
Research Article
Thromb Haemost Res. 2017; 1(1): 1002.
Point of Care Testing (POCT) to Assess Drug Concentration in Patients Treated with on Vitamin K Antagonist Oral Anticoagulants (NOACs)
Padayattil Jose S¹, Carraro P², Haleh A², Rossi K³, Nante G³, Denas G¹, Zoppellaro G¹, Bracco A¹, Pengo V¹ and Banzato A¹*
¹Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Italy
²Department of Laboratory Medicine, St Antony Hospital, Italy
³Department of Medicine (DIMED), University of Padua, Italy
*Corresponding author: Alessandra Banzato, Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Via Giustinani, 2 - 35128 Padova –Italy
Received: May 31, 2017; Accepted: August 08, 2017; Published: August 18, 2017
Abstract
Background: Non–vitamin K antagonist oral anticoagulants (NOACs) do not need routine laboratory monitoring but measurement of drug concentration is important in emergency conditions. Specific laboratory tests are not readily available or not implemented in every hospital. Point-of-Care Tests (POCT) may bridge this gap and be used as a bedside solution.
Objectives: Feasibility of POCT to assess plasma levels of dabigatran, rivaroxaban and apixaban.
Patients/Methods: Activated Coagulation Time-Low Range (ACT - LR) using a portable Hemochron Signature Elite for dabigatran and prothrombin time (expressed as INR) by Coaguchek XS Pro for rivaroxaban and apixaban were obtained at trough and peak in 136 consecutive patients taking NOACs (70 on dabigatran, 45 on rivaroxaban and 20 on apixaban). Using a paired study design, drug concentrations were concurrently determined by functional specific tests.
Results and Conclusion: The correlation between NOACs concentration and the values obtained using the POCTs was high for dabigatran and rivaroxaban (r=0.80 and r=0.82, respectively) and low for apixaban (r=0.21). ACT-LR = 188 seconds better detected dabigatran levels = 50ng/ml, with a sensitivity of 87.5% and a specificity of 84.1%. ACT-LR values >217 seconds better discriminated value of dabigatran >200ng/ml, with a sensitivity of 86.7% and a specificity of 81.4%. INR Coaguchek values = 1.2 better identified patients with rivaroxaban values <100ng/ml, with sensitivity of 90%, specificity of 88.5%. This analysis was not possible for apixaban.
Conclusion: in emergency situations POCT use may provide useful immediate information on dabigatran and rivaroxaban concentration.
Keywords: Apixaban; Concentration; Dabigatran; Emergency; Point of care test; Rivaroxaban
Introduction
Non–vitamin K antagonists oral anticoagulants (NOACs) are widely used in patients with Atrial Fibrillation (AF) and venous thromboembolism. Currently, four NOACs are available, i.e. a direct thrombin inhibitor (dabigatran etexilate) [1] and three direct Factor Xa (FXa) inhibitors (apixaban, edoxaban and rivaroxaban) [2-4].
The main advantage of NOACs resides in their use at fixed doses without routine monitoring [5]. However, measuring drug concentration may be useful in emergency situations such as active bleeding, urgent surgery, ischemic stroke requiring thrombolysis or drug over dose [6]. Different specific tests are available: diluted Thrombin Time (dTT) and Ecarin Clotting Time (ECT) are considered the tests of choice for the determination of dabigatran concentration [7,8]. On the other hand, a specific calibrated anti Xa assay is recommended for the determination of rivaroxaban, apixaban and edoxaban concentrations [7,8]. These specific tests are not implemented in every hospital and might be time consuming, thus, a readily available drug concentration tool would be worth. The aim of our study was to examine whether an appropriate Point of Care Test (POCT) can provide reliable information about NOACs concentration to get a ready to use information in critical/emergency situations.
Materials and Methods
Study design and settings
This is a single centre, paired study design. All consecutive patients starting NOACs (naïve or shifted from classic anticoagulants) followed in an anticoagulation clinic affiliated to a tertiary level university hospital were included. Patients treated with edoxaban were not included because, at the time of the study, this drug had just been introduced into Italian market. Informed consent was obtained from all patients.
Sample collection
Blood samples were collected at steady state after a period varying from 2 to 4 weeks since initiation of NOACs therapy. Blood samples were collected at trough level (12 hours after intake for dabigatran and apixaban and 24 hours for rivaroxaban) and at peak level (2-4 hours after drug intake). Blood was drawn with minimal stasis in 0.019M sodium citrate (9:1) and plasma was obtained after centrifugation at 2000g for 10 minutes and stored at -30°C until used. A direct finger puncture using a lancing device was used for POCT tests.
Laboratory tests
The Activated Clotting Time- Low Range (ACT-LR) for the determination of dabigatran activity was obtained with the portable meter Hemochron Signature Elite (Cremascoli& Iris, Milan, Italy). Prothrombin Time (International Normalized Ratio, INR Coaguchek) for the determination of rivaroxaban and apixaban activity was determined with Coaguchek XS Pro (Roche Diagnostics, Monza, Italy). The functional specific tests to obtain the plasma concentration of the three NOACs were Hyphen Hemoclot for dabigatran (modified diluted Thrombin Time, Dasit, Milan, Italy) and Hyphen DiXal for rivaroxaban and apixaban (chromogenic antiXa, Dasit, Milan, Italy). All these tests were calibrated using commercial plasmas from the same supplier and analysed on Sysmex CA 7000 (Siemens Health Care, Milan, Italy). Activated thromboplastin time (aPTT, Actin FS Siemens) and prothrombin time (PT-INR, Innovin, Siemens)in plasma samples were determined on Sysmex CA 7000 (Siemens Health Care, Milan, Italy).
Definition of clinically relevant NOACs concentration
A clear definition of therapeutic concentration of NOACs is lacking. In the RELY study the relative risk of ischemic stroke was increased by 50% at dabigatran plasma level of 28 ng/ml while the rate of major bleeding was doubled at a concentration of 210 ng/ml [9]. Concentration of dabigatran <50 ng/ml, rivaroxaban <100 ng/ml and apixaban <10 ng/ml are considered as safe to allow thrombolysis in treatable stroke patients [10]. We analysed the performance of POCT to detect the values <50 ng/ml for dabigatran and values <100 ng/ml for rivaroxaban as well as values of dabigatran concentration >200 ng/ml.
Statistics
NOACs concentrations obtained by specific tests at trough and at peak concentrations are expressed as median and interquartile ranges. Results obtained by ACT-LR (seconds) and Coaguchek (INR) were compared to NOACs plasma concentration by linear regression analysis. Receiver Operating Characteristic curve (ROC curve) was used to illustrate the performance of POCT at relevant cut-off points. Sensitivity and specificity, positive and negative predictive values at the defined cut-off points were expressed accordingly.
Graph Pad Software (San Diego, CA, USA) was used for statistical analysis and a two-sided p-value =0.05 was considered as statistically significant.
Results
From October 2013 to October 2015, one hundred thirty six consecutive patients were considered and accepted to participate in the study. One patient taking dabigatran was excluded for incorrect timing adherence (drug was taken 24 hrs before sample collection). Patients taking NOACs were distributed as follows: 70 on dabigatran, 45 on rivaroxaban and 20 on apixaban.
Median trough values were 102 (IQR 65-136.5) ng/ml, 30.5 (IQR 15.5- 44.7) ng/ml and 46.5 (IQR 33.5-84.5) ng/ml for dabigatran, rivaroxaban and apixaban, respectively. Median peak values were 206 (IQR 134-292) ng/ml, 198.5 (IQR 142.8- 283.3) ng/ml and 111.5 (IQR 79.2-147.8) ng/ml for dabigatran, rivaroxaban and apixaban, respectively (Figure 1).
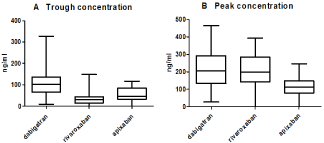
Figure 1: Trough (A) and peak (B) concentration of dabigatran, rivaroxaban and apixaban.
Trough and peak values were combined to compute the correlation analysis with POCT results. The linear regression between dabigatran concentration (determined by dTT) and the values obtained using the POCT ACT-LR (seconds) was r=0.80 (Figure 2A). The linear regression between rivaroxaban and apixaban plasma levels (determined by anti- Xa assays) and the values obtained using the POCT (INR Coaguchek) were r=0.82 and r=0.21, respectively (Figure 2B and Figure 2C). The linear regression was also calculated for NOACs concentration and common coagulation tests (aPTT for dabigatran and PT-INR for rivaroxaban and apixaban). Results were r= 0.73, r = 0.76 and r=0.31 for dabigatran, rivaroxaban and apixaban, respectively.
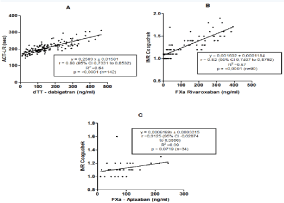
Figure 2: A) correlation of concentration of dabigatran measured by dTT and ACT-LR, B) correlation of concentration of rivaroxaban determined by anti Xa assay
and INR Coaguchek, C) correlation of concentration of apixaban determined by anti Xa assay and INR Coaguchek.
The evaluation of ACT-LR as diagnostic tool by ROC curve for low and high threshold levels of dabigatran (50 ng/ml and200 ng/ml) are shown in Figures 3&4.
Area under the curve for ACT-LR using a cut off value of dabigatran of 50ng/ml is 0.92 (95% CI, 0.861 to 0.958, P<0.0001), significance level (Figure 3). ACT-LR = 188 seconds, better detected dabigatran levels < 50ng/ml, with a sensitivity of 87.5% and a specificity of 84.1%. Positive predictive value is 41% and negative predictive value is 97%. As shown in Figure 4, the area under the curve for ACT-LR using a cut-off value of 200ng/ml is 0.89 (95%CI, 0.821 to 0.933, P<0.0001). ACT-LR values>217 sec better discriminate value of dabigatran >200ng/ml, with a sensitivity of 86.7% and a specificity of 81.4%. Positive predictive value is 67% and negative predictive value is 92%.
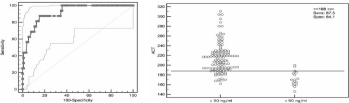
Figure 3: ROC curve and distribution of plasma levels< 50ng/ml according to a ACT-LR cut-off value of 188 seconds.
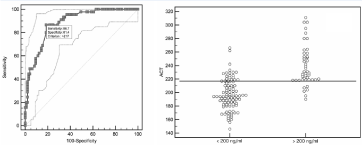
Figure 4: ROC curve and distribution of dabigatran plasma levels > 200 ng/ml according to ACT-LR cut-off value of 217 seconds.
The evaluation of CoaguCheck INR as a diagnostic tool to detect low rivaroxaban concentrations is shown in Figure 5. The area under the curve for Coagucheck-INR using a cut-off value of rivaroxaban of 100ng/ml is 0.94 (95% CI, 0.87 to 0.98, P <0.0001)(Fig 5).INR Coaguchek values = 1.2 better identified patients with rivaroxaban values <100ng/ml, with sensitivity of 90%, specificity of 88.5%. Positive predictive value is 86% and negative predictive is92%. Due to the poor linear regression, ROC curve analysis was not applicable for apixaban.
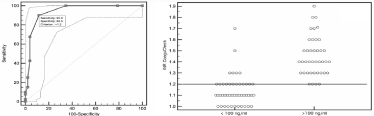
Figure 5: ROC curve and distribution of rivaroxaban plasma level <100ng/ml according to INR Coaguchek values = 1.2.
Discussion
In this study, we found a good correlation between the results obtained by two specific POCT (ACT-LR and CoaguChek) and plasma levels of dabigatran and rivaroxaban, respectively. The median trough and peak concentrations of NOACs were similar to that reported in previous studies [11]. Although routine monitoring is not required for NOACs, the determination may be useful in special situations [12]. Specific coagulation assays for NOAC are not readily available in every hospital, and might delay clinical decision-making. Therefore, POCT may emerge as feasible alternative to specific assays [13-15]. POCT have been described as useful tool to differentiate the trough and peak levels [16]. A portable INR system (CoaguChek) was also evaluatedto guide thrombolysis decisions in stroke patients taking NOACs [11]. Conversely, as shown by Ebner et al. [11], a poor relationship was found between the concentration of Apixaban and POCT results. Moreover, the correlation of values obtained by POCT with drug concentration is better than that obtained using the common coagulation tests (PT and aPTT). The higher sensitivity of POCT to determine NOAC concentration has been reported in previous studies [17-19].
Data defining relevant clinical concentrations of NOACs were assessed in an exposure response analysis from RELY study [9]. Reilly et al. showed a correlation with trough plasma level of dabigatran and clinical events. In that study a concentration of dabigatran of 28 ng/ ml was associated with 50% increased risk of thrombotic events and concentrations >210 ng/ml doubled bleeding events. Similar studies for NOACs other than dabigatran are not available. A possible cut off levels for safe thrombolysis in stroke patients is <50 ng/ml for dabigatran, <100 ng/ml for rivaroxaban and <10 mg/ml for apixaban, according to a consensus paper [10]. We used these cut off levels to estimate the sensitivity and specificity of POCT in detecting NOACs concentrations below these threshold values. Values of ACT-LR = 188 seconds picked up few patients with less than 50ng/ml of dabigatran (low positive predictive value) while value above 188 seconds indicated that most patients have a dabigatran concentration above 50ng/ml (high negative predicting value). In other words, ACT-LR values of more than 188 seconds may advice not to perform thrombolytic therapy in patients on dabigatran with an ischemic stroke. On the other hand, emergency physician may be confident in performing thrombolysis in a patient with ischemic stroke while on rivaroxaban when the Coagucheck INR is = 1.2 (positive predictive value is 86%). In case of major bleeding in patients on dabigatran, ACT-LR value >217 predict with good precision (positive predicted value 67%) a drug concentrations above 200ng/ml.
These findings have relevant clinical implications. In patients on treatment with NOACs, thrombolysis for ischemic stroke may be considered only at very low plasma levels. Under these premises and considering the results of our study, POCT is a time saving and reliable tool to guide decision-making. Another field of application is urgent surgery. Antidotes for dabigatran are now available and for other NOACs are on the way. Considering the cost of these therapies, a rapid test to detect relevant concentration of NOAC may be helpful to reduce inappropriate use of these agents.
Conclusion
In emergency clinical conditions, when specific tests are not readily available, POCT tests (ACT-LR for dabigatran and CoaguChek for rivaroxaban) can help in decision-making with better precision than common coagulation tests.
Essentials
• In certain clinical situations NOACs monitoring may be of value
• Peak and trough POCT and plasma concentration levels of NOACs were assessed
• There was a good correlation between POCT and plasma levels of dabigatran and rivaroxaban
• POCT might be a feasible tool to guide decisions certain situations in NOACs receiving patients
Addendum
Padayattil Jose S and Pengo V conceived the study and wrote the paper. Carraro P and Haleh A performed the coagulation studies. Rossi K, Nante G, Denas G, Zoppellaro G, Bracco Aand Banzato A followed up patients on NOACs and critically reviewed the manuscript.
References
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361: 1139-1151.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365: 981-992.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365: 883-891.
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369: 2093-2104.
- Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012; 126: 2381-2391.
- Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015; 17: 1467-1507.
- Tripodi A. The laboratory and the direct oral anticoagulants. Blood. 2013; 121: 4032-4035.
- Kitchen S, Gray E, Mackie I, Baglin T, Makris M, committee B. Measurement of non-coumarin anticoagulants and their effects on tests of Haemostasis: Guidance from the British Committee for Standards in Haematology. Br J Haematol. 2014; 166: 830-841.
- Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol. 2014; 63: 321-328.
- Steiner T, Bohm M, Dichgans M, Diener HC, Ell C, Endres M, et al. Recommendations for the emergency management of complications associated with the new direct oral anticoagulants (DOACs), apixaban, dabigatran and rivaroxaban. Clin Res Cardiol. 2013; 102: 399-412.
- Ebner M, Peter A, Spencer C, Hartig F, Birschmann I, Kuhn J, et al. Pointof- Care Testing of Coagulation in Patients Treated With Non-Vitamin K Antagonist Oral Anticoagulants. Stroke. 2015; 46: 2741-2747.
- Pengo V, Crippa L, Falanga A, Finazzi G, Marongiu F, Palareti G,et al. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA). Thromb Haemost. 2011; 106: 868-876.
- Hawes EM, Deal AM, Funk-Adcock D, Gosselin R, Jeanneret C, Cook AM, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013; 11: 1493-1502.
- Francart SJ, Hawes EM, Deal AM, Adcock DM, Gosselin R, Jeanneret C, et al. Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross-sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost. 2014; 111: 1133-1140.
- Eller T, Busse J, Dittrich M, Flieder T, Alban S, Knabbe C, et al. Dabigatran, rivaroxaban, apixaban, argatroban and fondaparinux and their effects on coagulation POC and platelet function tests. Clin Chem Lab Med. 2014; 52: 835-844.
- Mani H, Herth N, Kasper A, Wendt T, Schuettfort G, Weil Y, et al. Point-of-care coagulation testing for assessment of the pharmacodynamic anticoagulant effect of direct oral anticoagulant. Ther Drug Monit. 2014; 36: 624-631.
- van Ryn J, Baruch L, Clemens A. Interpretation of point-of-care INR results in patients treated with dabigatran. Am J Med. 2012; 125: 417-420.
- Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, et al. Assessment of laboratory assays to measure rivaroxaban--an oral, direct factor Xa inhibitor. Thromb Haemost. 2010; 103: 815-825.
- Hillarp A, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Stigendal L, Sten- Linder M, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011; 9: 133-139.