
Rapid Communication
Austin Therapeutics. 2016; 3(1): 1025.
The High Phagocytic Activity of Macrophages against Blood Stream Isolates of Enterococci
Jarzembowski T¹*, Daca A², Witkowski JM³, Bry¹ E2, Rutkowski B4, Świeć K¹
¹Department of Microbiology, Medical University of Gdansk, Gdansk, Poland
²Department of Pathology and Experimental Rheumatology, Medical University of Gdansk, Poland
³Department of Pathophysiology, Medical University of Gdansk, Gdansk, Poland
4Department of Nephrology, Transplantology and Internal Diseases, Medical University of Gdansk, Poland
*Corresponding author: Tomasz Jarzembowski, Department of Microbiology, Medical University of Gdansk, Gdansk, Poland
Received: February 29, 2016; Accepted: May 19, 2016; Published: May 25, 2016
Abstract
The incidence of serious enterococcal infections is increasing while its pathogenesis remains not sufficiently understood. This study aimed to evaluate the resistance of blood strains and commensal isolates of enterococci to phagocytosis in-vitro.
Enterococcal strains were isolated from blood of patients hospitalized in Invasive Medicine Centre of the Medical University of Gdansk and from faeces of healthy volunteers. The strains were cultured in BHI medium in 37°C for 24h (planktonic form) and 72h (biofilm form cultured in 6-wells culture plates). Bacterial cells were dispersed, stained with fluorescent reporters (SYTO9, PI) and incubated with phagocytes. Macrophage phagocytosis was studied using THP-1 derived macrophages (activation with 6uM PMA). Phagocytosis level was assessed using microscope automated system - Pansy’s 3000 and analysed with Cell Profiler 2.1.1 software which yielded the number of phagocytosed bacteria per phagocytic cell (load index, LI). Phagocytosis maintained by macrophages was more effective against planktonic cells of blood stream isolates (LI 0,55) than against commensal strains or biofilm released cells (LI 0,29 and 0,19 respectively). The results demonstrated differences in phagocytosis susceptibility of blood stream and commensal enterococcal isolates and proved that the macrophages may eradicate bacterial biofilm.
Keywords: Enterococcal; Macrophages; Phagocytic cell
Introduction
Macrophages play an important role in host defense against infection and contribute to the initial inflammatory response [1,2]. They combine phagocytosis, antigen processing and secretion of pro and anti-inflammatory cytokines [3]. However, survival within macrophages despite phagocytosis may increase spread of enterococci and cause systemic infection. Enterococcal infections are believed to be mainly endogenous [4]. The fact that no differences between intestinal and blood isolates has been found by PFGE (Pulsed- Field Gel Electrophoresis) supports this opinion [5]. Shay et al. [6] proved that, amongst 11 paired stools and blood VRE isolates, 8 were identical. Similarly, Montecalvo et al. [7] found closely related stool and blood isolates in 3 patients during an outbreak in an oncology ward. On the other hand, since 2005, Willems at al. [8] and Leavis’ team [9] proposed the identification of high risk clonal complex of Enterococcus faecium, indicating the existence of at least two different genetic lineages of these bacteria.
So far, little is known also about how the host immune system responds during invasive enterococcal infections [10], especially how the bacterial biofilm affect the host defense mechanisms [11]. Due to the complexity of the biofilm matrix, interaction of biofilm with the host immune system may involve completely different mechanisms when compared to their planktonic counterpart. Microbial communities encased within a complex matrix biofilm develop on natural body surfaces such as epithelium, lung and heart as well as implanted medical devices such as central venous and urinary catheters, intra-uterine devices, and prosthetic heart valves [10]. In our previous study, significant differences in molecular properties between enterococcal isolates from different sites of infection have also been proved [12]. Additionally, the differences between commensal and RTx (renal transplantation) patients’ enterococcal isolates include susceptibility to phagocytosis [13]. The current study aimed to compare macrophages’ activity against intestinal and blood stream isolates of enterococci, considering biofilm and planctonic form.
Materials and Methods
Nine blood stream isolates were collected from patients hospitalized at Medical University of Gdansk. As a reference group, 10 enterococcal strains of E. faecalis were isolated from healthy volunteers from Gdansk region. The isolates were identified to species level by strep ID test (BioMerieux) and classified as different strains of E. faecalis by biochemical and resistance profiles. All bacterial strains were stored at (-70°C) in Brain Heart Infusion (BHI) broth with 25% (vol/vol) glycerol. Biofilms of these strains were obtained by culturing at 37°C on flat-bottom wells (TRP, Switzerland) for 72h in BHI medium. After another 28h, medium was replaced with fresh 2ml of BHI. Bacterial cells were dispersed, permeabilized by sonication on ice and stained with fluorescent reporter (PI). Fluorescence of bacteria was standardized by measure of FL3 signal by using a FAC Scan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, and USA).
Figure 1: Fluorescence of macrophages as a result of phagocytosis of PI
stained enterococci.
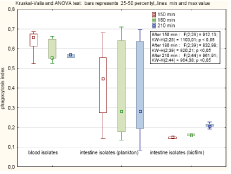
Figure 2: Effectives of phagocytosis of strain of different origin.
Macrophages’ phagocytosis was studied using THP-1 derived macrophages (activation with 6uM PMA). The cells were cultured in RPMI-1640 medium supplemented with 2mM L-glutamine, 100U/ml penicillin, 100μg/ml streptomycin and 10% (vol/vol) heat-inactivated Foetal Bovine Serum (FBS) (all from Sigma-Aldrich). Phagocytosis level was assessed using microscope automated system (Pansy’s 3000, Pan Biotech GmbH, Germany) and analysed with Cell Profiler 2.1.1 software (Broad Institute, Cambridge, Great Britain) which yielded the number of phagocytised bacteria per phagocytic cell (load index, LI). The phagocytosis was analysed after 150,180 and 210 min of incubation, the differences were tested by Analysis of Variance (ANOVA) by Stat Soft software (Statistica 10, USA).
Results
During the observation period the number of macrophages which succeed in phagocytosis almost triplicaded (from geomean 29 up to 84). Phagocytosis of PI stained bacteria result in fluorescence of macrophages since 0, 08 to 0, 7 FU in relation to number of phagocytised bacteria. Output of phagocytosis differed significantly depending on the origin of the strains. The highest ratio was observed for blood stream isolates. The mean value of 0, 549 was constant in time. The commensal strains were phagocytosed at the mean value of 0, 28, and the load index decreased from 0, 35 at 150 min to 0, 27 in 210 min while the number of macrophages with engulfed bacteria increased. In contrast to this observations, the phagocytosis of biofilm cells increased in time (from 0, 15 to 0, 21) with the mean of 0, 19.
Discussion
One may expect that the host defense system can discriminate between pathogens and commensal, so colonization with bacteria does not necessarily elicit an immune response [14]. For example, the reaction of neutrophils differs in relation to the origin of the strain [13]. The differences in virulence between enterococcal strains results from many factors. In previous study we have observed significant difference in biofilm composition and metabolic activity of biofilm formed by commensal and virulent strains [15].
Until recently it was assumed that, biofilm cells resist phagocytosis by immune cells [10] and formation of biofilm protect bacteria from being eradicated. In fact the resistance of enterococci to phagocytosis was described many years ago and the role of some of the virulence traits in this phenomenon was proved [16]. The previous study had shown that Enterococcus faecalis recovered from biofilm better survive inside monocytes in-vitro than their planktonic counterparts [17]. Additionally, monocytes exposed on biofilm produce lower amounts of proinflammatory cytokines compared to monocytes infected with planktonic bacteria [10,12]. However, Leidet al. [18] documented penetration in an in-vitro study of freshly isolated human leukocytes into Staphylococcus aureus biofilms, under static and flowing fluid conditions that mimic physiological shear. Leukocytes also penetrated 7-day-old S. aureus biofilms under laminar-shear conditions. Furthermore, studies of Daw and others [10], comparing the uptake of E. faecalis biofilm cells to their planktonic counterparts, revealed that biofilm cells may be taken up efficiently by immune cells such as macrophages and dendritic cells. On the other hand that survival of enterococcal cells within macrophages is common and lets them spread and create distant site of infection [16]. The classic forms of the activation of macrophages results in generation of large amounts of reactive oxygen species and inflammatory cytokines, which all serve to augment killing of microbes and local cell-mediated immune response. However, it should be noted that all markers of monocytes/macrophages activation in response to bacteria invasion reported above, were based primarily on monocytes’ interaction with freely suspended cells or their components, which may not reflect true monocytes response to biofilm-bound bacteria.
In our study, we observed high phagocytosis of bloodstream isolates. If we follow the assumption that phagocytes can serve as ‘vehicles’ allowing them to spread throughout the body [19] we may explain why strain with the strongest attraction to macrophage can cause sepsis. From this point of view, low phagocytosis index of the enterococcal strain seems to be more profitable for the host.
Conclusion
The difference in phagocytosis mediated by macrophages was also related to the biological form of strain. As observed by Daw and others [10], biofilm released cells were phagocytosed by macrophages, but in contrast to previous observation, the index was definitely lower than for planctonic cells. Also the time needed for efficient phagocytosis is longer while compared with planctonic cells. However one should notice that our observation regard commensal strains while Daw and others studied UTI etiological agent. We may put the hypothesis that the resistance to phagocytosis is an element of adaptation of the commensal strains to colonize the human body.
References
- Topley N, Mackenzie RK, Williams JD. Macrophages and mesothelial cells in bacterial peritonitis. Immunobiology. 1996; 195: 563-573.
- Broche F, Tellado JM. Defense mechanisms of the peritoneal cavity. Curr Opin Crit Care. 2001; 7: 105-116.
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008; 112: 935-945.
- Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009; 155: 1749-1757.
- Olivier CN, Blake RK, Steed LL, Salgado CD. Risk of vancomycin-resistant Enterococcus (VRE) bloodstream infection among patients colonized with VRE. Infect Control Hosp Epidemiol. 2008; 29: 404-409.
- Shay DK, Maloney SA, Montecalvo M, Banerjee S, Wormser GP, Arduino MJ, et al. Epidemiology and mortality risk of vancomycin-resistant enterococcal bloodstream infections. J Infect Dis. 1995; 172: 993-1000.
- Montecalvo MA, Horowitz H, Gedris C, Carbonaro C, Tenover FC, Issah A, et al. Outbreak of vancomycin, ampicillin and aminoglycoside-resistant Enterococcus faecium bacteremia in an adult oncology unit. Antimicrob Agents Chemother. 1994; 38: 1363-1367.
- Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005; 11: 821-828.
- Leavis HL, Bonten MJ, Willems RJ. Identification of high-risk enterococcal clonal complexes: global dispersion and antibiotic resistance. Curr Opin Microbiol. 2006; 9: 454-460.
- Daw K, Baghdayan AS, Awasthi S, Shankar N. Biofilm and planktonic Enterococcus faecalis elicit different responses from host phagocytes in vitro. FEMS Immunol Med Microbiol. 2012; 65: 270-282.
- Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008; 100: 1-18.
- Daca A, Jarzembowski T, Witkowski JM, Bryl E, Rutkowski B, et al. Prophages in enterococcal isolates from renal transplant recipients: renal failure etiologies promote selection of strains. Biomed Res Int. 2014; 514689.
- Jarzembowski T, Daca A, Witkowski JM, Bryl E, Rutkowski B. The high PMNs phagocytosis resistance of enterococcal isolates from RTx patients. Biomed Res Int. 2015; 2015: 432579.
- Hirschfeld J. Dynamic interactions of neutrophils and biofilms. J Oral Microbiol. 2014; 6: 26102.
- Meissner W, Jarzembowski TA, Rzyska H, Botelho C, Pałubicka A. Low metabolic activity of biofilm formed by Enterococcus faecalis isolated from healthy humans and wild mallards (Anas platyrhynchos). Ann Microbiol. 2013; 63: 1477-1482.
- Süssmuth SD, Silberhorn AM, Wirth R, Susa M, Marre R, Rozdzinski E. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect Immun. 2000; 68: 4900-4906.
- Mathew S, Yaw-Chyn L, Kishen A. Immunogenic potential of Enterococcus faecalis biofilm under simulated growth conditions. J Endod. 2010; 36: 832- 836.
- Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002; 70: 6339-6345.
- Wells CL, VandeWesterlo EM, Jechorek RP, Erlandsen SL. Effect of hypoxia on enterocyte endocytosis of enteric bacteria. Crit Care Med. 1996; 24: 985- 991.