
Review Article
Austin Therapeutics. 2015; 2(2): 1021.
Albumin: A Versatile Drug Carrier
Bairagi U, Mittal P and Mishra B*
Department of Pharmaceutics, Banaras Hindu University, Indian Institute of Technology, India
*Corresponding author: Mishra B, Department of Pharmaceutics, Banaras Hindu University, Indian Institute of Technology, Varanasi-221005, Uttar Pradesh, India
Received: October 20, 2015; Accepted: October 30, 2015 Published: November 17, 2015
Abstract
Albumin is a multifaceted, highly soluble, stable, non toxic, non poisonous, biocompatible and biodegradable plasma protein. Because of its versatile nature, it can be used for the delivery of the drugs, hormones, metals and fatty acids by binding to its specific binding sites. The structure, location, size, charge and hydrophobicity of these drug binding sites are very important to optimize the interaction of drugs with albumin. These findings can be a useful device for the analysis and estimation of drug–drug and drug-carrier interactions or protein binding in various diseased states. Various studies reveled that albumin can be used to increase the circulating half-life and bioavailability of drug molecules which are smaller than the renal filtration threshold and are rapidly lost from the circulation leading to limiting therapeutic potential. This article presents a review of the special features of albumin as a drug carrier, and how the understanding of these features is currently being employed to optimize the circulatory half-life and bioavailability of drugs having the ability to bind/ conjugate/genetically fuse to albumin.
Keywords: Albumin; Binding site; Drug carrier; Drug delivery; Nanoparticles; Cancer therapy
Abbreviations
CBER, USFDA: Center for Biologics Evaluation and Research, United State Food and Drug Administration; HAS: Human Serum Albumin; GLP: Glucagon-Like Peptides; SCFv: Single Chain Antibody; G-CSF: Granulocyte Colony Stimulating Factor; IFNa-2b: Interferon Alpha 2b; EPR: Enhanced Permeability and Retention; NO: Nitric Oxide; SPARC: Secreted Protein, Acidic and Rich in Cysteine; IL-1RA: Interleukin-1 Receptor Antagonist; Anti-EGFR: Anti- Epidermal Growth Factor Receptor; SNO-HAS: S-Nitrosylated Human Serum Albumin
Introduction
Albumin is the main human blood plasma protein which constitutes about 55-60% of all plasma proteins which is synthesized in the liver. It is transported as a single non-glycosylated chain, reaching a blood concentration of about 7.061074M. Its distribution is primarily intravascular [1]. Albumin is a major constituent of the blood in mammalian and avian species, and its structure across these immensely different organisms shows great similarity [2]. The mature albumin protein is constituted by a single polypeptide chain of 585 residues having molecular weight of 66,438Da. Thus, it is a medium sized highly soluble compound which is small enough to pass through the fenestrated endothelium, such as in the nephron. Human Serum Albumin (HSA) undergoes several modifications throughout its life time which could affect its binding and anti-oxidant properties. HSA contains a single Trp residue at position 214. Other components such as Met, Gly, and Ile residues are less in HSA, but Cys, Leu, Glu, and Lys are more abundant. Due to the presence of a large number of ionized residues, HSA has a high total charge (i.e., 215 ions per molecule at pH 7.0), which assist its solubility. Besides these, the acidic amino acid residues are more than the basic ones, resulting in net negative charge per molecule. The disulfide bridges significantly contribute to the stability of HSA and facilitate its long biological lifetime. The secondary structure of HSA is dominated by a-helices (68%), without any β-sheet element. HSA is arranged in a globular heart-shaped conformation containing three homologous domains generally indicated as I (1–195), II (196–383), and III (384–585). The three domains are further divided into sub-domains A and B (Figure 1). These three domains are similar in the amino acid sequence as well as in the secondary and tertiary structure. Exceptionally, the HSA conformation is grossly maintained even in the presence of a wide variety of ligands and is common to the structure of serum albumin of all vertebrates [3]. Albumin is a concentrated solution of protein, which is obtained from healthy donors and is administered intravenously to restore plasma volume depleted by shock, trauma, surgery, and burns. Albumin is marketed as fractionated plasma products (licensed product) under blood and blood products by the Center for Biologics Evaluation and Research (CBER), US FDA (United State Food and Drug administration). Its 5% or 25% solution is supplied in vials having capacity 20mL, 50mL, 250mL or 500mL depending upon the percentage of solution (Table 1).
Albumin (5%, 25%)
Size (mL)
Manufacturer
Albumin
AlbuKed®
AlbuRx®
Albutein®
Buminate
Plasbumin®
50, 250
50,250
50,100,250,500
50,100,250,500
20,50,100,500
50,100,250,500
Octapharma
Kedrion
CSL Behring
Grifols
Baxter Healthcare
Grifols
Table 1: Marketed products of albumin worldwide.
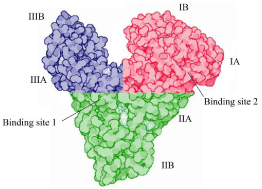
Figure 1: The structure of Human Serum Albumin (HSA).
The three domains of HSA are colored as follows: domain I: red; domain II:
green; domain III: blue.
Advantages of albumin
• Albumin is important for various physiological processes like maintenance of colloidal osmotic pressure, immunomodulation, endothelial stabilization, solubilizing long chain fatty acid, delivery of nutrients to cells, and balancing plasma pH [2].
• It is responsible for the antioxidant property of human serum, either directly or by binding to radical scavengers, or by sequestering transition metal ions with pro-oxidant activity [1].
• Albumin acts as a NO depot and carrier, leading to covalent modification of molecules. Moreover, heme-HSA mutants have been proposed as O2-carriers not only for red blood cell substitutes but also for O2-therapeutic reagents [1,2].
Disadvantages of albumin
In spite of its wide range of properties and endogenous origin, it shows potential for allergic reactions and transmission of infections. Hyper oncotic albumin may cause kidney damage [2].
Special Features of Albumin as a Drug Carrier
Albumin is best known for its remarkable ligand binding capacity. It is a versatile protein with antioxidant, immunomodulatory, detoxifying properties and can act as a potent drug carrier. Albumin can be used as an exogenous or endogenous protein for the treatment of various diseases like cancer, rheumatoid arthritis, diabetes and hepatitis. Albumin-based drug delivery systems include albumindrug nanoparticles, albumin fusion protein, pro-drugs and peptide derivatives that bind covalently to albumin as well as physically bind to the antibody fragments and therapeutically active peptides. During the last decade, the use of albumin has been a source of the debate focusing on whether albumin can act as a better drug carrier for delivery of macro-molecules or not [1,4]. Human albumin has the capacity to bind an extraordinarily diverse range of molecules. Following are the main features which explain its specificity as drug carrier-
• Albumin provides a depository for a wide variety of compounds that may be available in quantities well beyond their bioavailability in plasma. This is feasible because the negative charge of HSA (Human Serum Albumin) facilitates electrostatic binding of various ligands with albumin, acting as a depot and carrier for many drug compounds [1,5].
• Albumin also performs transport functions through the binding sites which are present in its tertiary structure. The substances transported by albumin includes large number of drugs, bilirubin, bile acids, hormones, metals, anions, long-chain fatty acids, L-thyroxine, nitric oxide, endotoxins and other bacterial products such as the protein G-like albumin-binding molecule [5].
• The methodology, selectivity and capacities of ligand binding to albumin are as assorted as the compounds, which has the capacity to bound [2]. The modes of binding include complex formation with metals, hydrophobic and electrostatic interactions and covalent binding. Several binding sites are present on albumin molecules. For fatty acids, seven binding sites have been described [6]. The most eminent sites for drug binding are the site I and site II located on the sub-domains IIA and IIIA, respectively [6,7].
• Complex formation and high-affinity binding are principally reversible, whereas covalent binding to albumin may occur in a reversible or irreversible manner.
The Mechanisms for the Improvement of Half Life of the Therapeutic Compounds by Albumin
Size of albumin is above the renal threshold so it has specific interaction and recycling by the specific receptors, which leads to its long circulatory half-life i.e. of 19 days [2]. Accordingly, to improve the pharmacokinetic profile, various drug molecules had been joined to albumin covalently and non-covalently.
Non-covalent binding of therapeutic molecules to albumin
Albumin reversibly binds to a broad range of endogenous ligands such as fatty acids, bilirubin, bile acids, thyroxine and exogenous ligands such as penicillins, warfarin and diazepam. Binding affects the pharmacokinetic properties of these molecules; generally it enhances their distribution and bioavailability. For example, albumin has several fatty acids binding sites and their binding affinity depends upon the fatty acid chain length. In recent technologies, drug molecules are attached to a fatty acid. On injection, the molecules bind to the endogenous albumin in the blood through its fatty acid binding sites from where it slowly dissociates, hence prolonging its half-life and bioavailability. This technology has been used to enhance the pharmacokinetics of insulin by forming insulin analog Levemir® (insulin detemir) [8-13]. Another examples of application of this technology includes products of GLP-1 (Glucagon-Like Peptides-1) and G-CSF (Granulocyte Colony-Stimulating Factor) [14-18], scFv (Single Chain Antibody), IFNa-2b, IL-1RA (Interleukin-1 receptor antagonist), Fab [8] and Anti-EGFR (Anti Epidermal growth factor receptor) (Table 2).
Mode of interaction
with albumin
Active pharmaceutical
ingredient
Half-life without albumin
Half-life with albumin
Clinical status
Clinical applications
References
Non-covalent binding
Insulin detemir
4-6min
5-7h
Approved
Diabetes
[13]
GLP-1
1.5-2min
11-15h
Approved
Diabetes
[13]
scFv
20min
40h
Preclinical
Tumor targeting
[19]
IFNa-2b
1.2h
22.6h
Preclinical
Hepatitis
[20]
Fab
0.8h
32.4h
Preclinical
Tumor targeting
[8]
Anti-EGFR
1h
44h
Preclinical
Tumor targeting
[21]
IL-1RA
2min
43h
Preclinical
Rheumatoid Arthritis
[20]
G-CSF
2.1h
23.7h
Preclinical
Cancer
[13]
Table 2: Products of albumin formed by non- covalent binding.
Covalent binding of therapeutic molecules to albumin
Recently, therapeutic compounds have also been generated by the covalent attachment of drug molecules to the albumin, either by conjugation, where proteins or small molecules are exogenously joined to albumin by a chemical bond, or by gene fusion where the gene for a protein is engineered to that of albumin and expressed in a suitable host resulting in the production of a single polypeptide. Conjugation of drug molecules to albumin has been used by researchers [9,10] in which small molecules (doxorubicin or methotrexate) are coupled to albumin for the development of anticancer and anti-rheumatic drugs. Products of albumin formed by covalent binding with active pharmaceuticals include GLP-1[13] and Exendin-4 (Table 3) [10].
Mode of interaction
with albumin
Active pharmaceutical
ingredient
Half-life without albumin
Half-life with albumin
Clinical status
Clinical applications
References
Covalent attachment
GLP-1
1.5-2min
9-15days
Phase I completed
Diabetes
[13]
Exendin-4
<1h
8days
2nd Phase II studies completed, a 3rd Phase II terminated
Diabetes
[10]
Table 3: Products of albumin formed by covalent binding.
Genetic joining (bonding) of therapeutic molecules to albumin
A large variety of proteins has been genetically joined to albumin for the production of biologically active molecules with half-life more than their unfused counterparts. The half-life of the albumin fusion is dependent mainly on the nature of the therapeutic protein fused to albumin. The potency of the therapeutic protein may be affected by fusion to albumin, but the same may also be enhanced by the use of linkers between the albumin and the therapeutic protein. This technology has been currently used in the clinical development of– Albiglutide™, Balugrastim, MM-111 and the albumin fusions to the clotting factors FVII and FIX [11]. GLP-1[22], G-CSF [23], FVIIa [23], FIX [24], scFv [8] and IFNa-2b [13] have been joined genetically to albumin to form different products (Table 4).
Mode of interaction with albumin
Active pharmaceutical
ingredient
Half-life without albumin
Half-life with albumin
Clinical status
Clinical applications
References
Genetic fusion
GLP-1
1.5-2min
5days
BLA submitted
Diabetes
[22]
G-CSF
2.5h
18-40h
Phase III completed
Cancer
[8]
F VIIa
39.5-45.6min
262.7min
Phase I completed
Coagulation factor
[23]
F IX
17.1h
92h
Phase III initiated
Coagulation factor
[24]
scF v
3.5h
27.7-30.8h
Preclinical
Tumor targeting
[8]
IFNa-2b
2-3h
140-159h
Phase III completed, BLA withdrawn
Hepatitis
[13]
Table 4: Products formed by genetic bonding.
By modulation of albumin’s affinity to FcRn receptors
The main key to this technology is the optimization of the albumin/ FcRn interaction – producing albumin analogue which have longer and shorter half-life than natural albumin. This approach extends the previous work by researchers in the identification of domain III in this interaction and allows specific molecular interactions to be predicted and tested [12].
Therapeutic Applications
The main technologies behind the use of albumin as a drug carrier are Drug-albumin complex, Drug-albumin conjugate, Drug-albumin nanoparticles, Drug-albumin ligands and antibody conjugates. Drugs, prodrugs or polypeptides can either be bound non-covalently or covalently through a ligand or protein-binding group to HSA. More complex systems are formed by the attachment of numerous targeting ligands and prodrugs to the protein surface, nanobodies, and bispecific antibodies, while nanoparticles, micellar structures, microbubbles with lipophilic drugs and diagnostic agents can be prepared as water-soluble suitable galenic formulations for intravenous injection [13,25-29].
In diabetes
The treatment of juvenile diabetes or advanced type 2 diabetes is done by counterbalancing the lack of insulin production in the body. Preferably, a long-acting form of insulin is used to decrease and normalize the blood glucose level over 24h. The recent technology includes the attachment of a fatty acid to insulin which subsequently binds to 5–7 fatty acid binding sites present in the HSA molecule and eventually enhances the bioavailability of insulin. Novo Nordisk has developed novel insulin analog Levemir® (Figure 2) for the treatment of diabetes in which myristic acid is chemically bound to the lysine amino acid at position B29. Levemir® (insulin detemir) was approved in 2004 for the treatment of diabetes mellitus 1 and 2 and is administered subcutaneously as a water-soluble solution with a prolonged pharmacokinetic profile and bioavailability that makes Levemir® suitable for the better treatment regimen of diabetes with duration of action of approximately 26h. The other option for the control of glucose levels in diabetes includes stimulation of insulin secretion. The peptide hormone GLP-1-(7-37) is produced from selective cleavage of the proglucagon molecule and increases insulin secretion in pancreatic cells, but has a half-life of 1.5– 2 min only due to degradation by enzymes. Due to which it has limited use. By the same technology of Levemir®, GLP-1-(7-37) is derivatized with a fatty acid, palmitic acid, at the e-amino position of lysine introduced at the N-terminal position of glutamic acid in the GLP-1 peptide sequence. The resulting new drug liraglutide (Victoza®) is an albumin-binding derivative of GLP-1 and is stable against metabolic degradation by enzymes due to albumin-binding and has a plasma half-life of 11–15 hours after subcutaneous administration.
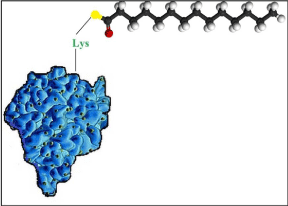
Figure 2: Structure of albumin-binding derivative Levemir® (insulin detemir)
formed by removing an amino acid, threonin, in position B29 and attaching
the fatty acid myristic acid to the introduced amino acid lysine.
The most recent development by Novo Nordisk is insulin degludec (Tresiba®), an advanced product to Levemir®, the difference being that palmitic acid is conjugated through a gamma-L-glutamyl spacer to the amino acid lysine at position B29 of the insulin molecule. Insulin degludec is an ultra-long-acting insulin analog having an extended duration of action that lasts up to 40 hours [13,14].
In hepatitis
Human Genome Science has developed a broadly applicable albumin fusion protein technology in which a therapeutically active protein or peptide can be genetically fused to recombinant human albumin. The lead compound of this technology has Albuferon®. Albuferon® is albumin conjugates for liver targeting. “Albinterferon- a-2b”, a fusion protein of albumin and interferon-a-2b (INFa-2b) which is currently used for the treatment of hepatitis C infection. INFa-2b has a molecular weight of ~19kDa. It has limited use as such and is required to inject frequently (daily or three times weekly). By genetic fusion of recombinant INFa-2b with recombinant HSA, a fusion protein with a molecular weight of 85.7kDa was generated. It was developed by Human Genome Sciences in collaboration with Novartis as a long-acting interferon for the treatment of chronic hepatitis C [15].
In oncology
Unique properties of albumin responsible for the accumulation in solid tumors are as follows:
a) The enhanced permeability and retention of macromolecules (albumin) in passive tumor targeting (EPR effect).
b) Presence of two albumin-binding proteins present on the tumor endothelium (the gp60 receptor) and the SPARC (Secreted Protein, Acidic, and Rich in Cysteine), which is a secreted glycoprotein with high binding affinity to albumin in the tumor interstitium, enhance its retention.
c) Serum albumin level got decreased in cancer patient due to the more demand of amino acids by the proliferating tumor mass, which is fulfilled by digesting albumin. The condition of decreased level of albumin is known as Hypoalbuminaemia. Hypoalbuminaemia is a characteristic feature of patients with advanced solid tumors hence they could especially profit from albumin-based drug delivery systems [16,27].
Abraxane® for treating solid tumors: Abraxane® is an albuminpaclitaxel nanoparticle system and the most advanced drug product of the nab technology developed by American Bioscience. In nab technology; a lipophilic drug and human serum albumin are passed through a jet under high pressure to form nab-paclitaxel nanoparticles with a mean particle size of 130nm. The resulting paclitaxel nanoparticle, Abraxane®, is water-soluble. Abraxane® is stable as a nanoparticle in its galenic formulation and dissolves rapidly after intravenous infusion resulting in soluble albumin-bound paclitaxel complexes having a size comparable to that of endogenous albumin. These albumin-paclitaxel complexes accumulate in the tumor through Enhanced Permeation and Retention (EPR) effect of solid tumors (Figure 3). Further albumin transport pathway mediated by the 60-kDa glycoprotein gp60, also known as albondin, located on the endothelial cell surface seems to be responsible for the tumor uptake and causing the even distribution of Abraxane® and the subsequent release of paclitaxel [17,18,26].
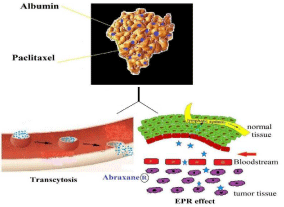
Figure 3: Uptake of albumin-paclitaxel nanoparticles mediated by the EPR
effect and the transcytosis pathway and subsequent binding to the tumor
extracellular matrix.
INNO-206 for sarcoma and gastric cancer (in clinical trials): Felix showed that a maleimide bearing prodrug of doxorubicin was rapidly and selectively bound to cysteine-34 position of endogenous albumin. On the basis of these studies, the acid-sensitive (6-maleimidocaproyl) hydrazone derivative of doxorubicin (DOXOEMCH, renamed INNO-206) is emerged as a clinical candidate due to its high plasma stability in its albumin-bound form. INNO-206 has superior efficacy against sarcoma and gastric cancer with reduced cardiotoxicity [14,28].
In rheumatoid arthritis
A novel antibody-based technology has been advent by the Belgium pharmaceutical company Ablynx. This technology includes the use of albumin-binding nanobodies (VHH), which led to the development of unique formulations that have reached clinical phase II studies for the treatment of rheumatoid arthritis. A trivalent antibody consisting of two anti-tumor necrosis factor-a (TNF-a) nanobodies (TR2) and one albumin-binding nanobody (ARI), named ATN-103 (now Ozoralizumab) with an MW~45 kDa has been successfully developed preclinically. TNF-a is one of the key mediators of the inflammatory response and has recently been used as the molecular target for the development of three approved immunoglobulins namely Enebrel® (etanercept), Remicade® (infliximab), and Humira® (adalimumab), used for the treatment o inflammatory diseases such as rheumatoid arthritis where they are used alone or in combination with methotrexate in latter [14].
In AIDS
Susceptibility of therapeutic peptides to degradation by peptidases, lack of its bioavailability and distribution to the target site, are some of the reasons which limit its use as a therapeutic agent. Recent efforts have concentrated on improving their pharmacokinetic profile; making use of the albumin-binding strategies described previously, i.e. the attachment of a maleimide moiety for covalent binding to albumin or myristic acid for physical binding to the fatty acid binding sites on albumin. This technology has been used for antiviral applications which show that the polarity of the respective peptide influences the efficacy of albumin-binding and the development of albumin-binding peptides enhances their therapeutic action [10].
PC-1505: An albumin-conjugate of an antiviral C34 peptide that inhibits HIV-1 entry: To overcome the drawbacks of currently available treatments for HIV infection, Roche Applied Sciences developed a novel antiretroviral drug marketed under the trade name Fuzeon®. Structurally, it consists of a synthetic peptide that targets gp41, a glycoprotein subunit that remains non-covalently bound to gp120 and facilitates the second step by which HIV enters T-cells and hence helpful in enhancing antiviral efficiency. Since its approval by the FDA in March 2003, Fuzeon® remains the only compound marketed to date that targets the conformational rearrangements of gp41 and thus inhibits HIV entry into uninfected cells [13].
In bacterial infections
Staphylococcus aureus (S. aureus) is a multidrug-resistant bacteria and main causative agent of nosocomial infections (hospital acquired infections). A class of new antibacterial inhibitors cationic peptide FARKGALRQ (nonapeptides) was found to be highly active against Staphylococcus aureus, but their use is often limited due to their susceptibility to degradation and very low bioavailability. A scientist at Diamond Light Source Ltd, Science Division derivatized them with myristic acid to form N-myristoylated nonapeptide RH01 (MyrFARKGALRQ). This technology was termed as SAFETY™. Its in vitro studies showed that nonapeptides bound to albumin were more active against S. aureus [13].
In diagnosis and imaging
An injectable radiopharmaceutical 99mTc aggregated albumin has been used in nuclear medicine for almost 30 years. It is a gamma emitting radionuclide imaging agent consists of a sterile aqueous suspension of 99mTc labeled to human serum albumin particles. It is available in several kits from a number of manufacturer under trade names 99mTc-Albures®, 99mTc-Nanocoll®, 99mTc- Human Serum Albumin®, Tc-99m-Microalbumin® and Technetium-99m Albumin Colloid®. These kits have been used for bone marrow scanning, inflammation scanning, and lung imaging to assess the presence of pulmonary emboli, diagnosis for various indications including cardiac function tests, lymphoscintigraphy, and sentinel node detection in breast cancer, other solid tumors, leg edema and rheumatoid arthritis.
AFL-HSA is a promising fluorescence marker for detecting brain tumors during surgery. It is a fluorescein-labeled albumin conjugate. AFL-HSA is manufactured by Orpegen Pharma (Heidelberg, Germany) by linking 5-([4, 6- dichlorotriazin-2 yl] amino) fluorescein to HSA.
Vasovist® from Beyer Schering, developed by Randy Lauffer and B-22956/1 from Bracco Imaging, are two albumin-binding gadolinium (III)-based MRI contrast agents exploited to acquire high-resolution MRI images with an improved delineation of vessel structure and vascular diseases. Vasovist® is mainly used as a contrast agent for detecting malignant lesions in humans and in preclinical tumor models [13].
Albumin as a carrier for Nitric Oxide (NO)
Nitric Oxide (NO) is involved in multiple cellular functions as a diffusible molecular messenger including vascular smooth muscle relaxation, inhibition of platelet aggregation, effects on neurotransmission, and regulation of immune function. Defects in NO production can lead to several diseases such as cardiovascular abnormalities, stroke, and malignancies. Such complications can be treated by replacing or supplementing endogenous NO production by the exogenous administration. But NO therapy is limited by its short half-life in vivo (~0.1 s), a lack of NO selectivity, limited bioavailability, and too much NO can induce apoptosis and cellular damage. So, there is a need to develop reliable NO donors with better pharmacological and pharmacokinetic properties to overcome these challenges. In previous findings, the use of NO-traffic proteins has been examined in several experiments as a promising approach to achieve this goal. A suitable NO traffic protein should have i) high efficiency of S-nitrosylation, ii) a high stability of the S-nitroso form in the circulation and iii) a high efficiency of S-Transnitrosation into cells which need NO. Albumin has received broad attention because it is the most abundant plasma protein and because NO binds to the cysteine 34 position of albumin forming S-Nitrosylated Human Serum Albumin (SNO-HSA). In addition, SNO-HSA has been reported to be more stable than low molecular weight S-nitrosothiols, and therefore, it has the potential to act as a circulating endogenous reservoir of NO and hence as an NO donor in therapeutic applications [9].
Conclusion and Perspectives
Albumin is turning out as one of the most important drug carrier for therapeutically active drugs, peptides, and antibodies. It is used especially for the treatment and diagnosis of malignant, inflammatory, metabolic and viral diseases. The elucidation of binding of serum albumin to FcRn receptors that control its half-life is a key point in designing of albumin-based therapeutic or diagnostic agents for optimizing their pharmacokinetics and drug targeting properties. The high abundance, versatility, stability, multiple binding sites, and very long half-life of serum albumin make it an ideal endogenous serum protein for improving the pharmacokinetic properties of therapeutically active drugs, peptides or small-sized antibody moieties. Considering the commercial success of products that use albumin as a drug carrier and the ongoing clinical trials as well as due to the advent of many diverse technologies for improving the pharmacokinetic profile and drug targeting potential of therapeutic and diagnostic peptides, albumin is attracting the interest of researchers and also the pharmaceutical and biotechnological companies. It is likely that the pharmaceutical, clinical and commercial use of albumin will be fully explored in the coming decade and the field will be expanded to further indications other than those discussed above.
References
- Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, et al. The extraordinary ligand binding properties of human serum albumin. IUBMB Life. 2005; 57: 787-796.
- Theodore P Jr. All about albumin. Amsterdam, Elsevier. 1995.
- Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012; 33: 209-290.
- Cistola DP, Small DM. Fatty acid distribution in systems modeling the normal and diabetic human circulation. A 13C nuclear magnetic resonance study. J Clin Invest. 1991; 87: 1431-1441.
- Lejon S, Frick IM, Björck L, Wikström M, Svensson S. Crystal structure and biological implications of a bacterial albumin binding module in complex with human serum albumin. J Biol Chem. 2004; 279: 42924-42928.
- Simard JR, Zunszain PA, Hamilton JA, Curry S. Location of high and low affinity fatty acid binding sites on human serum albumin revealed by NMR drug-competition analysis. J Mol Biol. 2006; 361: 336-351.
- Sudlow G, Birkett DJ, Wade DN. Further characterization of specific drug binding sites on human serum albumin. Mol Pharmacol. 1976; 12: 1052-1061.
- Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013; 1830: 5526-5534.
- Kratz F, Elsadek B. Clinical impact of serum proteins on drug delivery. J Control Release. 2012; 161: 429-445.
- Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008; 132: 171-183.
- Wang W, Ou Y, Shi Y. AlbuBNP, a recombinant B-type natriuretic peptide and human serum albumin fusion hormone, as a long-term therapy of congestive heart failure. Pharm Res. 2004; 21: 2105-2111.
- Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006; 45: 4983-4990.
- Elsadek B, Kratz F. Impact of albumin on drug delivery--new applications on the horizon. J Control Release. 2012; 157: 4-28.
- Kratz F. A clinical update of using albumin as a drug vehicle - a commentary. J Control Release. 2014; 190: 331-336.
- Fiume L, Di Stefano G, Busi C, Mattioli A, Bonino F, Torrani-Cerenzia M, et al. Liver targeting of antiviral nucleoside analogues through the asialoglycoprotein receptor. J Viral Hepat. 1997; 4: 363-370.
- Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006; 14: 999-1011.
- Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009; 27: 3611-3619.
- Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol. 1992; 262: H246-254.
- Sabrina T, Christoph D, Katharina F, Alessandra V, Fabian B, Dario N. New strategy for the extension of the serum half-life of antibody fragments, Bioconjug. Chem. 2009; 20: 2286–2292.
- Holt LJ, Basran A, Jones K, Chorlton J, Jespers LS, Brewis ND, et al. Anti-serum albumin domain antibodies for extending the half-lives of short lived drugs. Protein Eng Des Sel. 2008; 21: 283-288.
- Roovers RC, Laeremans T, Huang L, De Taeye S, Verkleij AJ, Revets H, et al. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Nanobodies. Cancer Immunol Immunother. 2007; 56: 303-317.
- Julio R, Jane R, Mark B, Fred Y, Murray S. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009; 32: 1880-1886.
- Weimer T, Wormsbächer W, Kronthaler U, Lang W, Liebing U, Schulte S. Prolonged in-vivo half-life of factor VIIa by fusion to albumin. Thromb Haemost. 2008; 99: 659-667.
- Elena S, Claude N, Robert K, Andreas T, Ingrid PF, Christine V, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood. 2012; 120: 2405-2411.
- Merlot AM, Kalinowski DS, Kovacevic Z, Jansson PJ, Lane DJ, Huang ML, et al. Making a case for albumin – a highly promising drug-delivery system. Future Med Chem. 2015; 7: 553-556.
- Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006; 7: 1041-1053.
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008; 14: 1310-1316.
- Abu Ajaj K, Graeser R, Fichtner I, Kratz F. In vitro and in vivo study of an albumin-binding prodrug of doxorubicin that is cleaved by cathepsin B. Cancer Chemother Pharmacol. 2009; 64: 413-418.
- Neumann E, Frei E, Funk D, Becker MD, Schrenk HH, Müller-Ladner U, et al. Native albumin for targeted drug delivery. Expert Opin Drug Deliv. 2010; 7: 915-925.