
Research Article
Austin Therapeutics. 2015; 2(1): 1015.
Design and Fabrication of Ordered Mesoporous Alumina Scaffold for Drug Delivery of Poorly Water Soluble Drug
Borbane SA, Pande VV*, Vibhute SK, Kendre PN and Dange VU
Department of Pharmaceutics, Sanjivani College of Pharmaceutical Education and Research, India
*Corresponding author: Pande VV, Department of Pharmaceutics, Sanjivani College of Pharmaceutical Education and Research, India
Received: February 26, 2014; Accepted: September 04, 2015; Published: September 06, 2015
Abstract
The aim of present study was to develop and evaluate Telmisartan (TEL) loaded Ordered Mesoporous Alumina (OMA) scaffold for enhanced solubility and dissolution of TEL. The Ordered Mesoporous Alumina acts as a carrier for drug and increases its solubility and dissolution rate. The OMA was synthesized by Evaporation Induced Self Assembly (EISA) mechanism and characterized by the FT-IR, SEM, TEM porosimetry and XRD. The total specific surface area on OMA was found to be 299 m²/g whereas total cumulative volume, average pore diameter and total porosity was found to be 1.3182 cc/g, 26 nm and 23% respectively. The Telmisartan (TEL) was loaded in the OMA by the Solvent Impregnation Method. The TEL: OMA compatibility was studied by FT-IR spectroscopy; where no significant interaction was observed. The drug loading was confirmed by UV and TGA; which was found to be 45% and 43% respectively. Finally the dissolution rate and drug release of TEL loaded OMA was compared to the pure crystalline Telmisartan. Significant increase in dissolution and drug release from OMA was observed. It is concluded that the OMA could be a promising drug carrier and platform for delivery of poorly water soluble drugs for improved oral bioavailability.
Keywords: Telmisartan; Ordered mesoporous alumina; Poorly water soluble drug; Oral bioavailability
Abbreviations
TEL: Telmisarton; ARB: Angiotensin II Receptor Antagonist; USFDA: United State Food and Drug Administration; BCS: Biopharmaceutical Classification System; OMA: Ordered Mesoporous Silica; EISA: Evaporation Induced Self Assembly; FTIR: Fourier Transform Infra-Red spectroscopy; KBr: Potassium Bromide; SEM: Scanning Electron Microscopy; TEM: Transmission Electron Microscopy; PXRD: Powder X- Ray Diffraction; Mpa: Mega Pascal; TGA: Thermo Gravimetric Analysis; IUPAC: International Union of Pure and Applied Chemistry.
Introduction
Many drugs exhibit poor water solubility hence low bioavailability; leading to limitations in potential formulation options and in some cases; resulting in drug candidates being rejected during the development process. Although several formulation approaches including solid dispersions [1]; emulsion based systems [2]; nanosizing [3] and cyclodextrin inclusion complexes [4] have led to promising in vitro results; mesoporous material based drug delivery systems have been investigated to enhance solubility [5] and thus improved bioavailability.
Mesoporous materials offer several attractive features for controlled release; such as a high adsorption capacity; the ability of the mesoporous channels to change the crystalline state of a drug to an amorphous one; the possibility of stabilizing loaded drugs within these pores and the ease of modifying the pore dimensions to control drug delivery kinetics. In the recent years; ordered mesoporous materials were investigated as materials for applications in many areas; such as catalysis; sorption; separation; drug delivery/controlled drug release [6].
Hypertension is an important public health challenge worldwide because of its high prevalence and concomitant increase in risk of disease [7]. In the year 2000; the world was estimated to have close to 1 billion people with hypertension and predicted an increase to 1.56 billion by 2025 [8]. The global economic burden of increased blood pressure was estimated to consume US$370 billion worldwide and 10% of healthcare expenditures [9].
Telmisartan (TEL) is an Angiotensin-II Receptor Antagonist (ARB) used in the management of hypertension [10] was approved by the US Food and Drug Administration (FDA) in 1998 [11]. It is indicated for the treatment of hypertension but Telmisartan’s dual mode of action may provide protective benefits against the vascular and renal damage caused by diabetes and cardiovascular disease [12]. TEL is a BCS II drug with major drawback of its poor water solubility of 0.09 μg/ml and unsatisfactory bioavailability of 45% [13].
The present study was an approach to develop and evaluate Telmisartan (TEL) loaded Ordered Mesoporous Alumina (OMA) for enhanced solubility and dissolution of TEL.
Materials and Methods
Materials
The chemicals used in this work were of AR grade. Telmisartan and Pluronic F127 were obtained as a gift sample from Glennmark India Ltd; Nasik and Hi-media Laboratories; Mumbai respectively. Aluminium isopropoxide; ethanol and nitric acid were procured from Yarrowchem Pvt. Ltd. Mumbai.
Synthesis of ordered mesoporous alumina (OMA) scaffold
The OMA scaffold was synthesized by Evaporation Induced Self Assembly (EISA) mechanism. Approximately 0.75 g of Pluronic F127 was dissolved in 99.5% absolute ethanol and allowed to stir at room temperature for 1 h. Then; 1.0213 g of 98% aluminium isopropoxide was added; followed by 0.8 ml of 70% nitric acid. The total amount of ethanol was varied from 20 to 40 ml. The synthesis mixture was continuously stirred at room temperature for 12 h. Solvent evaporation was performed at 60°C in drying oven without stirring. After two days aging; the solution turned into a light yellow solid and further the resulting samples were calcined in muffle furnace by heating at 1°C /min to 70°C and then held at that temperature for 6 h [14].
Characterization of the ordered mesoporous alumina scaffold
Infrared spectroscopy: FT-IR spectroscopy used to determine the molecular interaction between excipients. Spectral analysis was carried out using FT-IR spectrophotometer (FT-IR 8400-S; Shimadzu; Japan); by the KBr pellet technique. The spectra were recorded at ambient temperature over a wave number range of 4000 to 400 cm-1. The samples were powdered and mixed with dry powdered potassium bromide. The powdered mixture was taken in sampler and scanned in FTIR spectrophotometer. A background scan was acquired before scanning the samples [15].
Scanning electron microscopy (SEM): The morphology of synthesized mesoporous alumina was examined by Scanning Electron Microscopy (SEM; JEOL; 5400; Tokyo; Japan). The samples were loaded on studs and applied fine gold coating with gold for 5 min at 10 mA ion current under a pressure of 0.1 Torr using ion sputter. The coated pellets were scanned and the micrographs were examined [16].
Transmission electron microscopy (TEM): The porous structure of sample was characterised by using Technai G2 20 (Philips; Holland). The sample was prepared on 300 mesh copper grid and scanned using TEM; operated at 200 kV [17].
Powder X-ray Diffraction (XRD): Powder X-ray Diffraction analysis was performed with a D5000 diffractometer (40 kV; 40 mA; Siemens/Bruker) using Cu-Ka radiation (l = 0.154 nm). About 500 mg sample was ground in a mortar and put on silicon sample holders with zero background prior to analysis [18,19].
Mercury porosimetry: The technique involves the intrusion of a non-wetting liquid (often mercury) at high pressure into a material through the use of a porosimeter. The pore size can be determined based on the external pressure needed to force the liquid into a pore against the opposing force of the liquid’s surface tension. The pore size; particle size distribution and surface area were determined by the mercury porosimetry technique by using the Pascal Mercury Porosimeter (Pascal 440; Thermo Electron Corporation; Italy). The intruded mercury volume was referred to the sample mass unit and was plotted versus applied pressure in Mpa. The sample was degassed in an oven at 120°C for 1 h [20].
Loading of telmisartan
The drug loading procedure involved a combination of adsorption equilibrium and solvent evaporation; which was developed in order to enhance the drug loading efficiency. The Telmisartan (TEL) was loaded in the OMA by the Solvent Impregnation Method. Briefly; Telmisartan (TEL) adsorption into Ordered Mesoporous Alumina (OMA) was carried out by soaking the OMA in an acetic acid solution of TEL (60 mg/ml). The mixture was then subjected to orbital shaking incubator. The loading was performed under ambient conditions (about 2°C) in closed containers to prevent evaporation of acetic acid during the loading period. Finally; the mixture was dried at 55°C under vacuum for 24 h [21]. The drug loaded sample was referred to as TEL: OMA.
Compatibility study of the TEL and OMA
After the successful drug loading in the OMA; there was need to determine the compatibility between the drug and the template material i.e. OMA. Molecular interaction between drug (TEL) and OMA was determined by FT-IR spectroscopy. Spectral analysis was carried out using FT-IR spectrophotometer (FT-IR 8400-S; Shimadzu; Japan); using the KBr pellet technique. The spectra were recorded at ambient temperature over a wave number range of 4000 to 400 cm-1. The samples were powdered and mixed with dry powdered potassium bromide. The powdered mixture was taken in sampler and scanned in FTIR spectrophotometer. A background scan was acquired before scanning the samples. The spectrum of the combination (TEL: OMA) was compared with the pure OMA and TEL [15].
Quantification of the drug
The Telmisartan loaded on the OMA was determined both by the UV spectrophotometry and Thermo Gravimetric Analysis (TGA). In UV spectroscopy the 20 mg of the TEL: OMA was solubilised in 100 ml of the phosphate buffer (pH 1.2) and kept under stirring for 24 h; then sonicated for 30 minutes. The sample was filtered through cellulose membrane and the amount of the drug was determined by UV spectrophotometer (UV1650; Shimadzu; Japan). The drug calibration curve was previously prepared in phosphate buffer (pH 1.2) (λmax=296; r=0.999). The Thermo Gravimetric Analysis was carried out by the thermal analyser (Pyris-1 TGA; Perkin Elmer) operating at heating rate 10°C /min and air flow of 30 ml/min [21].
Dissolution study
The drug release from OMA loaded sample was studied by using a USP Type II dissolution apparatus (Electrolab TDT-08L). The release study was carried out under sink conditions by using 900 ml 0.1 N HCl at 37°C at 100 rpm. At predetermined sampling intervals; 5 ml aliquots were taken and filtered through membrane filter. The removed sample was instantly replaced with an equal volume of fresh dissolution medium [22].
Results and Discussion
Characterization of the OMA scaffold
FT-IR study: As depicted in (Figure 1); the broad band at 3400 - 3500 cm-1 was assigned to the stretching vibration of the –OH group bonded to Al cation and peak at 1645 cm-1 corresponds to water of hydration. The shoulder band at 1404 cm-1 corresponds to -NO3 group from HNO3 added for acid treatment; while peak at 450-500 cm-1 is assigned to the bending vibrations of Al-O. The pattern of this bending band is an indication of alumina with gamma phase [15].
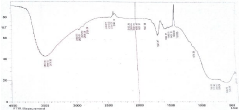
Figure 1: FT-IR spectrum of the OMA.
Scanning electron microscopy (SEM): The morphologies of the samples as depicted in (Figure 2); observed on SEM. It could be seen that all samples were porous in nature. And with smooth surface.

Figure 2: SEM images of OMA.
Transmission electron microscopy: The TEM images of the calcined mesoporous alumina (Figure 3) reveals that the pores of the alumina support have a wormhole or sponge-like appearance which implies the advantage of having a highly inter-connected pore system [23].

Figure 3: TEM images of the Mesoporous Alumina.
Powder XRD of TEL
The main peaks at 2θ; 36.3°; 46.09° and 66.5° are due to γ -Al2O3. It is clear that the resulted catalyst has highly crystalline structure. It also shows nano-size nature as seen from the broadening of the peaks due to the presence of small crystallite sizes (Figure 4).
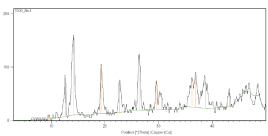
Figure 4: XRD graph of the TEL.
Mercury porosimetry
The porosity of a material affects its physical properties and subsequently its behavior in its surrounding environment. The adsorption; permeability; strength; density; and other factors influenced by a substance’s porosity determine the manner and fashion in which it can be appropriately used. The mercury porosimetry was performed; in which the intruded mercury volume was referred to the sample mass unit and was plotted versus the applied pressure in Mpa. The N2 adsorptions (Figure 5) indicates type IV sorption isotherm according to IUPAC classification; which is given by many mesoporous industrial adsorbents. They exhibit well defined H1 hysteresis loop which are typical for mesoporous materials. The presence of H1 hysteresis type confirms that they have open ended cylindrical mesoporous. The fact that both isotherms have hysteresis loop with sharp branches indicates narrow pore size distribution [24]. The findings of different parameters are as follows. The total cumulative volume and surface area were found to be 1.31cc/g and 299m2/g respectively. The average pore diameter and porosity were found to be 26 nm and 23% respectively. At the same time the particle size distribution was studied as the graph plotted as cumulative volume versus pore diameter as shown in Figure 5 and Figure 6. The clear peak observed was the characteristic of the mesoporous material which was at diameter 7-11 nm.
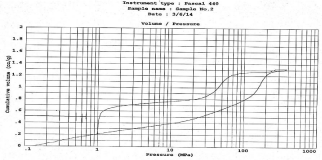
Figure 5: Mercury Intrusion-Extrusion Curve.
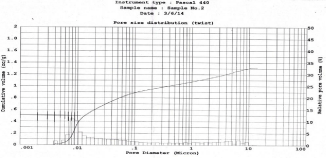
Figure 6: Pore size distribution plot.
Compatibility study of the TEL and OMA
The broad peak of mesoporous alumina from 3200-3600 cm-1 get narrowed due to the major peaks of the Telmisartan OH stretch at 3350 cm-1; the other peaks like 2956 cm-1 of aliphatic C-H stretch; the major peaks at 1359 cm-1 of C-H stretching of COOH; 1473 C-H bend; 1581 C=N stretch was observed. No major changes observed in the peaks of pure drug (TEL) individual and in the mixture (TEL: OMA) as shown in (Figure 7). Hence it can be concluded that TEL and OMA are compatible with each other as no significant interaction between them was observed.
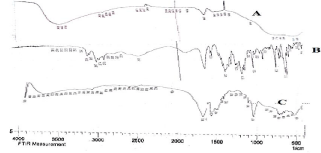
Figure 7: Combined IR spectra of OMA (A); TEL (B) and TEL: OMA(C).
Drug loading quantification
The drug loading by UV method was found to be 45 %. In TGA the TEL: OMA thermal behaviour showed initially a reduction in mass under 100–11°C that could be attributed to the loss of adsorbed humidity. Then there was a gradual reduction in mass between 200 and 40°C that was attributed to initial drug degradation and a third step at 50°C-70°C; there was the steady graph observed which suggests that there was no more effect of temperature on OMA. Hence we can conclude the total degraded amount of TEL loading in OMA was found to be approximately 43% (Figure 8).
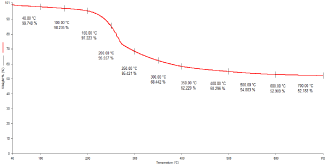
Figure 8: TGA graph of the TEL: OMA complex.
Powder XRD of TEL: OMA
The XRD pattern of the drug loaded sample was recorded to determine whether the crystalline TEL phase was retained as it is in OMA or not. The X-ray diffraction pattern of TEL shows that it was highly crystalline in nature as indicated by the numerous sharp peaks. The six peaks at 6.7; 14.1; 15.0; 19.0; 22.3; 23.8 shows the TEL crystalline structure was absent in the TEL: OMA mixture and hence it may be indication of amorphous form [25] (Figure 9).
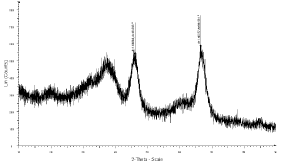
Figure 9: XRD spectra of the TEL: OMA complex.
Dissolution study
Unsatisfactory dissolution rate of poorly soluble drug is major concern in dosage form design and drug delivery. Most important prerequisite in this regard is enhancement of dissolution rate of such drugs in biological fluids. As compared to the crystalline drug; the mesoporous materials can reduce the lattice energy and improve the wettability resulting in enhanced dissolution behavior. On immersion of mesoporous material (loaded with drug) into biological medium; the medium gets penetrated into the mesoporous system driven by capillary forces. Then drug molecules get dissolved in the medium inside the pores followed by diffusion of drug molecules out of the mesoporous owing to concentration gradients. Finally; the drug molecules get diffused and conventionally transported in the release medium [21]. In this context; the term “enhanced dissolution rate” is frequently used typically to refer “enhanced release” with respect to mesoporous carriers. In dissolution study of TEL and TEL: OMA; initial burst like release pattern was observed for TEL from OMA. Such release was observed in the first 15 min and around 39%. After attaining a maximum level of release; the dose maintenance was begin (from 75 min) to release the drug for a remaining study period; obeying the first order release mechanism. Similar phenomenon is reported in previous study [26]. At the end of 2 h; the release of TEL from OMA and pure TEL was up to 81.77 % and 15.68 % respectively. This indicates enhanced dissolution rate of OMA loaded TEL which is attributed to inclusion of the TEL within the pore channels of OMA resulted in an efficient particle size reduction (Figure 10).
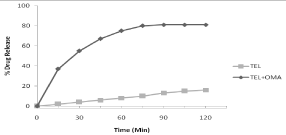
Figure 10: Drug release profile of TEL and TEL: OMA.
Conclusion
In the present study attempt was made to synthesize Ordered Mesoporous Alumina (OMA) with high surface area for loading of model drug Telmisartan (TEL). The TEL was efficiently loaded in synthesized OMA leading to its enhanced dissolution rate as compared to the pure crystalline TEL; confirming the potential of the mesoporous alumina as a platform for drug delivery system. The dissolution improvement was associated with mesoporous alumina converting the loaded drug from the crystalline state to the amorphous form. Since drug release is a crucial and limiting step for drug absorption; particularly for drugs with low solubility and high permeability; mesoporous alumina may offer a larger window of absorption for the drug by improving the dissolution rate of poorly water soluble drugs. These release profiles and drug-loading efficiencies are of great interest for pharmaceutical application in order to improve the delivery of drugs for longer and sustained action as well as to reduce the risk of dose dumping due to multiple doses.
Acknowledgement
The authors are thankful to the Principal, Sanjivani College of Pharmaceutical Education and Research; Kopargaon, for providing the necessary facility to carry out this study. The gift samples from Glennmark India Ltd; Nasik and Hi-media Laboratories; Mumbai are gratefully acknowledged.
References
- Ladan A, Singh G, Singh G, Kimia F. Solid Dispersion: Methods and polymers to increase the solubility of poorly soluble drugs. J Appl Pharm Sci. 2012; 2: 170-175.
- Mandal S. A platform for improving dissolution rate of poorly water soluble drug. Int J Pharm Sci Nanotech. 2011; 3: 1214-1219.
- Nanjwade B, Derkar G, Bechra H, Manvi F. Nanosized technological approaches for the delivery of poorly water soluble drugs. Iran J Pharm Sci. 2010; 6: 149-162.
- Srikanth M, Murali G, Rao N, Sunil S, Balaji S, Ramanamurthy K. Dissolution rate enhancement of poorly soluble bicalutamide using β-cyclodextrin inclusion complexation. Int J Pharm Sci. 2010; 2: 191-198.
- Ramli Z, Chandren S. Effect of templates on the synthesis of organized mesoporous alumina. The Malay J Analy Sci. 2007; 1: 110-116.
- Yuan Q, Duan H, Li L. Homogeneously dispersed ceria nanocatalyst stabilized with ordered mesoporous alumina. Adv Mater. 2011; 22: 1475-1478.
- Whelton P. Epidemiology of hypertension. Lancet 1994;344: 101–106.
- Kearney P, Whelton M, Reynolds K. Global burden of hypertension: analysis of worldwide data. Lancet. 2005; 365: 217–23.
- Gaziano T, Bitton A, Anand S. The global cost of nonoptimal blood pressure. J Hypertens 2009; 27: 1472–1477.
- Cadeddu C, Pira,s A, Mantovani G, Deidda M, Dessì M, Madeddu C, et.al, 2010. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am. Heart J. 2010; 3: 1-7.
- Telmisartan Briefing Document 2009, Cardiovascular and Renal Drugs Advisory Committee Briefing Document (Accessed on 10/10/2013).
- Benson S, Pershadsingh H, Ho C, Chittiboyina A, Desai P, Pravenec M, Qi N. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR -modulating activity. Hypertension 2004; 43: 993–1002.
- Tran P, Tran H, Lee B. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J Control Release. 2008; 129: 59-65.
- Grant S. Polymer templating synthesis, adsorption and structural properties of alumina-based ordered mesoporous materials. Colloids and Surfaces A: Physicochem. Eng. Aspects 2011; 385: 121-125.
- Ramli Z, Saleh R. Preparation of ordered mesoporous alumina particles via simple precipitation method. J. Funda. Sci. 2008; 4: 435-443.
- Hartmann S, Sachse A, Galarneau A. Challenges and strategies in the synthesis of mesoporous alumina powders and hierarchical alumina monoliths. Mat. 2012; 5: 336-349.
- Liu J, Huang F, Liao X, Shi B. Synthesis of thermally stable mesoporous alumina by using bayberry tannin as template in aqueous system. Bull. Korean Chem. Soc. 2013; 34: 2651-2656.
- Harry G. Brittain H. Physical characterization of pharmaceutical solids. Informa Healthcare 1st ed. New York, 1995; 253-280.
- Ishii Y, Nishiwaki Y, Al-zubaidi A, Kawasaki S. Pore Size Determination in Ordered Mesoporous Materials Using Powder X-ray Diffraction. J. Phys. Chem. C. 2013; 117: 18120–18130.
- Giesche H. Mercury Porosimetry: A general practical overview. Part. Part.Syst. Charact. 2006; 23: 1-.
- Zhang Y, Wang J, Bai X, Jiang T, Zhang Q, Wang Y. Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs. Mol. Pharmaceutics 2012; 9: 505-513.
- USFDA. Dissolution methods database.
- Satterfield C. Heterogenous catalysis in practice. Mc Graw Hill, New York. 1980.
- Sing K.. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure & Appl. Chem. 1982; 54: 2201-22l8.
- Yang H, Coombs N, Ozin G. Morphogenesis of shapes and surface patterns in mesoporous silica. Nature. 1997; 386: 692–695.
- Vallet-Regi, M, Ramila A, Del Real R, P, Pérez-Pariente J. A new property of MCM-41: drug delivery system. Chemistry of Materials. 2001; 13: 308–311.