
Research Article
Austin Therapeutics. 2019; 5(1): 1032.
Piper Cubeba and Vitamin C, A Fruitful Combination against Drug Induced Nephrotoxicity-A Histopathological Analysis
Asgher A1, Saeed U1, Iftikhar S2*, Talat H3, Rana M4 and Zia A1
1Department of Pharmacology, FMH College of Medicine and Dentistry, Pakistan
2Department of Pharmacology, Rashid Latif Medical and Dental College, Pakistan
3Senior Demonstrator, King Edward Medical University, Pakistan
4Department of Pathology, Rashid Latif Medical and Dental College, Pakistan
*Corresponding author: Salman Iftikhar, Department of Pharmacology, Rashid Latif Medical and Dental College, Lahore, Pakistan
Received: October 18, 2019; Accepted: November 12, 2019; Published: November 19, 2019
Abstract
Aminoglycosides are frequently used antibiotics for life threatening gramnegative bacterial infections. Gentamicin binds to the cell wall phospholipids, blocks phosphatidylinositol pathway leading to production of Reactive Oxygen Species (ROS) and impairment of cell integrity that contributes to its toxicities including nephrotoxicity. Fifty male rabbits were divided into five groups A, B, C, D and E each containing ten with group a being normal control. In this study microscopic examination in gentamicin-only treated group B 80% rabbits showed severe inflammation, 70% showed severe tubular necrosis and 70% showed severe glomerular congestion. Kidney sections from rabbits in group C, and D treated with Piper cubeba and vitamin C alone respectively, showed only mild to moderate inflammation, tubular necrosis and glomerular congestion. Maximum improvement was seen in Group E where Piper cubeba and vitamin C were co-administered. A significant increase in proximal tubular dilatation was found in group B. Kidney sections from rabbits in Group C, and D and E showed almost normal architecture of proximal tubules. The difference in mean tubular diameter in-group A, C, D and E was insignificant.
Conclusion: Piper cubeba appears to be more nephroprotective in comparison to vitamin C. Likewise, co-administration of both Piper cubeba and vitamin C augmented the nephroprotective effect as compared to either Piper cubeba or vitamin C given alone respectively.
Keywords: Piper Cubeba; Vitamin C; Aminoglycosides; Nephrotoxicity
Introduction
Despite the advent of new highly potent and broad-spectrum antibiotics, aminoglycosides have been classified as critical antimicrobials by World Health Organization (WHO) to address the serious bacterial infections, which may result in significant morbidity and mortality if left untreated. All aminoglycosides are now classified as Critically Important Organization [1]. This is primarily because of their chemical stability, rapid bactericidal effect, synergy with beta lactam antibiotics, and low resistance in community and healthcare settings Takahashi and Igarashi [2]. They inhibit protein synthesis by binding 30S subunit of bacterial ribosome and the concentration dependent killing effect of aminoglycosides persists even after they have been discontinued Wargo and Edwards [3]. Nephrotoxicity is a therapeutic limitation of aminoglycosides that appears in 10-25 % of patients despite rigorous monitoring Riviere, [4]. Tubular toxicity is the consequence of many interconnected actions triggered by accumulation of drug in the proximal tubular epithelial cells with the help of a receptor complex formed by megalin and cubilin Nagai and Takano [5]. Studies have demonstrated excessive production of Reactive Oxygen Species (ROS) cause oxidative stress which is responsible for tubular damage as increased amounts of super oxide anion (O2–), hydroxyl radicals (OH), hydrogen peroxide (H2O2), and reactive nitrogen species are produced Fatima and Sultana, [6]. Oxidative stress directly damages some macromolecules e.g. lysosome and mitochondria, via several mechanisms including peroxidation of membrane lipids, protein denaturation and DNA damage Lopez-Novoa et al [7]. With revival of herbal medicine and their incorporation in treatment of various ailments it is of interest to note that Piper cubeba (tailed pepper, java pepper, kabab chini) has been traditionally used to treat renal disorders, gonorrhea, syphilis, abdominal pain, enteritis, and asthma in Unani Medicine de Lima et al [8]. Chemical constituents of Piper cubeba like polyphenols (phenolic acid and flavonoids), a-cubebene, tanins, and dihydrocubebin have shown significant antioxidant activity [9].
Vitamin C is a naturally occurring water soluble substance that acts as non-enzymatic chain breaking antioxidant and free radical scavenger. It has shown protection against gentamicin induced nephrotoxicity by decreasing lipid peroxidation Motamedi et al [10]. In clinical situations the rate of aminoglycoside nephrotoxicity is very high usually occurs after one week of treatment and is attributed to oxidative stress Nagai and Takano [5]. The role of different agents such as Piper cubeba and vitamin C, due to their antioxidant effect had been evaluated internationally for providing nephroprotection against drug (aminoglycoside) induced nephrotoxicity. But none of the agent had been able to provide complete nephroprotection against such damage.
The purpose of this study is to find out the concomitant effect of the Piper cubaba for the first time locally and Vitamin C in aminoglycoside induced nephrotoxicity and to look for their effects alone and in combination, whether both are together more effective than either of the single agent. The study might be used to develop safe, efficacious and cheap nephroprotective agents.
Materials and Methods
The study was conducted in the KEMU and University of Veterinary and Animal Sciences, Lahore.
Preparation of the herbal cocktails
Dried berries of Piper cubeba (Java pepper) were bought from local market. Proper identification was carried out by Botany department Government College University, Lahore. They were finely powdered in an electric grinder. Mucilage of 5 grams of gum acacia in 100 ml of distilled water was prepared. Piper cubeba 800 mg/ kg of body weight was weighed for each animal and mixed in 2 ml of mucilage. The test drug was prepared fresh every time and given once daily in the morning through oral route for 21 days [11].
Drug dosage
Gentamicin was administered in doses ten times larger than the corresponding human clinical dose to produce experimental nephrotoxicity Tulkens, [12]. It was given in a dose of 80mg/kg of the body weight, intramuscularly in two equally divided doses with an interval of 12 hours, for 21 days Saleem et al [13]. Vitamin C 250mg/ kg/day was dissolved in 2 ml of distilled water and given orally once a day Rehman et al [14].
Experimental procedure
Rabbits were procured from local market and divided into five groups (A, B. C, D, and E). They were housed in clean cages, provided green fodder, and water ad libitum. Animals were kept at room temperature with natural day and night cycle. A period of ten days was given them to get acclimatized before the start of experiment.
Fifty rabbits were divided into five groups randomly, with 10 rabbits in each group.
Group A: Normal control group. 0.5 ml of isotonic saline was injected I/M twice a day, for 21 days.
Group B: Gentamicin 80 mg/kg/day was injected I/M in two equally divided doses with an interval of 12 hours, for 21 days.
Group C: Gentamicin 80 mg/kg/day was injected I/M in two equally divided doses with an interval of 12 hours & Piper cubeba in a dose of 800 mg/kg/day was given orally once a day, for 21 days.
Group D: Gentamicin 80 mg/kg/day was injected I/M in two equally divided doses with an interval of 12 hours & vitamin C 250 mg/kg/day was given orally, for 21 days.
Group E: Gentamicin 80 mg/kg/day was injected I/M in two equally divided doses with an interval of 12 hours. Piper cubeba in a dose of 800 mg/kg/day & vitamin C 250mg/kg/day was given orally once a day, for 21 days.
Histopathological procedure
On 21st day the rabbits were sacrificed, and both the kidneys were removed Ullah et al [15].
Fixation of tissue
Clean and dry plastic tissue jars were labeled properly with group no. and rabbit number. The renal tissue was immersed in 10% formalin (formaldehyde) for fixation in those jars. Formalin was changed every hour for three times. Then the tissue was left for 72 hours for proper fixation 95.
Dehydration
Alcohol was used to dehydrate renal tissue. Dehydration was carried out in a slow, step-wise manner by passing the tissue blocks through 80%, 90%, 95%, 100%, 100% graded strength of alcohol, step incubation period was kept 1 hour for each strength. Clearing was done in three steps using xylene. In each step tissue was incubated for two hours.
Infiltration and Embedding
Tissue was allowed to equilibrate in an incubator at 580°C for two hours with liquid paraffin being changed after an hour. After infiltration, the tissue was allowed to solidify in a mold, embedded within a small cube of paraffin.
Sectioning
Sectioning was done by using microtome in a thickness of 5μm. The sections were flattened by floating them on water held at 450°C. The sections were then mounted on individual, properly labeled microscope slides.
Staining
Slides were stained with hematoxylin and eosin stains. They were rehydrated by dipping them for 30 seconds in decreasing concentration of alcohol i.e. 100%, 90%, 80% and 70% each. Then they were dipped in hematoxylin for 2 to 3 minutes and were washed with running water. They were kept in 1% acid alcohol and again washed with water. Slides were then dipped in eosin for 1 to 2 minutes and washed under tap water. After rinsing they were rehydrated by increasing concentrations of alcohol 90%, 100% for 2 to 3 minutes and cleared with xylene. They were covered carefully with coverslip and were placed to dry for 24 hours 95. The stained slides were then examined under microscope to evaluate proximal tubular dilatation, glomerular congestion, inflammation, and necrosis. Semi-quantitative grading of Histopathological changes was done as following:
_No pathological change
+Mild (less than 25% of the tissue affected)
++Moderate (25-50 % of the tissue affected)
+++Severe (more than 50% of the tissue affected)
Tubular diameter was measured by using NIS (Nikon imaging software) Element “D” (documentation) software installed in decahead microscope in Pathology Lab. KEMU. Diameter of five tubules per high field was measured in each slide both for right and left kidney and their mean was taken.
Statistical analysis
Data was analyzed by using Statistical Package for Social Studies (SPSS) software. Quantitative data was presented in form of mean ± S.D. ANOVA was applied to compare mean values within all study groups. Post Hoc Tukey’s was applied for pairwise comparison among groups. Histopathological changes were expressed as frequencies and percentages; Kruskal Wallis ANOVA Test was applied for evaluating qualitative data in all study groups. Mann Whitney test was applied for pairwise significant differences among different groups. ‘P’ value less than 0.05 was considered significant.
Results
Tubular diameter
Mean inner tubular diameter of proximal convoluted tubules of kidney for gentamicin only treated group increased significantly in comparison to group A, C, D, and E. ANOVA showed the difference with a significant p value of ‹0.001. There was a significant increase in the outer tubular diameter of proximal convoluted tubules of rabbit kidney in group B in comparison to group A, C, D and E, with a p value of ‹0.001. The difference between group A, C, D and E was insignificant.
Multiple comparisons by Post hoc Tukey’s test revealed that inner tubular diameter of group B was significantly dilated in comparison to other groups. There was no significant difference between the mean inner tubular diameter of group A, C, D, and E. Likewise, outer tubular diameter of group B was also significantly dilated when compared to group A, C, D, and E. There difference between the mean outer tubular diameter of group A, C, D, and E was insignificant.
Tubular necrosis
There was no pathological change found in group A. In-group B, 3(30%) showed moderate and 7(70%) showed severe tubular necrosis. In-group C, 7(70%) showed mild and 3(30%) showed moderate tubular necrosis. In-group D, 6(60%) showed mild and 4(40%) showed moderate tubular necrosis. In-group E, 4(40%) showed no pathological change, 4(40%) showed mild, and 2(20%) showed moderate necrosis. ‘Kruskal Wallis’ showed significance difference with a p value of ‹ 0.001 (Table 3).
Tubular Diameter
Group A Mean±SD
Group B Mean±SD
Group C Mean±SD
Group D Mean±SD
Group E Mean±SD
P value ANOVA
Inner
22.05 ± 2.24
50.70 ± 10.77
29.44 ± 7.69
32.13 ± 11.46
27.32 ± 5.86
<0.001
Outer
41.21 ± 2.40
78.15 ± 19.32
48.48 ± 11.5
50.61 ± 9.17
46.26 ± 5.56
<0.001
Table 1: Mean tubular diameter (μm) for group A, B, C, D, and E. (n=10).
Dependent Variable
(I) group
(J) group
Mean Difference (I-J)
Summary
B
-28.66
***
A
C
-7.39
Ns
Tubular Diameter Inner μm
D
-10.08
Ns
E
-5.28
Ns
C
21.26
***
B
D
18.58
***
E
23.38
***
C
D
-2.69
Ns
E
2.12
Ns
D
E
4.8
Ns
B
-36.94
***
A
C
-7.27
Ns
Tubular Diameter Outer μm
D
-9.4
Ns
E
-5.05
Ns
C
29.68
***
B
D
27.54
***
E
31.9
***
C
D
-2.13
ns
E
2.21
ns
D
E
4.34
Ns
*p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
Table 2: Post hoc Tukey’s test for multiple comparisons of mean tubular diameter (μm) among groups A, B, C, D, and E.
Tubular Necrosis
Group A n=10
Group B n=10
Group C n=10
Group D n=10
Group E n=10
p Value
n (%)
n (%)
n (%)
n (%)
n (%)
No Pathological change
10(100)
0(0)
0(0)
0(0)
4(40)
Mild
0(0)
0(0)
7(70)
6(60)
4(40)
<0.001
Moderate
0(0)
3(30)
3(30)
4(40)
2(20)
Severe
0(0)
7(70)
0(0)
0(0)
0(0)
p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
Table 3: Tubular necrosis among groups A, B, C, D, and E.
Pairwise comparison by Mann Whitney showed significant tubular necrosis in group B, C, D, and E in comparison to group A. Tubular necrosis in group C, D and E was significant in comparison to group B. Tubular necrosis among group C, D, and E was insignificant among different groups (Table 4).
Groups
Mean rank
Z
p value
B
5.5
-4.15
< 0.001
A
C
5.5
-4.15
< 0.001
D
5.5
-4.12
< 0.001
E
7.5
-2.81
0.005
C
15.05
-3.65
< 0.001
B
D
14.9
-3.53
< 0.001
E
15.2
-3.69
< 0.001
C
D
10
-0.46
0.648
E
12.4
-1.55
0.111
D
E
12.7
-1.81
0.07
Table 4: Pairwise comparison of tubular necrosis among various groups by Mann Whitney U Test.
Inflammation
No pathological change was found in group A. In group B, 2(20%) showed moderate and 8(80%) showed severe inflammation. In group C, 3(30%) showed no pathological change, 4(40%) showed mild and 3(30%) showed moderate inflammation. In group D, 1(10%) showed no pathological change, 5(50%) showed mild and 4(40%) showed moderate inflammation. In group E, 5(50%) showed no pathological change, 3(30%) showed mild, and 2(20%) moderate inflammation. ‘Kruskal Wallis’ showed significance difference with a p value of ‹ 0.001 (Table 5).
Inflammation
Group A
Group B
Group C
Group D
Group E
p value
n (%)
n (%)
n (%)
n (%)
n (%)
< 0.001
Nil
10(100)
0(0)
3(30)
1(10)
5(50)
<25%
0(0)
0(0)
4(40)
5(50)
3(30)
25-50%
0(0)
2(20)
3(30)
4(40)
2(20)
>50%
0(0)
8(80)
0(0)
0(0)
0(0)
Table 5: Inflammation among groups A, B, C, D, and E.
Pairwise comparison by Mann Whitney showed significant inflammation in group B, C, D, and E in comparison to group A. Inflammation in group C, D and E was significant in comparison to group B. Inflammation among group C, D, and E was insignificant.
Glomerular congestion
There was no pathological change found in group A. In-group B, 3(30%) showed moderate and 7(70%) showed severe glomerular congestion. In-group C, 3(30%) showed no pathological change, 5(50%) showed mild and 2(20%) showed moderate glomerular congestion. In-group D, 2(20%) showed no pathological change, 4(40%) showed mild and 4(40%) showed moderate glomerular congestion. In-group E, 4(40%) showed no pathological change, 3(30%) showed mild, and 3(30%) moderate glomerular congestion. ‘Kruskal Wallis ANOVA’ showed significance difference with a p-value of ‹0.001 (Table 7).
Groups
Mean rank
Z
p value
B
5.5
-4.2
< 0.001
A
C
7
-3.13
0.002
D
6
-3.76
< 0.001
E
8
-2.5
0.013
C
15.2
-3.72
< 0.001
B
D
15.1
-3.68
< 0.001
E
15.3
-3.8
< 0.001
C
D
9.45
-0.86
0.392
E
11.55
-0.85
0.397
D
E
12.6
-1.69
0.091
Table 6: Pairwise comparison of inflammation among various groups by Mann Whitney U Test.
Glomerular Congestion
Group A
Group B
Group C
Group D
Group E
p value
n (%)
n (%)
n (%)
n (%)
n (%)
< 0.001
Nil
10(100)
0(0)
3(30)
2(20)
4(40)
<25%
0(0)
0(0)
5(50)
4(40)
3(30)
25-50%
0(0)
3(30)
2(20)
4(40)
3(30)
>50%
0(0)
7(70)
0(0)
0(0)
0(0)
*p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
Table 7: Glomerular Congestion in-group A, B, C, D, and E. p value calculated by Kruskal Wallis ANOVA.
Table 8: Pairwise comparison of glomerular congestion among various groups by Mann Whitney U Test.
Pairwise comparison by Mann Whitney showed significant glomerular congestion in group B, C, D, and E in comparison to group A. glomerular congestion in group C, D and E was significant in comparison to group B. glomerular congestion among group C, D, and E was insignificant.
Urine microscopy
Numerous triple phosphate crystals per high power were seen on urine microscopy, which is normal for rabbit urine.
Discussion
Aminoglycosides are frequently used antibiotics for life threatening gram-negative bacterial infections. Gentamicin (a prototype aminoglycoside) binds to the cell wall phospholipids, blocks phosphatidylinositol pathway leading to production of Reactive Oxygen Species (ROS) and impairment of cell integrity. Renal tissue has abundance of polyunsaturated fatty acids, which makes it an organ susceptible to ROS. Studies have proved that antioxidants protect tissues from ROS Chashmi et al [16]. We used Piper cubeba in our study to counter this toxicity as previous studies showed that it can increase the activity of anti-oxidant enzymes like catalase, glutathione reductase, and superoxide dismutase in animal model. Piper cubeba possesses free radical scavenging activity e.g. nitric oxide, hydroxyl and hydrogen peroxide radicals Ahmad and Hasan, Pachpute and Deshmukh, [17,18]. Vitamin C (ascorbic acid) was also used alone in in combination with Piper cubeb. It is also a naturally occurring organic compound, which acts as a chain-breaking antioxidant and free radical scavenger. It has a protective effect against gentamicin, induced nephrotoxicity Abdel-Daim et al, Ali et al [19]. Several studies have suggested more effectiveness of combination therapy by cosupplementation of two antioxidants (Kadkhodaee et al., Chandran et al., [20,21]. On microscopy in gentamicin only treated group B 80% rabbits showed severe inflammation, 70% showed severe tubular necrosis and 70% showed severe glomerular congestion (Figure 3,4). Kidney sections from rabbits in group C, and D treated with Piper cubeba and vitamin C alone respectively, showed only mild to moderate inflammation, tubular necrosis and glomerular congestion (Figure 5,6). These findings are in accordance with previous studies showing the preservation of normal renal architecture with the use of Piper cubeba and vitamin C. Ahmad et al. [11], observed a significant nephroprotective effect of Piper cubeba in both pre-treated and post-treated groups. They stated that Piper cubeba possesses significant benefit against gentamycin-induced nephrotoxicity. The maximum improvement was seen in Group E where Piper cubeba was co-administered with vitamin C (Figure 7,8). Similar results were observed by Kadkhodaee et al. where they co-administered moderate doses of vitamins C and E and observed positive effects on renal preservation in aminoglycoside induced nephrotoxicity [20]. A significant increase in proximal tubular dilatation with a p value of ‹.001 was found in group B (Figure 3). This finding is in accordance with Chen et al [22] they witnessed gentamicin causing a significant dilatation in lumen of proximal tubules. Kidney sections from rabbits in Group C, and D and E showed almost normal architecture of proximal tubules this is in keeping with the studies conducted by Ahmad et al and Alhusaini et al [11,23,24]. Where they observed similar results concomitant use of antioxidants. The difference in mean tubular diameter in group A, C, D and E was insignificant. Current study evaluated the nephroprotective effect of Piper Cubeba and vitamin C alone and in combination against aminoglycoside induced nephrotoxicity in rabbits. The study revealed that concomitant use of Piper cubeba and vitamin C alone or in combination along with gentamicin significantly reversed the nephrotoxicity observing the Histopathological parameters.
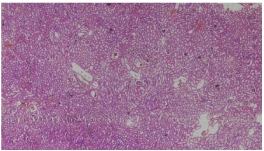
Figure 1: Photomicrograph showing normal architecture of rabbit kidney.
Group A (10 x magnification; H&E).
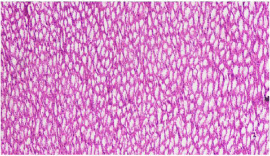
Figure 2: Photomicrograph showing normal tubules in renal medulla of rabbit
kidney. Group A (10 x magnification; H&E).
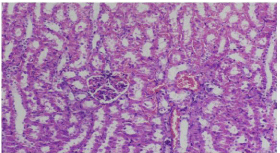
Figure 3: Photomicrograph showing tubular dilatation, severe glomerular
congestion. Group B (20 x magnification; H&E).
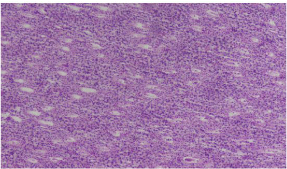
Figure 4: Photomicrograph showing severe inflammation. Group B (10 x
magnification H&E).
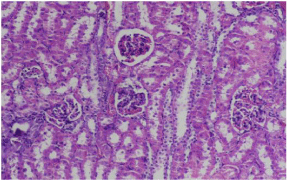
Figure 5: Photomicrograph showing moderate glomerular congestion and
tubular dilatation. Group C. (20 x magnification H&E).
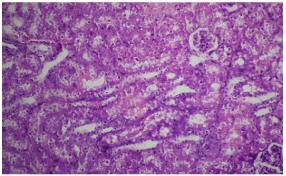
Figure 6: Photomicrograph showing mild to moderate glomerular congestion,
inflammation and tubular necrosis. Group D. (20 x magnification H&E).
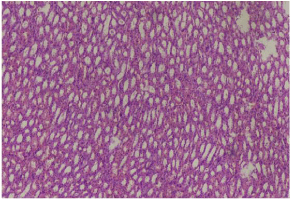
Figure 7: Photomicrograph showing mild inflammation. Group E (10 x
magnification H&E).
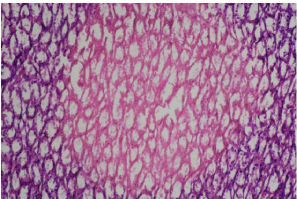
Figure 8: Photomicrograph showing mild tubular necrosis in renal medulla.
Group E. (20 x magnification H&E).
Conclusion
Piper cubeba was observed to be more nephroprotective in comparison to vitamin C. Likewise, co-administration of both Piper cubeba and vitamin C significantly augmented the nephroprotective effect of each other as compared to either Piper cubeba or vitamin C alone.
References
- Organization WH. Critically important antimicrobials for human medicine: ranking of antimicrobial agents for risk management of antimicrobial resistance due to non-human use. 2017.
- Takahashi Y, Igarashi M. Destination of aminoglycoside antibiotics in the ‘post-antibiotic era’. The Journal of antibiotics. 2018; 71: 4-14.
- Wargo KA, Edwards JD. Aminoglycoside-induced nephrotoxicity. Journal of pharmacy practice. 2014; 27: 573-577.
- Riviere JE. Aminoglycoside-induced toxic nephropathy. Handbook of animal models of renal failure. CRC Press. 2018.
- Nagai J, Takano M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochemical pharmacology. 2014; 90: 331-337.
- Fatima N, Sultana A. Renoprotective and anti-oxidant effects of Coleus forskohlii against gentamicin induced nephrotoxicity in albino wistar rats. Acta Pharmaceutica Sciencia. 2018; 56.
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011; 79: 33-45.
- De Lima RG, Barros, Da Silva Laurentiz MTR. Medicinal Attributes of Lignans Extracted from Piper Cubeba: Current Developments. Chemistry Open. 2018; 7: 180-191.
- Zahin M, Khan MS, Abul Qais F, Abulreesh HH, Ahmad I. Antioxidant properties and anti-mutagenic potential of Piper Cubeba fruit extract and molecular docking of certain bioactive compounds. Drug and chemical toxicology. 2018; 41: 358-367.
- Motamedi M, Iranmanesh A, Teimori A. Kidney regeneration in Aphanius furcatus (Teleostei: Aphaniidae) after damage induced by gentamicin and evaluation of vitamin C in toxicity reduction. Iranian Journal of Ichthyology. 2019; 6: 65-72.
- Ahmad QZ, Jahan N, Ahmad G. Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi Journal of Kidney Diseases and Transplantation. 2012; 23: 773.
- Tulkens PM. Nephrotoxicity of aminoglycoside antibiotics. Toxicology letters. 1989; 46: 107-123.
- Saleem U, Ahmad B, Rehman K, Mahmood S, Alam M, Erum A. Nephroprotective effect of vitamin C and Nigella sativa oil on gentamicin associated nephrotoxicity in rabbits. Pak J Pharm Sci. 2012; 5: 727-730.
- Rehman K, Akash M, Azhar S, Khan S, Abid R, Waseem A, et al. A biochemical and histopathologic study showing protection and treatment of gentamicin-induced nephrotoxicity in rabbits using vitamin C. African Journal of Traditional, Complementary and Alternative Medicines. 2012; 9: 360-365.
- Ullah N, Khan MA, Khan T, Asif AH, Ahmad W. Mentha piperita in nephrotoxicity-a possible intervention to ameliorate renal derangements associated with gentamicin. Indian journal of pharmacology. 2014; 46: 166.
- Chashmi NA, Emadi S, Khastar H. Protective effects of hydroxytyrosol on gentamicin induced nephrotoxicity in mice. Biochemical and biophysical research communications. 2017; 482: 1427-1429.
- Ahmad W, Hasan A. An Appraisal of Medicinal Properties of Kabab Chini (Piper cubeba). Unimed Kulliyat. 2012; 5-7: 13-18.
- Pachpute AP, Deshmukh TA. Antioxidant and Hepatoprotective Activity of an Ethanol Extract of Piper Cubeba Fruits. Int J Res Dev Pharm L Sci. 2013; 2: 321-329.
- Abdel-Daim MM, Ahmed A, Ijaz H, Abushouk AI, Ahmed H, Negida A, et al. Influence of Spirulina platensis and ascorbic acid on amikacin-induced nephrotoxicity in rabbits. Environmental Science and Pollution Research. 2019; 26: 8080-8086.
- Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Experimental physiology. 2005; 90: 571-576.
- Chandran R, Kim T, Mehta SL, Udho E, Chanana V, Cengiz P, et al. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. Journal of Cerebral Blood Flow & Metabolism. 2018; 38: 1818- 1827.
- Chen Q, Cui Y, Ding G, Jia Z, Zhang Y, Zhang A, Huang S. PEA3 protects against gentamicin nephrotoxicity: role of mitochondrial dysfunction. American journal of translational research. 2017; 9: 2153-2162.
- Alhusaini AM, Faddah LM, El Orabi NF, Hasan IH. Role of Some Natural Antioxidants in the Modulation of Some Proteins Expressions against Sodium Fluoride-Induced Renal Injury. Bio Med Research International, 2018; 2018: 5614803-5614803.
- Ali S, Hussain S, Khan R, Mumtaz S, Ashraf N, Andleeb S, et al. Renal toxicity of heavy metals (cadmium and mercury) and their amelioration with ascorbic acid in rabbits. Environmental Science and Pollution Research. 2019; 26: 3909-3920.