease start to produce vasodilators intensively, primarily NO and adrenomedulin. Noris et al. suggest that thrombocytes can also significantly contribute to NO production [24]. We also found serum levels of ET-1 to be statistically higher in HD patients compared to CAPD patients and control group (figure 3 and 4). The literature regarding physiology and pathophyisiology of ET-1 in ESRD patients treated with one of dialysis modes provides conflicting data, although most authors state that ET-1 serum levelsare higher in these patients compared to healthy subjects [12].
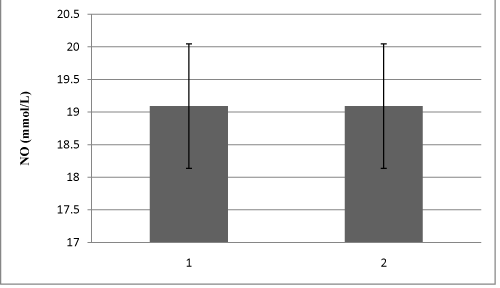
Figure 2: Mean serum levels of NO in 1-HD patients and 2-CAPD patients.
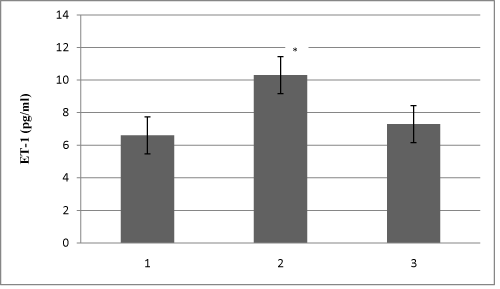
Figure 3: Mean serum levels of ET-1 in all studied subjects (1- Healthy subjects; 2-HD patients; 3-CAPD patients).
* p < 0,05.
In the glomeruli affected by sclerosis, endothelial injury leads to the increased secretion of endothelin-1 and consequent vasoconstriction, increased intraglomerular pressure, and decreased glomerular filtration for which it is suspected to be one of main reasons for elevated serum ET-1 levels [21,26,27]. On the other hand slight increase ET-1 in peritoneal cavity and application of human recombinant erythropoietin can contribute to increase serum levels of ET-1. Besides increased production of ET-1, Lebel et al. found lower elimination of ET-1 via peritoneal membrane [28-32].
From our results we concluded that imbalance in production of vasoactive substances (NO and ET-1) is present in HD and CAPD patients. This imbalance can lead to disturbance in local blood flow control. These pathophysiological mechanism can cause significant hemodynamic disturbance that may lead to hypertension and atherosclerosis.
References
- Carracedo J, Buendía P, Merino A, Soriano S, Esquivias E, Martín-Malo A, et al. Cellular senescence determines endothelial cell damage induced by uremia. See comment in PubMed Commons below Exp Gerontol. 2013; 48: 766-773.
- Meenakshi SR, Agarwal R. Nitric oxide levels in patients with chronic renal disease. See comment in PubMed Commons below J Clin Diagn Res. 2013; 7: 1288-1290.
- Brunini TM, Moss MB, Siqueira MA, Santos SF, Lugon JR, Mendes-Ribeiro AC. Nitric oxide, malnutrition and chronic renal failure. See comment in PubMed Commons below Cardiovasc Hematol Agents Med Chem. 2007; 5: 155-161.
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. See comment in PubMed Commons below Nature. 1980; 288: 373-376.
- Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. See comment in PubMed Commons below Am J Physiol. 1985; 248: C550-556.
- Hirst DG, Robson T. Nitric oxide physiology and pathology. See comment in PubMed Commons below Methods Mol Biol. 2011; 704: 1-13.
- Derentowicz P, Markiewicz K, Wawrzyniak M, Czerwinska-Kartowicz I, Bulawa E, Siwinska-Golebiowska H. [Nitric oxide (NO)--Nobel prize in medicine and physiology for 1998]. See comment in PubMed Commons below Med Wieku Rozwoj. 2000; 4: 209-217.
- de Leeuw PW. Pathophysiology of hypertension in patients on renal replacement therapy. See comment in PubMed Commons below Blood Purif. 1994; 12: 245-251.
- Davies DL, Beevers DG, Briggs JD, Medina AM, Robertson JI, Schalekamp MA, Brown JJ. Abnormal relation between exchangeable sodium and the renin-angiotensin system in malignant hypertension and in hypertension with chronic renal failure. See comment in PubMed Commons below Lancet. 1973; 1: 683-686.
- Campigano JL, Ramirez-Muzo O, Ramirez-Gonzales R. Normal renin uremic hypertension: Study of cardiac hemodynamics, plasma volume, extracellular fluid volume, and the renin- angiotensin system. Arch Intern Med. 1976; 136: 17–23.
- Tuckman J, Benninger JL, Reubi F. Haemodynamic and blood volume studies in long-term haemodialysis patients, and in patients with successfully transplanted kidneys. See comment in PubMed Commons below Clin Sci Mol Med Suppl. 1973; 45 Suppl 1: 155s-7.
- Lightfoot BO, Caruana RJ. Endothelin-1 in continuous ambulatory peritoneal dialysis and hemodialysis patients: a preliminary study. See comment in PubMed Commons below Perit Dial Int. 1993; 13: 55-58.
- Reyes AA, Karl IE, Klahr S. Role of arginine in health and in renal disease. See comment in PubMed Commons below Am J Physiol. 1994; 267: F331-346.
- Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. See comment in PubMed Commons below Lancet. 1992; 339: 572-575.
- Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. See comment in PubMed Commons below Kidney Int. 2000; 58: 1261-1266.
- Hand MF, Haynes WG, Webb DJ. Hemodialysis and L-arginine, but not D-arginine, correct renal failure-associated endothelial dysfunction. See comment in PubMed Commons below Kidney Int. 1998; 53: 1068-1077.
- Kari JA, Donald AE, Vallance DT, Bruckdorfer KR, Leone A, Mullen MJ, et al. Physiology and biochemistry of endothelial function in children with chronic renal failure. See comment in PubMed Commons below Kidney Int. 1997; 52: 468-472.
- Morris ST, McMurray JJ, Rodger RS, Jardine AG. Impaired endothelium-dependent vasodilatation in uraemia. See comment in PubMed Commons below Nephrol Dial Transplant. 2000; 15: 1194-1200.
- Mittermayer F, Schaller G, Pleiner J, Vychytil A, Sunder-Plassmann G, Hörl WH, et al. Asymmetrical dimethylarginine plasma concentrations are related to basal nitric oxide release but not endothelium-dependent vasodilation of resistance arteries in peritoneal dialysis patients. J Am Soc Nephrol. 2005; 16: 1832-1838.
- Madore F, Prud'homme L, Austin JS, Blaise G, Francoeur M, Léveillé M, Prud'homme M. Impact of nitric oxide on blood pressure in hemodialysis patients. See comment in PubMed Commons below Am J Kidney Dis. 1997; 30: 665-671.
- Potter GS, Johnson RJ, Fink GD. Role of endothelin in hypertension of experimental chronic renal failure. See comment in PubMed Commons below Hypertension. 1997; 30: 1578-1584.
- Davenport A, Fernando RL, Varghese Z. Intraperitoneal nitric oxide production in patients treated by continuous ambulatory peritonal dialysis. See comment in PubMed Commons below Blood Purif. 2004; 22: 216-223.
- Davenport A, Fernando RL, Robson R, Varghese Z. Nitric oxide production by human peritoneal mesothelial cells. See comment in PubMed Commons below Int J Artif Organs. 2004; 27: 15-23.
- Noris M, Benigni A, Boccardo P, Aiello S, Gaspari F, Todeschini M, et al. Enhanced nitric oxide synthesis in uremia: implications for platelet dysfunction and dialysis hypotension. See comment in PubMed Commons below Kidney Int. 1993; 44: 445-450.
- Su YJ, Liao SC, Cheng BC, Hwang JC, Chen JB. Increasing high-sensitive C-reactive protein level predicts peritonitis risk in chronic peritoneal dialysis patients. See comment in PubMed Commons below BMC Nephrol. 2013; 14: 185.
- Imai Y, Abe K, Otsuka Y, Sato M, Haruyama T, Ito T, et al. Blood pressure regulation in chronic hypotensive and hypertensive patients with chronic renal failure. See comment in PubMed Commons below Jpn Circ J. 1981; 45: 303-314.
- Büssemaker E, Passauer J, Reimann D, Schulze B, Reichel W, Gross P. The vascular endothelin system is not overactive in normotensive hemodialysis patients. See comment in PubMed Commons below Kidney Int. 2002; 62: 940-948.
- Kang DH, Yoon KI, Han DS. Acute effects of recombinant human erythropoietin on plasma levels of proendothelin-1 and endothelin-1 in haemodialysis patients. See comment in PubMed Commons below Nephrol Dial Transplant. 1998; 13: 2877-2883.
- Lebel M, Moreau V, Grose JH, Kingma I, Langlois S. Plasma and peritoneal endothelin levels and blood pressure in CAPD patients with or without erythropoietin replacement therapy. Clin Nephrol. 1998; 49: 313-318.
- Kourti P, Zarogiannis SG, Liakopoulos V, Karioti A, Eleftheriadis T, Hatzoglou C, et al. Endothelin-1 acutely reduces the permeability of visceral sheep peritoneum in vitro through both endothelin-A and endothelin-B receptors. See comment in PubMed Commons below Artif Organs. 2013; 37: 308-312.
- Kourti P, Zarogiannis S, Liakopoulos V, Hatzoglou C, Giannopoulou M, Chronopoulou I, et al. Effect of endothelin-1 on the transmesothelial resistance of isolated visceral sheep peritoneum. See comment in PubMed Commons below Adv Perit Dial. 2007; 23: 38-42.
- Morgera S, Kuchinke S, Budde K, Lun A, Hocher B, Neumayer HH. Volume stress-induced peritoneal endothelin-1 release in continuous ambulatory peritoneal dialysis. See comment in PubMed Commons below J Am Soc Nephrol. 1999; 10: 2585-2590.