
Review Article
Austin Therapeutics. 2016; 3(1): 1027.
Scaffolds: Porous Scaffold for Modulated Drug Delivery
Pande V, Kharde A, Bhawar P*and Abhale V
Department of Pharmaceutics (PG), Sanjivani College of Pharmaceutical Education and Research, India
*Corresponding author: Pramila Bhawar, Department of Pharmaceutics (PG), Sanjivani College of Pharmaceutical Education and Research, Kopargaon, India
Received: April 16, 2016; Accepted: June 27, 2016; Published: July 01, 2016
Abstract
Scaffolds are counterfeit extracellular networks which are fit for supporting cell development and three-dimensional tissue arrangement, in this way being imperative segments for tissue building. Scaffolds are embeds or infuses, which are utilized to convey cells, medications, and qualities into the body. Different types of polymeric scaffold for cell/drug delivery are accessible: a run of the mill three-dimensional permeable lattice, a nanofibrous scaffold, a thermo touchy sol-gel move hydro gel, and a permeable microsphere. Scaffolds are utilized for medication delivery as a part of tissue designing’s as this structure is exceptionally permeable to permit tissue development. A scaffold gives a suitable substrate to cell connection, cell multiplication, separated capacity, and cell movement. Scaffold lattices can be utilized to accomplish drug delivery with high stacking and productivity to particular destinations. Scaffolds can be prepared by no of techniques like solvent extraction, freeze drying, freeze extraction, freeze gelation, powder compaction, phase inversion and so on polymeric scaffolds also has to full fill ideal criteria for drug delivery system like strength loading capacity etc. Scaffolds are prepared from different types of biodegradable polymer and bioceramics.
Keywords: Scaffold; Tissue engineering; Drug delivery system
Introduction
Scaffolds are counterfeit extracellular lattices which are equipped for supporting cell development and three-dimensional tissue arrangement, in this way being critical parts for tissue engineering [1]. Scaffolds are utilized to convey cells, drugs and qualities into the body which are either embed or infuse. Different types of polymeric scaffolds for cell and medication delivery are available: [1] an typical three-dimensional permeable lattice, [2] a nanofibrous network, [3] a thermo sensitive sol-gel transition hydrogel, and [4] a permeable microsphere. Scaffold is utilized for medication delivery as a part of tissue building’s as this structure is highly permeable to permit tissue growth [2]. A scaffold gives a suitable substrate for cell connection, cell multiplication, separated capacity and cell migration. Scaffold networks can be utilized to accomplish drug delivery with high stacking and efficiency to particular site.
Scaffolds have been created to enhance bone in growth and recovery in the treatment of bone deformities. To meet the challenges of recovering bone lost to ailment or injury, biodegradable scaffolds are being explored as an approach to recover bone without the requirement for an auto-or allograft [3]. Because of the poor blood circulation in the rigid deformity locales, a level of medications, for example, anti-toxins, antimicrobials, and development components, should be supplied to the affected region. Keeping in mind the end goal to be successful as a DDS, the carrier needs to full fill the prerequisites of security, more prominent viability, predictable therapeutic response, and controlled and delayed discharge period. A few bearers have been created to exemplify medications, for example, biodegradable polymers (engineered or common) and bioactive earthenware production, as particulates, films, and permeable lattice [4-7]. Among those, Hydroxyapatite (HA) scaffold has improved enthusiasm as a medication conveyance transporter because of its osteoconductivity and biocompatibility [4-7].
The conventional techniques for supplying a patient with pharmacologic dynamic substances experience the ill effects of being inadequately specific, so harm can jumps to the healthy tissues and organs, unique in relation to the proposed target. Also, high medication dosages can be required to accomplish the wanted effect. Hence highly permeable scaffold is an ideal medication delivery system [8]. Due to excellent biocompatibility with living body and bioactivity, Calcium Phosphate (CaP) earthenware scaffold are generally utilized as biomedical insert materials [9]. Biomaterials utilized for preparation of scaffold might be regular polymers such as alginate, proteins, collagens, gelatin, fibrins, and egg whites, or manufactured polymers such as polyvinyl liquor and polyglycolide. Bio-earthenware production, for example, hydroxyl-apatite and tricalcium- phosphates additionally are utilized. Strategies utilized for manufacture of a scaffold incorporate particulate leaching, solidify drying, supercritical liquid innovation, thermally impelled stage separation, rapid prototyping, powder compaction, sol-gel, and liquefy forming. These procedures allow the planning of permeable structures with conventional porosity.
In biomedical exploration, readiness of permeable scaffolds from cutting edge biomaterial for healing bone imperfections speaks to another methodology for tissue engineering. Tissue building is an interdisciplinary and multidisciplinary field that goes for the advancement of organic substitutes that restore, keep up, or enhance tissue function [9]. Scaffold are utilized effectively as a part of different fields of tissue designing, for example, bone arrangement, periodontal recovery, repair of nasal and auricular mutations, ligament improvement, as fake corneas, as heart valves, in tendon repair , in ligament substitution, and in tumours. They additionally are utilized as a part of joint pain aggravation, diabetes, coronary illness, osteochondrogenesis, and twisted dressings. Their application recently has stretched out to conveyance of medications and hereditary materials, including plasmid DNA, at a controlled rate over a long period of time [10].
Types of scaffold
a) Highly porous well interconnected pore structure
b) Nanofibrous matrix prepared by electro spinning
c) Injectables matrices such as hydrogel
d) Porous microspheres [2] (Figure 1)
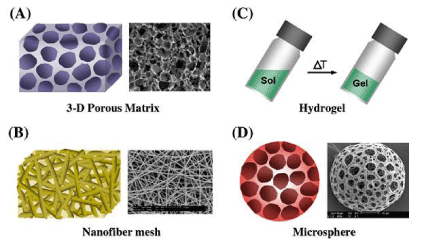
Figure 1: Different forms of polymeric scaffolds for cell/drug/gene delivery:
three-dimensional porous matrix (A) 3-d porous matrix (B) hydrogel (C)
nanofibre mesh (D) microspheres.
Material used for Scaffold Preparation
The material of choice for the preparation of matrices and scaffolds for cells growth varies widely depending on their application [11] number of biodegradable polymeric scaffolds was prepared by using a combination of natural (collagen) and synthetic (Poly-Caprolactone) (PCL) polymers in different compositions. These scaffolds were soft, spongy, porous and transparent in nature. The ideal polymer should be:
a) Biodegradable
b) Biocompatible
c) Highly cell adhesive
d) Porous, mechanically stable 3-D structure [2]
A number of different categories of biomaterials are generally used as scaffold for cell and drug delivery.
Natural polymers
Natural polymers contains alginate, proteins, collagens, gelatin, fibrins, egg whites, elsinan, pectin (pectinic corrosive), galactan, curdlan, gellan, levan, emulsan, dextran, pullulan, gluten, elastin, fibroin, hyarulonic corrosive, cellulose, starch, chitosan (chitin), scleroglucan, heparin, silk, chondroitin 6-sulfate, and polyhydroxyalkanoates. They can be utilized as biomaterials for medication/cell/quality conveyance purposes. Points of interest of characteristic polymers incorporate their biocompatibility, commercial accessibility, simple preparing, and they all the more nearly mimic the normal ECM of tissues; notwithstanding, impediments are short supply, cost, clump to group variety, and vulnerability to cross-contamination [12].
Synthetic polymers
Synthetic polymers are mainly divided into two categories: biodegradable and non biodegradables. Biodegradable polymers are polyglycolide, polylactide and its copolymer
Poly(lactide-co-glycolide), polyphosphazene, polyanhydride, Poly(propylene fumarate), polycyanoacrylate, polycaprolactone, polydioxanone, and polyurethanes Non-biodegradable.
Polymers include polyvinyl alcohol, polyhydroxyethymethacrylate, and Poly(N-isopropylacrylamide). Advantages of this scaffold are easily controlled physicochemical properties and quality, no immunogenicity, processing with various techniques, and continuous supply of large quantities.
Bioceramics
Liquefying inorganic crude materials to set up a amorphous or crystalline strong body is known as bioceramics, and these permeable last items are utilized primarily for scaffolds. Bioceramics named [1] nonresorbable (generally dormant), for instance alumina, zirconia, and silicon nitride; and [2] bioactive or surface dynamic (semiidle), for instance glass earthenware production, for example, thick hydroxyapatites [9CaO•Ca(OH)2•3P2O5], and biodegradable or resorbable (non inert, for example, calcium phosphates, aluminum calcium phosphates, coralline, tricalcium phosphates (3CaO•P2O5), zinc calcium phosphorus oxides, zinc sulfate calcium phosphates, ferric calcium phosphorus oxides, and calcium aluminates [13].
Fabrication Techniques
In the body, cells and tissue are organized into three-dimensional architecture. To engineer these functional tissue and organs, scaffolds have to be fabricated by different technology to facilitate the cell distribution and guide their growth into three-dimensional space. The main methodology for scaffolds fabrication are mentioned below [13],
- Solvent casting/Particulate-leaching techniques [14]
- Gas foaming
- Phase separation
- Electrospinning
- Porogen leaching
- Fibre mesh
- Fibre bonding
- Self assembly
- Rapid Prototyping (RP)
- Sol-gel technique
- Melt molding
- Membrane lamination
- Freeze drying [14]
- Freeze extraction
- Freeze gelation [15]
- Supercritical assisted phase-inversion process [15]
Solvent-casting and particulate leaching technique
Solvent casting and particulate draining is a straightforward and most commonally utilized technique for manufacturing frameworks for tissue designing. This technique includes blending water of dissolvable salt (e.g. sodium chloride, sodium citrate) particles into a biodegradable polymer arrangement. The blend is then cast into the mold of the desirable shape. After the dissolvable is evacuated by vanishing or lyophilisation, the salt particles are filtered out to acquire a permeable structure. This strategy has points of interest of straightforward operation, sufficient control of pore size and porosity by salt/polymer proportion and molecule size of the additional salt .used this technique to create biodegradable polymer platforms to build trabecular bone. In any case, the pore shape is constrained to the cubic crystal shape of the salt. The trouble of expelling solvent particles from the inside of a polymer framework makes it difficult to create thick 3D platforms. Truth be told, the majority of the permeable materials arranged by dissolvable throwing and particulate draining technique are constrained to thickness running from 0.5 to 2mm. What’s more, their restricted between pore network is disadvantageous for uniform cell seeding and tissue development. Broken PLGA/salt composite materials into little pieces and pressure shaped them into thicker specimens and afterward disintegrated the salt to produce required platforms for bone tissue designing studies in bioreactors. In any case, cell development and mineralization were constrained to the outside of the frameworks, which was credited to restricted interior supplement transport conditions by the specialists. Our gathering built up a strategy to make biodegradable polymer platforms with round pore shape and well controlled interpose network. Paraffin circles are picked as pore-creating materials. The made new framework has a homogeneous froth skeleton. The control of porosity and the pore size can be accomplished by changing the grouping of the polymer arrangement, the quantity of the throwing steps, and the span of the paraffin circles. The principle point of preference of this strategy is that it can guarantee the production of a completely interconnected pore system in the polymer platform, which is basic to uniform cell seeding, tissue in growth, and recovery. Also, the paraffin circle get together can be broken down in some natural solvents however not water. Thusly, certain water-dissolvable polymers can be included in creating such platforms. Be that as it may, what is the perfect pore size and inter pore network of such platforms for bone tissue designing is yet to be researched.
Gas-foaming process
Gas frothing procedure can be utilized to create exceptionally permeable polymer froths without the utilization of organic solvents. In this methodology, carbon dioxide (CO2) is generally utilized as a specialists for the development of polymer froth. Strong polymer circles are presented to high weight CO2 to allow saturation of CO2 in the polymer. Thermodynamic unsteadiness is then made by quickly discharging CO2 gas from the polymer framework, trailed by the nucleation and development of gas rises in the material. Polymer sponges with a pore size of 100¹m and porosity up to 93% can be created utilizing this method. The weakness of this strategy is that it yields generally a nonporous surface and shut pore structure, with just 10-30% of interconnected pores The porosity and interpose availability can be fundamentally enhanced by joining particulate filtering procedure with the gas-frothing procedure albeit totally disposing of shut pores remains challenging [16].
Electrospinning
The electrospinning procedure for the frameworks planning uses the electrostatic power for the creation of polymeric fibre extending from nanoscale to micro scale. This procedure is control by high power electric field between two anodes having electric charges of inverse extremity. One terminal is put in the polymer arrangement and other is put in gatherer. For the most part polymer arrangement is pumped as result in framing a drop of arrangement. A short time later, electric field is created, which plans to deliver a power, because of this the beads results to defeat the surface strain of the arrangement. A plane of polymer is shot out, which delivers the filaments, same moment the dissolvable begins dissipating because of plane arrangement and proceeds after the nanofibers are saved to gatherer. More than 200 polymers are utilized for electrospinning like silk fibroin, collagen, chitosan, and gelatin and so on. In the field of tissue building electrospinning strategy is connected for the arrangement of nanofiber platform plan. The procedure is exceptionally flexible as far as utilization of polymers, non-intrusive and does not require the utilization of coagulation science or high temperature for fiber era. Essentially, in this procedure a high voltage is utilized to make an electrically charged plane of polymer arrangement or melt, which frames polymer fiber in the wake of drying or cementing. One of the fundamental favorable circumstances of this method is that it can create the framework with primary auxiliary element suitable for development of the cell and ensuing tissue association. It can create the ultra fine fibers with uncommon introduction, high perspective proportion, high surface zone, and having control over pore geometry. These qualities are good for better cell development for in- vitro and in-vivo in light of the fact that they straightforwardly impact the cell bond, cell expression, and transportation of oxygen, supplements to the cells. This gives spatial environment to the development of new tissue with proper physiological capacities. Cell seeding is the primary issue of electrospining innovation. This is overcome by conciliatory biopolymer or cryospinning, which permits making the gap of sought size in electrospun lattices.
Porogen leaching
Porogen leaching is a standout amongst the most widely recognized strategies utilized for arrangement of platforms with controlled porosity. The particulate filtering technique is completely based upon the dispersion of Porogen either in fluid particulates or powdered materials by the procedure of vanishing, cross connecting or other response fluid might be cemented. These porogens go about as spot holder for pore and interconnection of the pores in the real frameworks creation strategy. Profoundly permeable framework with porosity up to 93% and pore width up to 500 micrometers can be set up by utilizing this system. Fundamental goal of this procedure is the acknowledgment of greater pore size and build pore interconnectivity. Primary favorable position of this system is its effortlessness, adaptability and simple to control the pore size and geometry. Pore geometry is control by the determination of the shape for particular Porogen specialists, where as pore size is control by sieving the Porogen molecule to the particular dimensional extent.
One of the primary disadvantages of this method is that it can just create slim wafers or film up to 3mm thick and extremely hard to outline the frameworks with precise pore between availability.
Fiber bonding
The fiber bonding method was first developed by who produced scaffolds made of Polyglycolic Acid (PGA) polymer. They took advantage of the fact that PGA was available as sutures and thus in the shape of long fibers. Mikos et al 191 improved the structural stability of the constructs developing a fiber-bonding technique in which the PGA fibers are joined at their cross-linking points by “sintering” above their melting point temperature. For example, PGA fibers have been bonded by embedding in PLLA solution, cooling, and subsequent removal of PLLA.189. The scaffolds were fabricated by bonding a collagen matrix to PGA polymers with threaded collagen fiber stitches. The main advantage of the fiber-bonding technique is the high surface area: volume ratio, which makes them ideal for tissue engineering applications and high porosity, which provides more surface area for cell attachment and sufficient space for the regeneration of ECM.
Disadvantages are poor mechanical integrity, residual organic solvents, lack of structural stability, that they can be used only to make small membranes, all the materials cannot be used for all the processes, membrane porosity is difficult to control, and morphology.
Fiber mesh
The fiber mesh technique for scaffold fabrication consists of individual fibers either woven or interwoven into a 3D pattern of variable pore size. It is prepared by the deposition of a polymer solution over a nonwoven mesh of another polymer followed by subsequent evaporation. 195 PGA is the first biocompatible and biodegradable polymer to be spun into fiber and used as a synthetic suture thread. The main advantage of this technique is the rapid diffusion of nutrient that is favorable for cell survival and growth and for high cell attachment because of the large surface area. One of the main drawbacks of this technique is a lack of structural stability. This problem can be overcome by hot drying of PLLA fibers to improve crystallinity and structure orientation.
Self assembly
Self assembly is the spontaneous organization of the molecule into well defines into an ordered structure required for specific function. Self assembly of natural or synthetic molecule produced nanoscale fibers known as nanofibers. Amphiphilic peptide sequence is a common method for the fabrication of 3D nanofibrous structure for tissue engineering. In aqueous solution the hydrophobic and hydrophilic domains within these peptides interact together with the help of weak non covalent bonds this produces distinct fast recovering hydrogel, with the hydrophobic interactions as the molecules come together. Instead of peptides synthetic polymer nanofibers are also prepared by self assembly of diblock polymers (AxBy) when the two blocks separate from one another in bulk due to their incompatibility, the volume formation of A and B can be controlled to obtain B domain of cylindrical shape with nanoscale diameter that is embedded into matrix A. Polymeric dendrimers can also self-assemble into nanofibers. The di-and tri-block Peptide Ampholites (PAs) are designed that are self-assembled into a rod-like architecture. So a new technique for the self-assembly of PAs into nanofibers by controlling pH and by engineering the peptide head group of the PAs is developed.
Rapid Prototyping (RP)
RP is also called as solid free-form technique. This technique is more advanced technique for scaffold fabrication. It is computer controlled fabrication technique. It can rapidly produce 3D object by using layer manufacturing method. RP technique generally comprises the design of scaffold model by using the Computer Aided Design (CAD) software, which is then expressed as a series of cross section. Corresponding to each cross section RP machine lays down a layer of material starting from the bottom and moving up a layer at a time to create the scaffolds. In typical example, image of bone defect in a patient can be taken and develop 3D CAD computer model. The computer then can reduced the model to slice or layers. The 3D objects are constructed layer by layer by using RP techniques such as Fused Deposition Modeling (FDM), Selective Laser Sintering (SLS), 3D Printing (3D-P) or stereo lithography. Now a day RP is an efficient way for generating the scaffolds of desired property, other advantage of this technique is to produce the parts with highly reproducible architecture and compositional variations. RP has advantage over other fabrication techniques, it has ability to control matrix architecture (size, shape, inter connectivity, branching, geometry and orientation) yielding biomimetic structure, that varying in design and material composition [13]. It has ability to control the mechanical property, biological effects and degradation kinetics of scaffolds
Sol-gel technique
Scaffolds are prepared by dissolving inorganic metal salts or metal organic compounds in a solvent, where a series of hydrolysis and polymerization reactions allow the formation of a colloidal suspension (sol); after casting the sol into a mold a wet gel is formed, and with further drying and heat treatment the gel is converted into dense ceramic or glass articles. Sol-gel techniques have become popular recently because of their high chemical homogeneity, low processing temperatures, and the possibility of controlling the size and morphology of particles. The sol-gel-derived materials provide excellent matrices for a variety of organic and inorganic compounds. One of the most important features of doped sol-gel materials is their ability to preserve chemical and physical properties of the do pants. The advantages of sol-gel technology can be used for construction of biomedical sensors, laser materials, or for delayed drug delivery. The disadvantages are the high cost of raw materials; large shrinkage during processing; residual fine pores, hydroxyl, and carbon; and a health hazard from the long processing time of the organic solution.
Melt molding technique
Scaffolds are prepared by melting polymers/ceramics in the presence of porogens (such as sodium chloride and sugar crystals); once the mixture has cooled, porosity is achieved by dissolving the porogens in water. Finally, the porous scaffolds are usually lyophilized. In 1995, Thompson etal.181 used the compression molding principle: a Teflon mold was used with PLGA and gelatin microspheres of a specific diameter; the mold was heated above the glass-transition temperature of PLGA while pressure was applied to the mixture. This treatment causes the PLGA particles to bond together. Once the mold is removed, the gelatin component is leached out by immersion in water, and the scaffold is then dried. The advantage of this technique is independent control of porosity and pore size, macroshape control, and pore interconnectivity and geometry, which is important for the exchange of nutrients/waste from pore to pore. The disadvantage is the high temperature required for nonamorphous polymers and residual porogens.
Membrane lamination
Membrane lamination is another SFF-like technique used for constructing three-dimensional biodegradable polymeric foam scaffolds with precise anatomical shapes. Membrane lamination is prepared by solvent casting and particle leaching and introducing peptide and proteins layer by layer during the fabrication process. The membranes with appropriate shape are soaked with solvent, and then stacked up in three-dimensional assemblies with continuous pore structure and morphology. The bulk properties of the final 3D scaffolds are identical to those of the individual membrane. This method generates the porous 3D polymer foams with defined anatomical shape, since it is possible to use the computer assisted modeling to design the template with desired implant shape. The disadvantage of this technique is that layering of porous sheets, result in lesser pore interconnectivity and other disadvantage of this technique is that it is a time consuming process since only thin membrane can be used in this process.
Freeze drying
Freeze drying technique is use for the fabrication of porous scaffolds. This technique is based upon the principle of sublimation. Polymer is first dissolved in a solvent to form a solution of desired concentration. The solution is frozen and solvent is removed by lyophilization under the high vacuums that fabricates the scaffold with high porosity and inters connectivity. These techniques are applied to a number of different polymers including silk proteins PGA, PLLA, PLGA, PLGA/PPF blends. The pore size can be controlled by the freezing rate and pH; a fast freezing rate produces smaller pores. Controlled solidification in a single direction has been used to create a homogenous 3D-pore structure. Main advantage of this technique is that, it neither requires high temperature nor separate leaching step. The drawback of this technique is smaller pore size and long processing time.
Freeze extraction and freeze gelation technique
PLLA was dissolved in dioxane and PLGA was dissolved in dioxane or DMSO (dimethyl sulfoxide) to form a 3wt% polymer solution. The polymer solution was placed in a glass petridish and frozen at 20°C. The solvent contained in the frozen solution was then removed either by freeze-drying or by freeze-extraction. The procedures of freeze-extraction are described below. The frozen polymer solution was immersed in an ethanol aqueous solution that was pre-cooled to 20°C. It should be noted that the ethanol concentration was 80wt% preparation of PLLA scaffolds, and 30wt% for PLGA. Due to the miscibility between the solvent (dioxane or DMSO) and the ethanol aqueous solution, the solvent was extracted out and replaced with ethanol aqueous solution, a non-solvent for PLLA and PLGA. After extraction, drying at room temperature was performed to remove the ethanol aqueous solution contained in the polymer matrix. PLLA and PLGA scaffolds could then be obtained after the drying stage.
For the freeze-extraction method (PLLA and PLGA), the idea was to remove the solvent by extraction with a non-solvent. After the removal of solvent, the space originally occupied by solvent was taken by non-solvent and the polymer was then surrounded with the nonsolvent. Under this circumstance, even at room temperature, the polymer would not dissolve. Hence, drying at room temperature could be carried out to remove the non-solvent, leaving space that became pores in the scaffolds. It should be noted that the extraction was performed at a temperature lower than the freezing point of the polymer solution to assure that the polymer would not redissolve during the extraction process. Hence the non-solvent should have a freezing point lower than that of the polymer solution, so that it could be kept at a liquid state during extraction. The nonsolvent used in this study was ethanol aqueous solution of which the freezing point can be changed by adjusting its composition. With a suitable composition of ethanol aqueous solution, the non-solvent bath (ethanol-water solution) was in liquid state during the extraction of dioxane out of the frozen PLLA and PLGA solutions. For chitosan it is not easy to extract out the solvent (aqueous solution of acetic acid) with a nonsolvent [15].
Supercritical assisted phase-inversion process
The phase inversion experiments were carried out in an apparatus especially for this purpose [11] apparatus is high pressure phase inversion apparatus (P-Pressure transducer; TIC-Temperature Controller; FM-Flow Meter; BPR-Back Pressure Regulator). Briefly, in each experiment a small amount (ca. 2ml) of the polymer solution is loaded in a stainless steel cap with 2cm diameter, which is placed inside the high pressure vessel. The vessel is heated in by means of an electric thin band heater (OGDEN) connected to a temperature controller, that maintains temperature within ± 1oC (TC). Carbon dioxide is pumped into the vessel using high pressure piston pump (P-200A Thar Technologies) until the operational pressure is attained. The pressure inside the vessel is measured with a Pressure transducer (P). The system was closed for 45 minutes to allow the occurrence of phase separation. Afterwards the system is flushed for another 45 minutes, with a stream of carbon dioxide at very low flow rate (5g/ min), in order to ensure complete drying of the scaffolds. The flow is regulated by a flow meter.
Powder compaction
Scaffolds are prepared by compressing the polymers/ceramics using projectiles or punch and dies; the velocity of compaction of the projectile or punch and dies is adjusted to achieve powder consolidation with the desired porosity. The process can include sintering as an alternative to use uniaxial or isostatic pressing. Polymerfoam replica method [17].
Thermally induced phase separation
Thermally Induced Phase Separation (TIPS) was first applied to PLA scaffolds by Schugens and later several other researchers applied this technique to prepare composite scaffolds. It consists of inducing a solid-liquid or liquid-liquid phase separation. This is done by dissolving the polymer in a solvent and quenching the solution at a certain temperature. The quenching induces a phase separation into a polymer-rich phase and a polymer-poor phase. In particular, TIPS uses thermal energy as the latent solvent to induce phase separation. The solvent must then be removed from the phase-separated solutions either by freeze-drying or solvent extraction. The solvent leaves behind microstructural foam.
The main advantage of the phase separation method is that pore morphology and orientation can be tailored by altering the thermodynamic and kinetic parameters of the processing, and it is a highly porous structure and permits incorporations of bioactive agents (hydrophilic or hydrophobic).
Correlation of Different Preparation Strategies for Scaffold
See Table 1.
Methods
Merits
Demerits
Solvent casting/
particulate
leaching
Control over
Porosity, pore size
and crystallinity
Limited mechanical
property, residual
solvents and Porogen
material
Porogen leaching
Controlled over
porosity and pore
geometry
Inadequate pore size
and pore
interconnectivity
Gas foaming
Free of harsh organic
solvents, control over
porosity and pore
size
Limited mechanical
property, inadequate
pore interconnectivity
Self assembly
Control over
porosity, pore size
and fiber diameter
Expensive material,
complex design
parameters
Electrospining
Control over
porosity, pore size
and fiber diameter
Limited mechanical
property, pore size
decrease with fiber
thickness
Phase separation
No decrease in the
activity of the
molecule
Difficult to control
precisely scaffold
morphology
Rapid Prototyping
Excellent control over
geometry, porosity,
no supporting
material required
Limited polymer type,
highly expensive
equipment
Fiber mesh
Large surface area for
cell attachment, rapid
nutrient diffusion
Lack the structural
Stability
Fibre bonding
High surface to
volume ratio, high
porosity
Poor mechanical
property, limited
applications to other polymers
Melt molding
Independent control
over porosity and
pore size
Required high
temperature for non
amorphous polymer
Membrane lamination
Provide 3D matrix
Lack required
mechanical strength,
inadequate pore
interconnectivity
Freeze drying
High temperature
and separate leaching
step not required
Small pore size and long
processing time
Table 1: Correlation of Different Preparation Strategies for Scaffold.
Properties of Scaffold Matrices in Cell/Drug Delivery
Scaffolds assume a vital part in tissue designing (Table 1). The functions of scaffolds are to direct the development of cells either seeded inside of the permeable structure of the scaffold or migrating from encompassing tissue. The dominant part of mammalian cell sorts are dock dependent, meaning they will die if the adhesion substrate is not provided. Scaffold matrices scan be utilized to accomplish cell conveyance with high stacking and productivity to specific sites. Along these lines, the scaffold must give a suitable substrate to cell connection, cell proliferation, separated capacity, and cell migration [18-20] to grant the vehicle of nutrients, waste, and organic flagging variables to take into account cell survival. The matrix material ought to be biodegrade at a controllable rate that approximates the rate of natural tissue recovery and ought to incite a negligible insusceptible and/or inflammatory response in vivo [21]. Tissue building scaffolds are intended to be colonized by cells and ought to transmit the substance and physical signs important to guarantee satisfactory tissue growth. Engineered polymer scaffolds might be utilized to convey proteins and development factors with or without cells locally to improve tissue repair and regeneration [22]. An perfect tissue engineering scaffold ought to full fill the accompanying requirements [23].
Biocompatibility
The scaffold should have satisfactory biocompatibility and lethality profile [24]. Biocompatibilities the capacity of the scaffold to perform in a particular application without inspiring an unsafe safe or incendiary reaction [25]. In the event that the scaffold is nontoxic and degradable, new tissue will eventually replace it, though on the off chance that it is nontoxic and biologically active, the scaffold will incorporate with the encompassing tissue. Not with standing, if the scaffolds naturally dormant, it might be exemplified by a stringy container; in the worst case, when the scaffold is harmful, rejection of the scaffold and restricted passing of the surrounding tissue can occur [26].
Biodegradability
The scaffold material should be biodegradable. Its degradation items should not be dangerous and should be disposed of effortlessly from the implantation site by the body [27]. Taking out the requirement for further surgery to remove it [27]. The scaffold’s degradation rate ought to be adjusted to coordinate the rate of tissue recovery with the goal that it has vanished totally once the tissue is repaired [24].
Mechanical properties
Mechanical properties of the scaffold ought to coordinate those of the tissue at the implantation site, or the mechanical properties at any rate ought to be adequate to shield cells from harming compressive or elastic powers without restraining fitting biomechanical cues [28,25] and to get by under physiological conditions. Instantly after implantation. A Novel Carrier for Cell and Drug Delivery the scaffold ought to give an insignificant level of biomechanical capacity that ought to improve progressively until ordinary tissue capacity has been restored, and soon thereafter the construct ought to have completely incorporated with the encompassing host tissue [24].
Structure
It should have a reproducible microscopic and macroscopic structure with a high surface: volume ratio suitable for cell/drug attachment [24].
Interface adherence
Interface adherence is how cells or proteins bind to a scaffold’s surface. The scaffold should support cell adhesion and proliferation, facilitating cell–cell contact and cell migration [29].
Porosity
Permeable structures take into consideration ideal cooperation of the scaffold with cells [13]. In particular, pore size decides the proficiency at which cells seed into the scaffold [29]; little pores keep the cells from infiltrating the scaffold, whilst extensive pores anticipate cell connection because of a diminished range and, in this manner, accessible ligand density [24]. The scaffold ought to have a sufficient porosity; this incorporates the greatness of the porosity, the pore size dispersion, and its interconnectivity. This likewise will permit cell indevelopment and vascularisation and advance metabolite transport [30]. A scaffold with an open and entomb associated pore system and a high level of porosity (>90%) is perfect for the scaffold to collaborate and incorporate with the host tissue [31].
Nature
mimicking the native extracellular grid (ECM), an endogenous substance that encompasses cells, permits them to bind into tissues and give signal that guide cell development and morphogenesis [25,31].
Processability
The scaffold should possess relatively easy processability and malleability into the desired shape, according to the need. They should be capable of being produced into a sterile product.
Loading capacity release kinetics
Loading limit release kinetics is characterized as the measure of medication that can be blended into the scaffold. The scaffold ought to have a greatest stacking limit so the medication discharged constantly for more length after insertion into the body. The medication should be dispersed homogenously all through the framework or in discrete regions and must maintain a strategic distance from an underlying burst impact. The medication discharge from the framework should be controlled to permit the suitable measurements of medication to achieve the cells over a given time period.
Binding affinity
Binding affinity is characterized as how firmly the medication ties the framework; this coupling liking must be adequately low to permit arrival of the medication; nonetheless, low binding affinity would prompt dose dumping, which in the long run may create harmful impacts.
Stability
The stability of the incorporated drug/cell at physiological temperature with respect to physical, chemical, and biological activity is to be assessed. They should posse’s dimensional stability, chemical stability, and biological activity over a prolonged period of time [22] (Figure 2).
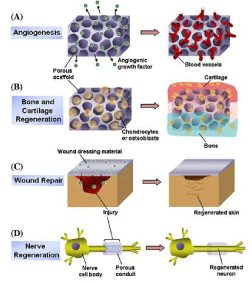
Figure 2: Multi-functional scaffolds for regeneration of various tissues:
Examples include (A) scaffolds delivering angiogenic growth factor to induce
blood vessel formation, (B) scaffolds seeded with osteoblasts/chondrocytes
for bone and cartilage regeneration, (C) growth factor releasing film as wound
dressing material, and (D) porous conduits for nerve regeneration [32-40].
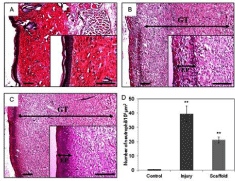
Figure 3: H&E stained sections for the morphological evaluation of skin
wounds.
Pharmacokinetics study of scaffold- David Rizik, et al, reported the Pharmacokinetics of Everolimus Eluted from the Absorb Bioresorbable Vascular Scaffold. The ABSORB III Pharmacokinetic (PK) study is a prospective, open label, non-randomized sub-study of the ABSORB III randomized trial designed to determine the PK of everolimus delivered by the Absorb Bioresorbable Vascular Scaffold (BVS) in subjects who only receive Absorb BVS with a maximum treatment of two de novo coronary artery lesions. Everolimus blood concentrations increased rapidly after Absorb BVS deployment, reaching maximum concentration between 0.17 and 2.37 hours (tmax), declining thereafter with a terminal half life ranging between 45.9 and 115 hours. By 4 hours, everolimus blood concentrations were below 3ng/ml (the chronic therapeutic level necessary to prevent organ rejection in transplant patients) in all subjects. Everolimus blood concentrations were low but measurable for up to 168 hours (7 days) after last scaffold deployment. The maximum observed everolimus concentration (Cmax) increased with dose and ranged from 1.085 to 4.460ng/ml across the dose range studied. Similarly, individual AUC (AUC24h: 12.09 to 44.22 ng*h/mL; AUClast: 25.37 to 104.6 ng*h/mL; AUC0-N: 33.15 to 120.8 ng*h/mL) increased proportionally with total scaffold dose [41-43].
In vivo study of scaffold - Mei-Ling Ho et al (2013) reported histological exam of the H&E stained skin section showed the granulation tissues of the control group without injury, -Figure A, the group without injury (Figure B) and the group with scaffold treatment (Figure C). The epidermis in the scaffold group was denser than the injury group, and proved that the skin repairing was facilitated by the scaffold. During the wound healing process, neutrophils secrete substances to accelerate KCs differentiation and to delay wound closure. Compared to the injury group, and there was less neutrophil infiltration in the scaffold treatment group (Figure D). By applying this scaffold, neutrophil infiltration would be decreased and wound closure would be more rapid. These evidences, including the improved healing speed, the increased epidermis density and reduced neutrophil infiltration, suggested that this scaffold is suitable for excision wound healing.
Concerning PLGA scaffolds, conflicting results were found after four weeks. Jiang et al., found remarkably less bone formation in their study within 5mm defects than Schneider et al., did in 6 mm critical defects. Although PLGA ratios were 85:15 in both studies, the materials differed in fiber diameter and scaffold architecture. While Jiang et al., used scaffolds with smooth nanofibers Schneider et al., used scaffolds in which even the fibers showed a porous structure. TCP nanoparticles increased fiber roughness additionally. Scaffold architecture, such as porosity and pore size, plays a critical role in cell migration and bone formation into a scaffold. A high porosity of nanofibers helps cell accommodation and facilitates the efficient exchange of nutrient substances between the scaffold and the environment. It was examined that a 100μm pore diameter is necessary for in vitro cell migration and a 300μm pore diameter is necessary for tissue in growth and nutrient diffusion However, the effects of scaffold architecture on bone formation can differ depending on the studied materials. Furthermore, there is also evidence that scaffold porosity can have no significant effect on bone formation [44].
Conclusion
Scaffold drug delivery scaffold is the most interesting drug delivery system. Scaffold drug delivery scaffold taking into account utilization of biodegradable material, for example, PLA or PLGA. Scaffold drug delivery is the most worthwhile medication delivery scaffold as it encourages the development of harm or unhealthy tissue by applying mix of biomaterials cells or bioactive atoms. By utilizing the biodegradable material tissue or organ are set up by basic implantation of scaffold then the biodegradable material is bioresorbable into the body. The field of biomaterials has assumed a critical part in the advancement of tissue-designed items. Despite this, few scaffolds are accessible financially, especially for cell/drug delivery. Looking into suitable and practicability, there is good scope in developing Injectables gel-sol scaffolds since they are easy to use, adaptable, and include the utilization of safe adjuvant; a number of them are as of now recorded in the Generally Recognized as Safe rundown or even have been endorsed by the Food and Drug Administration.
References
- Bombaldi de Souza, Fernanda C, Renata F, Moraes, Angela M. Chitosan/ Pectin Scaffolds for Tissue Engineering Applications. 2.
- Gaikwad V, Patil AB, Gaikwad M. Scaffolds for drug delivery in tissue engineering. International journal of pharmaceutical science and nanotechnology. 2008; 1.
- Chesnutt BM, Viano AM, Yuan Y, Yang Y, Guda T, Apple ford MR, et al. Design and characterization of a novel chitosan/ nanocrystalline calcium phosphate composite scaffold for bone regeneration. Journal of biomedical materials research. 2009; 88: 491-502.
- Di Silvio L, Bonfield W. Biodegradable drug delivery system for the treatment of bone infection and repair. J Mater Sci Mater Med. 1999; 10: 653-658.
- Shinto Y, Uchida A, Korkusuz F, Araki N, Ono K. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J Bone Joint Surg Br. 1992; 74: 600-604.
- Gittens SA, Uludag H. Growth factor delivery for bone tissue engineering. J Drug Target. 2001; 9: 407-429.
- Tabata Y. Necessity of drug delivery systems to tissue engineering. In: Park KD, editor. Biomaterials and drug delivery toward the new millennium. Seoul: Han Rim Won Publisher. 2000; 531.
- Locaa D, Locs J, Salma K, Gulbis J, Salma I, Cimdina LB. Porous Hydroxyapatite Bio ceramic Scaffolds for Drug Delivery and Bone Regeneration. Materials Science and Engineering. 2011; 18: 192019.
- Hossana J Md, Gafurb MA, Kadirb MR, Mainularima Md. Preparation and Characterization of Gelatine-Hydroxyapatite Composite for Bone Tissue Engineering. International Journal of Engineering& Technology. 2014; 14.
- Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, et al. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci USA. 2011; 108: 67-72.
- Abd Razak SI, Ahmad Sharif NF, Abdul Rahman WAW. Biodegradable Polymers and their Bone Applications: A Review. International Journal of Basic & Applied Sciences IJBAS-IJENS. 2012; 12: No: 01.
- Lyons F, Partap S, O’Brien FJ. Part 1: scaffolds and surfaces. Technol Health Care. 2008; 16: 305-317.
- Subia B, Kundu J, Kundu SC. Biomaterial scaffold fabrication techniques for potential tissue engineering applications. Tissue Engineering. 2010.
- Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007; 59: 249-262.
- Ho MH, Kuo PY, Hsieh HJ, Hsien TY, Hou LT, Lai JY, et al. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials. 2004; 25: 129-138.
- Duartea ARC, Manob JF, Reis RL. Preparation of Chitosan Scaffolds for Tissue Engineering using Supercritical Fluid Technology. Materials Science Forum Vols. 2010; 636-637.
- Garg T, Singh O, Arora S, Murthy R. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 2012; 29: 1-63.
- Khang G, Lee SJ, Kim MS, Lee HB. Biomaterials: tissue engineering and scaffold. In Webster J .Encyclopedia of Medical Devices and Instrumentation. 2006; 2: 366-383.
- Mandal BB, Kundu SC. Non-bioengineered high strength three-dimensional gland fibroin scaffolds from tropical non-mulberry silkworm for potential tissue engineering applications. Macromol Biosci. 2008; 8: 807-818.
- Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009; 30: 2956-2965.
- Lyons F, Partap S, O’Brien FJ. Part 1: scaffolds and surfaces. Technol Health Care. 2008; 16: 305-317.
- Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007; 59: 187-206.
- Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007; 59: 249-262.
- Khang G, Lee SJ, Kim MS, Lee HB. Biomaterials: tissue engineering and scaffold. In Webster J. Encyclopedia of Medical Devices and Instrumentation. 2006; 2: 366-383.
- Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007; 59: 249-262.
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002; 54: 3-12.
- Hench LL. Bioceramics. J Am Ceram Soc. 1998; 81: 1705-1728.
- Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998; 33: 111-139.
- Karande ST, Agrawal MC. Functions and requirement of synthetic scaffolds in tissue engineering. In: Laurencin CT, Nair LS editors. Nanotechnology and Tissue Engineering: The Scaffolds. 2008; 53.
- Papkov MS, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Advanced Drug Delivery Reviews. 2007; 59: 187-206.
- Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008; 60: 184-198.
- Mark Saltzman W, Baldwin SP. Materials for protein delivery in tissue engineering. Adv Drug Deliv Rev. 1998; 33: 71-86.
- Kim HW, Knowles JC, Kim HE. Hydroxyapatite/poly(epsilon-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials. 2004; 25: 1279-1287.
- Drouin, Bernard, Mantovani, Diego. Chitosan/Pectin Scaffolds for Tissue Engineering Applications. 2013.
- Inga Narkevica, Laura Stradina, Vladimir Yakushin, Jurijs Ozolins. Preparation and Characterization of Porous Titania Ceramic Scaffolds. Material Science and Applied Chemistry. 2001.
- Papkov MS, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Advanced Drug Delivery Reviews. 2007; 59: 187-206.
- Ho MH, Kuo PY, Hsieh HJ, Hsien TY, Hou LT, Lai JY, et al. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials. 2004; 25: 129-138.
- Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004; 32: 477-486.
- Mandal BB, Kundu SC. Osteogenic and adipogenic differentiation of rat bone marrow cells on non-mulberry and mulberry silk gland fibroin 3D scaffolds. Biomaterials. 2009; 30: 5019-5030.
- Widmer MS, Mikos AG. Fabrication of biodegradable polymer scaffolds. In: Patrick CW, Mikos AG, McIntire LV, editors. Frontiers in Tissue Engineering. 1998; 107-120.
- Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996; 17: 1417-1422.
- Freyman TM, Yannas IV, Gibson LJ. Cellular materials as porous scaffolds for tissue engineering. Prog Mater Sci. 2001; 46: 273-282.
- Rizik D, Cannon LA, Ellis S, Stone GW, Kennedy M, Ruster KP, et al. ABSORB III PK: Pharmacokinetics of Everolimus Eluted from the Absorb Bioresorbable Vascular Scaffold (BVS). Journal of the American college of cardiology. 2015; 66: 15.
- Wang HM, Chou YT, Wen ZH, Wang CZ, Chen CH, Ho ML. Novel biodegradable porous scaffold applied to skin regeneration. PLoS One. 2013; 8: e56330.