
Review Article
Thromb Haemost Res. 2017; 1(2): 1006.
Principles for Accurate Diagnosis of Deep Vein Thrombosis (DVT) and Prevention of DVT Recurrence and the Post-Thrombotic Syndrome in the Primary Care and Hospital Medicine
Jan Jacques Michiels1,2*, Wim Moosdorff¹, Janneke Maria Michiels2,3 and Martino Neumann HA4
¹Primary Care Medicine Medical Diagnostic Center, The Netherlands
²Goodheart Institute & Foundation in Nature Medicine, The Netherlands
³Primary Care Medicine Practice, The Netherlands
4Department of Dermatology, Erasmus University Medical Center, The Netherlands
*Corresponding author: Jan Jacques Michiels, Multidisciplinary Internist & Investigator, Good Heart Institute, Blood coagulation and Vascular Medicine Research Center, Erasmus Tower, Veenmos 13, 3069 AT Rotterdam, The Netherlands
Received: August 23, 2017; Accepted: October 10, 2017; Published: October 20, 2017
Abstract
The requirement for a safe diagnostic strategy of Deep Vein Thrombosis (DVT) should reach an overall objective post incidence of Venous Thromboembolism (VTE) of less than 1% during 3 months follow-up. The combined use of Complete Compression Ultrasonography (CCUS) followed by D-dimer testing and clinical score assessment safely rule in and out DVT. A negative ELISA VIDAS safely excludes DVT and VTE with a NPV between 99 and 100% and a low clinical score of zero. The combination of low clinical score and a less sensitive D-dimer test (Simplify) is not sensitive enough to exclude DVT and VTE in routine daily practice. Complete recanalization within 3 to 6 months and no reflux in one third of post-DVT patients is associated with a low or no risk of PTS obviating the need of MECS 6 months after DVT. Incomplete recanalization after 3 to 9 months due to valve destruction has been documented in two third of post-DVT patients. Absence of residual vein thrombosis (RVT = partial recanalization) at 3 months post-DVT and no reflux is predicted to be associated with no recurrence of DVT (1.2%) during follow-up obviating the need of wearing medical elastic stockings and anticoagulation at 3 to 4 months post-DVT. The presence of RVT or reflux at 3 months post-DVT is complicated by a high risk of DVT recurrence of about 30% and associated with induction and aggravation of symptomatic PTS indicating the compelling need to resume and extend anticoagulation with Direct Oral Anticoagulants (DOACS). We addressed four unanswered questions in the treatment of DVT and PTS. Which DVT patient has a clear indication for long-term compression stocking therapy to prevent PTS after the initial anticoagulant treatment in the acute phase of DVT? Is 3 months the appropriate point in time to determine candidates at risk to develop DVT recurrence and PTS? Which high risk symptomatic PTS patients are in need to extend anticoagulant treatment with DOACs.
Keywords: Deep venous thrombosis ; Ultrasonography ; Post-thrombotic syndrome ; ELISA D-dimer ; Medical elastic stockings ; Anticoagulation
Introduction
Deep-vein thrombosis
A normal quantitative ELISA VIDAS D-dimer test (cut-off <500 ug/L) was reported to have a 100% sensitivity when compared with phlebography in two studies [1,2]. In large prospective studies of outpatients with suspected Deep Vein Thrombosis (DVT), the sensitivity varied between 98% and 99.9% in 2239 patients, irrespective of clinical score [3-5]. In two large outcome studies, the sensitivity of a normal turbidimetric assay (Tinaquant, cut-off <500 ug/L for the exclusion of DVT varied from 91% to 98% and the specificity from 44% to 51% [4-6]. The qualitative D-Dimer test SimpliRed has a sensitivity of 89%, a specificity of 77% and a NPV of 96% for the exclusion of DVT [7]. Similarly, the quantitative ELISA VIDAS test at a cut of level of 1000 ug/ml has a sensitivity of 88% to 89%, a specificity of 56% to 68% and a NPV of 96% [8-10].
The general application of DVT exclusion by a negative SimpliRed (Simplify) by the combination of a negative CUS and low clinical score is not safe enough mainly because the prevalence of DVT in the low clinical score group varied widely (3% to 12%) [5,9]. After a first negative CUS the prevalence of DVT is uniformly low, 2% to 3%[8,9-14]. Consequently, the combination of a first negative CUS and a D-dimer level of ELISA VIDAS <1000, Tinaquant <800 ug/ml or negative SimpliRed (Simplify) will exclude deep vein thrombosis with a NPV of more than 99% in 4 prospective outcome studies[9,11-13]. A moderate to high probability in combination with a increased ELISA D-dimer (VIDAS >1000 or Tinaquant >800 ug/ml) or a positive qualitative D-dimer (SimpliRed or Simplify) should be followed by a second CUS of the legs after one week to detect a thrombus in about 3% to 10% of patients with suspected DVT [8,9,11-14].
Deep vein thrombosis and the post-thrombotic syndrome
After initial thrombosis, lysis of the leg vein clot (thrombus) immediately starts at time of anticoagulation. Propagation of the thrombus also occurs; the two processes occur simultaneously, whereby recanalization and the formation of a new thrombus are competing processes. Recanalization may be completed after 3 to 6 months without reflux or may be delayed up to more than 1 year with a high incidence of reflux development and DVT recurrence [15,16]. During these processes venous valves are destroyed in the majority of post-DVT patients and residual obstruction of the vein persisted in about 10% [17]. Loss of valve competence leading to Ambulatory Venous Hypertension (AVP) and diversion of venous flow through incompetent perforans veins appear to play an important role in the development of late complications of the Post-Thrombotic Syndrome (PTS) [15,16]. Anatomic studies have described the most distribution of venous valves to be a single valve in the Common Femoral Vein (CFV) above the sapheno-femoral junction, a relatively constant deep valve just before its termination in the CFV, three to four valves in the superficial femoral vein with relatively constant locations at the mid-thigh and adductor canal, one or two valves in the Popliteal Vein (PPV) and one to two valves with the terminal 2 to 2 cm of the Greater Saphenous vein (GSV). Among the calf veins, the Popliteal Vein (PPV) appears to be of primary importance in the development of the post-thrombotic syndrome, by virtue of both its importance in the calf muscle pump and its communications with the posterior arch vein. Meissner et al. studied the relationship between complete recanalization (lysis time) and the development of reflux in patients with a first episode of DVT at 3 months interval during the first year [15]. Duplex criteria for complete occlusion were defined as the absence of detectable flow, either spontaneous or with augmentation, in an incompressible venous segment. Partial occlusion was defined as normal or diminished flow either spontaneous or with augmentation, in an incompletely compressible venous segment. Complete resolution (lysis) of the leg vein clot (recanalization) was presumed to have occurred when spontaneous phasic flow returned and the vein was completely compressible [15]. Flow detected after distal augmentation in a completely compressible vein as accepted as evidence of complete recanalization (lysis of the leg vein clot). The median time from DVT to complete recanalization (lysis time) was about 3 months (100 days) for patients without reflux in all segments. In contrast, the median time from DVT to complete recanalization (lysis time) of all segments was about 9 to 12 months (more than 6 months) for DVT patients who developed reflux as the main determinant of PTS. In the study of 123 legs with DVT (107 patients) by Markel et al, about two third of the involved legs had developed valve incompetence [16]. The distribution of reflux at the end of the first year follow-up in this study was the following: popliteal vein, 58%, superficial femoral vein, 37%, greater saphenous vein, 25% and posterior popliteal vein, 18%. Reflux appeared to be more frequent in the segments previously affected by DVT [16].
From these two prospective clinical research studies [15,16] it may be concluded that complete recanalization within 3 months and no reflux is associated with a low or no risk of PTS obviating the need of MECS at 3 months after DVT (Table 1). On the other hand, partial and complete recanalization at 6 to 12 months is usually complicated by reflux due to valve destruction. Reflux seems to be a main determinant for not only for PTS and but also for DVT recurrence, the latter as a main contributing factor in worsening PTS. This hypothesis is supported by the relation between the persistent residual vein thrombosis (RVT = partial recanalization) and the risk of VTE recurrence in two prospective studies [18,19]. In a prospective outcome study, RVT at 3 months post-DVT was absent in 30%, which was associated with low recurrence of DVT (1.2% patient/years) during two years follow-up [18]. The presence of RVT at 3 months post-DVT was associated with a DVT recurrence rate of 27% during two years follow-up after discontinuation of anticoagulant treatment [18]. The proportion of provoked vs unprovoked DVT was 64% and 36% in patients with complete recanalization within 3 months and 23% vs 77% in the patient with RVT (incomplete recanalization) at 3 months post-DVT indicating that the distinction provoked vs unprovoked DVT is artificial in terms of risk on DVT recurrence.
In a prospective study of 313 consecutive DVT patients, Prandoni et al. have shown that RVT at any time post-DVT is a risk factor for recurrent VTE [19]. In this study, CUS of the common femoral and popliteal veins was performed at 3, 6, 12 24 and 36 months post DVT. The cumulative incidence of normal CUS (no RVT) was 39%, 58%, 69% and 74% at 6, 12, 24 and 36 months post DVT respectively. Of 58 VTE recurrent episodes, 41 occurred at time of RVT. The hazard ratio for recurrent VTE was 2.4 with persistent RVT versus those with earlier complete vein recanalization [19].
Scoring systems for PTS
The fundamental pathophysiologic disturbance with severe leg symptoms or sign after distal and proximal DVT is sustained venous hypertension, which can be measured with invasive venous pressure measurement (ambulant venous pressure: AVP). AVP can be regarded as the gold standard, since it directly measures the pressure in the venous system of the lower extremity. This technique requires special equipment, is invasive, time consuming and cumbersome and therefore only suitable for basic research and scientific studies.
Identification of no, early and late PTS in patients after a first or recurrent DVT is not reflected by the CEAP classification and remains a challenge for clinicians and phlebologists. Several means of measuring and classifying the early clinical signs and symptoms of PTS and its long-term sequelae of PTS exist. Most scoring systems for PTS are based on the presence or absence clinical signs and symptoms during the first year post-DVT and typical signs of CVI one or few years later. At least five definitions for early and/ or late PTS exist for the early or long-term complications after an episode of documented DVT. For the prevention and management of PTS, it is crucial that the natural history and treatment outcome of the disease should be documented by additional objective tools including Residual Vein Thrombosis (RVT) on DUS, and reflux and/ or obstruction on color ultrasonography [20-25]. At the baseline visit the clinicians should carefully examine the patient’s leg to classify the clinical category and to assess the severity of early PTS or late Chronic Venous Insufficiency (CVI) using the different scoring systems. The five scoring systems including the clinical classifications by Brandjes et al. [24] and by Prandoni et al. (known as the Vilalta score, Table 2) [25-28]) for early signs and symptoms of PTS during the first year post-DVT, and the CEAP (Table 3), Widmer and VCS classifications (Table 4) to assess various degrees CVI as late onset sequelae of PTS (Tables 2,3,4) [29-31]. Three objective classifications for PTS have been used by dermatologists and phlebologist the CEAP (Clinical- Etiology-Anatomic-Pathophysiologic, Table 3) [26] Widmer et al. (Table 4) [27] and the Venous Clinical Severity (VCS) score (Table 4) [28]. Clinical symptoms of PTS occurs in about half of the patients within one year post-DVT when the subjective clinical Villalta scoring is applied, which may vary from considerably from subjective complaints without objective PTS to a broad range of scarcely visible skin changes, pigmentation changes, pain, discomfort, venous ectasia, edema, and ulceration. A Dutch study prospectively evaluated the incidence and severity of PTS in 93 DVT patients under careful clinical survey using the CEAP classification and confirmed previous studies that half of DVT patients do develop PTS [32]. The cumulative incidence of PTS in that articular increased from 49% after one year to 55% and 56% after 2 and 6 years, but class 5 and 6 (healed) ulcers did not occur while on treatment with MECS.
Score versus:
Therapeutic implication
Score 0 at 6 months:
No MECS and no anticoagulant treatment (ACT)
Score 1 to 4 at 6 months:
MECS and discontinuation ACT
Score >4 and normal D-dimer:
MECS randomization ACT versus no ACT
Score >4 and abnormal D-dimer:
MECS and continuation of ACT according to the PROLONG
Plus Study
Table 2: Scoring system according to Prandoni for the assessment of postthrombotic syndrome in the early period 3 to 12 months post-DVT known as the Vilalta score [29-31].
Subjective symptoms
Objective signs
Heaviness
Pretibial oedema
Pain
Induration of the skin
Cramps
Hyperpigmentation
Pruritus
New venous ectasia
Paraesthesia
Redness
Pain during calf compression
Ulceration of the skin (= severe)
Table 3: Clinical-Etiology-Anatomic-Pathophysiologic (CEAP) classification after DVT in patients with signs and symptoms of the post-thrombotic syndrome [26].
Classification
Symptom
C0 (C = clinical)
No visible varicose veins
C1
Spider or reticular veins
C2
Varicose veins
C3
Oedema
C4a
Pigmentetion or eczema
C4b
Lipodermatosclerosis or atrophie blanche
C5
Skin changes with healed ulceration
C6
Skin changes with active ulceration
S
Symptomatic, including aches, pain, tightness, skin irritation, heaviness, muscle cramps, and other complaints attributable to venous dysfunction
A
Asymptomatic
C = Clinical symptom
E = Etiology
Post-DVT
A = Anatomic distribution
Deep, perforator, or superficial vein, alone or in combination
P = Pathophysiologic
dysfunction
Reflux or obstruction, alone or in combination
Table 4: The Venous Clinical Severity (VCS) Score system of PTS or CVI [28].
Prevention of DVT recurrence and PTS
Palareti et al. and other studies showed that normal versus increased D-dimer levels one month after discontinuation of regular anticoagulation is associated with an incidence of about 5% pt-years and 10 to 15% pt/years respectively [20-22]. This difference was independent from other factors like thrombophilia or residual venous occlusion [31,32]. In the PROLONG study, extended anticoagulation reduced the risk of DVT recurrence from 11% patient/years to less than 2% patient/years, whereas the incidence of DVT recurrence was still increased, 4.4% patient/years, in post-DVT patients with a normal D-dimer (Simplify) [23]. These data has to be interpreted in view of two other key observations: first the incidence of DVT recurrence after complete recanalization within 3 months and no reflux is very low [15,16,18]. Second the incidence of PTS in the control arm of two randomized clinical trials was about 50% within 6 months and did not significantly increase thereafter, whereas MECS decreased the incidence of PTS from around 50% to 25% after two years follow-up [24,25]. This may implicate that DVT recurrence in those patients with either a normal or increased D-dimer do occur in those with incomplete or complete RVT after 6 months with reflux (Table 1). The hypothesis in Table 1 that the Rotterdam scoring system for PTS will have therapeutic implications has to be tested by the use of objective measurements of RVT and reflux related to clinical score for PTS in prospective management and outcome studies.
Follow-up studies of patients one and two years after an episode of DVT are recommended. Complaints, clinical signs of PTS using objective scores for PTS and CEAP score measurement, and especially pre-tibial edema are to be investigated. In general, DVT patients are instructed to use the MECS directly at time of symptomatic acute DVT and discontinued at time of complete recanalization and absence of PTS at any time post-DVT [24,25]. In retrospect however, about half of the DVT patients do not develop PTS after one year obviating the need of wearing MECS lifelong [33]. Consequently, a duplex ultrasound should be performed at 1,2,3 and 6 months to one year and two years post-DVT, to determine whether there is still a need for wearing MECS and if additional treatment is necessary in cases of symptomatic PTS. If no pathologic changes remain (complete recanalization, no reflux the venous system functions normally and no PTS exist or will occur, the MECS do not need to be worn any longer and the asymptomatic patient can be discharged from the follow-up (Figure 1) [33]. We propose a prospective randomized clinical outcome study in patients with acute DVT with a PTS follow-up documentation at 1,3,6 and 12 months post-DVT with subsequent outcome for 4 to 5 years. Patients with provoked and unprovoked DVT at time of diagnosis are included. All provoked and unprovoked DVT patients will according to the standard immediately receive anticoagulant and compression therapy. In case of pronounced oedema, compression therapy will consist of short stretch bandages until the oedema is relieved. In case of minor or no oedema, compression therapy with MECS will be prescribed. MECS should be “flat knitted” stockings pressure class II and if complicated by oedema class III with a high resistance coefficient [33]. Objective documentation will consist of phlebology controls, duplex ultrasound imaging and ambulant venous pressure measurements (when indicated) and will take place at 0,1,3,6 and 12 months and subsequently every year. Based on these objective measurements and assessments, DVT patients will be risk stratified at 6 months post- DVT for continuation or discontinuation of compression therapy with MECS according to the study design.
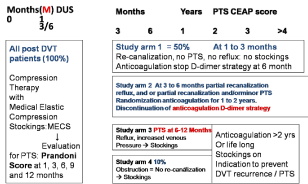
Figure 1: The European DVT - PTS Bridging the Gap study design 2014-
2017 designed by Michiels Moossdorff and Neumann [34,36].
Erasmus DVT diagnosis and DVT recurrence prevention study design
In view of all of the above we designed the Erasmus study to prevent DVT recurrence and PTS with MECS (Figure 1).
Study arm 1. Post-DVT patients with complete re-canalisation at 3 months, no reflux, and asymptomatic (no PTS) will not continue MECS, stop anticoagulant treatment, and will be remained in followup for at least 4 years.
Study arm 2. Post-DVT patients with reflux but normal venous pressure (no venous hypertension) and no PTS will be randomized for MECS vs no treatment for 2 years.
Study arm 3. Symptomatic Patients (PTS) with partial or complete recanalization but with reflux and increased venous pressure (venous hypertension) will receive compression therapy (MECS) for 2 years. After 2 years randomization will take place for continuation vs. discontinuation of MECS for another 2 years.
Study arm 4. All patients with obstruction on duplex ultrasound imaging (no re-canalisation) will receive compression therapy (MECS) for 2 years. After 2 years a randomization will take place for continuation and discontinuation of MECS for at least another 2 years.
PTS patients in study arm 3 and 4 are to be treated according to the PROLONG study54 if indicated according to the concept in Figure 1.
Evaluation procedures at time of inclusion 1 month and 3 months after DVT
Evaluation of clinical findings and details of positive echogram for DVT from the records of various hospitals or medical diagnostic centers where the diagnosis of DVT was made Blood collection (plasma, serum and DNA samples in deep freezer) for risk factor evaluation in retrospect.
Evaluation at time points 1 month, 6 months, 1 year, and 2 years post-DVT
1. Complete analysis for PTS according to the combined use of subjective Prandoni (Villalta, Table 2) score and according to objective CEAP score (Table 3).
2. DUS colour at 3 and 6 months for assessment of the degree of recanilization, reflux and obstruction
3. Allocation of PTS patients at 6 months to each of the four study arms.
4. Randomization of study arm 2 at time point 6 months into no MECS versus MECS
5. At time point 2 years randomization of PTS patient’s arm 3 and 4 into MECS versus no MECS with the exception of those who need active treatment for PTS based on objective measurements.
6. Repeat all measurements for PTS according to subjective Prandoni (Vilalta) score, and according to the objective CEAP classification, and assess the degree of recanalization, reflux and obstruction by DUS and colour Doppler at 9,12,18 and 24 months during follow-up.
Real life documentation of DVT patients and the need of extended anticoagulation
All patients with provoked and unprovoked DVT will be treated immediately with Direct Oral Anticoagulants (DOACs) for 6 months (Figures 1 and 2). This duration of DOAC treatment is based on risk stratification according to current recommendations [34]. All DVT patients will undergo a complete evaluation for PTS at 3 and 6 months post-DVT. Four types of PTS at 6 months post-DVT are distinguished depending on objective measurement criteria for PTS (Table 2) and allocated to the four study arms of the study design (Figures 1 and 2). DVT patients with delayed re-canalisation of veins at 3 and 6 months post-DVT will be risk stratified and subdivided in those without reflux and those with reflux on duplex ultrasound imaging. DVT patients with no re-canalization (obstruction) at 6 months will undergo invasive testing according to the Rotterdam approach. The diagnostic and treatment work-up of post-DVT patients should follow the Rotterdam Approach to PTS in the setting prospective randomized clinical outcome study with a followup period of 1 to 2 years (Figure 1). Patients with DVT at time of diagnosis are included. All acute DVT patients will according to the standard immediately receive anticoagulant and compression therapy. In case of pronounced oedema, compression therapy will consist of short stretch bandages until the oedema is relieved. In case of minor or no oedema, compression therapy with MECS will be prescribed. MECS should be “flat knitted” stockings pressure class II and if complicated by oedema class III with a high resistance coefficient. Objective documentation will consist of subjective and objective PTS score assessments and duplex ultrasound imaging (plus ambulant venous pressure measurements when indicated at time of making a therapeutic decision) will take place at 1,3,6,9 and 12 months and subsequently every year. Based on these objective measurements and assessments of PTS, DVT patients will be risk stratified at 6 months post-DVT for continuation or discontinuation of compression therapy with MECS and anticoagulation according to the proposed study design followed by discontinuation when no evidence of reflux obstruction or PTS symptoms are present.
Palareti et al. and other studies showed that normal versus increased D-dimer levels one month after discontinuation of regular anticoagulation is associated with an incidence DVT recurrence of about 5% patient-years and 10 to 15% patient/years respectively [23]. This difference was independent from other factors like thrombophilia or presence or absence of Residual Venous Thrombosis (RVT). Such post-DVT patients with increased sensitive D-dimer after discontinuation surely belong to the group of symptomatic post- DVT patients at high risk to develop PTS (score >3, Table 2 integrated in the algorithm [23,35]. In the prolong study, extended anticoagulation in post-DVT patients with increased D-dimer above the upper limit of normal will reduced the risk of DVT recurrence from 11% patient/ years to less than 2% patient/years, whereas the incidence of DVT recurrence was still increased, 4.4% patient/years, in post-DVT patients with a normal D-dimer on month after discontinuation of regular anticoagulation (Figure 2) [23,34]. Similar results have been produced in the DULCIS study using the standard cut off levels of quatitative D-dimer assays [35]. DVT recurrence in those patients with either a normal or increased D-dimer very likely do occur in those with incomplete or complete recanalization of the leg veins after 6 months with reflux score 3 or more (Table 1). This important observation has been confirmed by Latella et al. [36] in a prospective study of 305 DVT patients selected for quantitative ELISA D-dimer (VIDAS) measurement 4 months post-DVT. Of these 305 post-DVT patients 46% developed PTS (mild 25%, moderate 13%, severe 7%) and 54% did not develop PTS during 24 months follow-up. Mean D -dimer level measured 4 months post-DVT were significantly higher in patients with PTS vs without PTS (712 vs 444 ug/L P= 0.02) [36]. At time of D-dimer measurement 213 were taken anticoagulants. The PROLONG [23] and DULCIS studies [35] demonstrated the need to continue anticoagulant treatment in post-DVT patients with increased D-dimer level during anticoagulant treatment and when D-dimer levels are above the upper level of normal one month after discontinuation of anticoagulant treatment (Figure 2) [34-37].
Objective score
Complete recanalization at 3 or 6 months and no reflux
0
Incomplete recanalization at 6 and 12 months
1
Complete recanalization after 6 months and reflux
1
Incomplete recanalization after 6 months and reflux
2
Obstruction after 1 year without or with reflux
2
Normal D-dimer after discontinuation of anticoagulant therapy
0
Increased D-dimer after discontinuation of anticoagulant thereapy
3
Clinical score PTS:
Absent
0
Mild
1
Moderate
2
Total Rotterdam score
12
Table 1: The Rotterdam objective scoring system for grading the risk and severity of PTS during the first two years post-DVT based on prospective studies [20-25]: therapeutic implications.
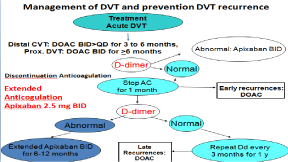
Figure 2: The European DVT - PTS Bridging the Gap study design 2014-
2017 designed by Michiels Moossdorff and Neumann [34,36].
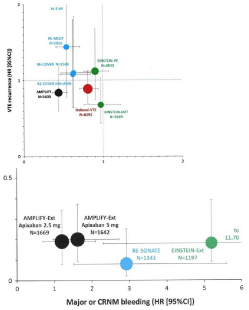
Figure 3: Meta-analysis of 3 to 6 months acute VTE treatment comparing
VKA against each of the DOACs for VTE recurrence vs MAJOR or CRNM
bleeding [38]. VTE versus CRNM bleeding rates (Hazard Ratios: 95% CI)
compared to VKA in patients 3 to 6 months treated in Einstein DVT and PE
with Rivaroxaban 15 mg BID (3 weeks) then 20 mg QD; in Recover I and II
with Heparin Dabigatran 150 mg BID; in AMPLIFY VTE with Apixaban 10mg
BID (7days) then 5 mg BID; and Hokusai-VTE with UFH Edoxaban 60 QD (or
30 mg QD adjustment).
Figure 3B: Lower figure VTE versus CRNM bleeding rates (Hazard ratios:
95% CI) compared to placebo in patients treated in AMPLIFY extension with
Apixaban 2.5 mg BID or 5 mg BID: in RE-SONATE with Dabigatran 150 mg
BID: and in Einstein extension with Rivaroxaban 20 QD.
Role of DOACs in acute DVT and prevention of DVT recurrence anno 2018 and beyond
The 2016 CHEST 10th edition on new antithrombotic guideline for treatment of Venous Thromboembolism (VTE) disease recommend Direct Oral Anticoagulants (DOACs) over Vitamine K Antogonist (VKA) for the initial 3 to 6 months treatment of patients with acute DVT or Pulmonary Embolism (PE). The Randimized Clinical Trials (RCTs) comparing DOACs versus VKA showed that DOACs are as effective as VKA therapy with reduced risk of bleeding and increased convenience for DVT and PE patients and for health care providers [38]. Five meta-analyses on the indirect comparison of the DOACs did show no significant differences in efficacy to prevent recurrent DVT and PE (VTE) including death from VTE between apixaban BID (twice daily), rivaroxaban OD (once daily) , LMWH-dabigatran and adjusted LMWH=edoxaban (Figures 3,4,5) [38-43]. Apixaban 10 mg BID (7 days) then 5 mg BID compared to Vitamin K Antagonist (VKA) in patients 3 to 6 months treated in the AMPLIFY study appearsto be associated with sihnificantly less bleeding (superiority in terms of major or Clinical Relevant Non-Major (CRNM) bleeds compared with rivaroxaban 15 mg BID (3 weeks) then 20 mg OD in the EISTEIN studies, Dabigatran 150 mg BID in the RE-COVER and edoxaban 60 mg OD (or adjusted 30 mg OD) compared to VKA (Figure 3 upper part) in the treatment of acute DVT and PE [38]. Apixaban vs VKA was in a similar indirect comparison of rivaroxaban vs VKA and dabigatran vs VKA also associated with less major bleeding for stroke prevention in patients with non-valvular atrial fibrillation (Figure 3) [38]. This overall evidence in subsequent large phase III DOAC trials and meta-analysis in two diseases VTE and AF is consistent [39-43]. Dabigatran inhibits thrombin (IIa) and apixaban, rivaroxaban and edoxaban directly blocks circulating Xa. The biological half life times of the Xa inhibitors apixaban, rivaroxaban and edoxaban is about 12 hours and do not differ significantly, but apixaban is administered BID as opposed OD for rivaroxaban and edoxaban (Figures 3,4,5) [38,40,42,43]. Theoretically, the Xa inhibitors apixaban, rivaroxaban and edoxaban are expected equally effective in terms of VTE reduction and bleeding complications compared to VKA when dosed BID according to their pharmacokinetic and pharmacodymic characteristics, it is repeatedly postulated that dosing differences of BID for apixaban versus OD for rivaroxaban and edoxaban account for the superiority of apixaban BID above rivaroxaban in terms of major and CRNM bleeds (higher peak levels with OD as compared to BID to reach equal efficacy in VTE reduction). This postulate has been confirmed by the demonstration that both apixaban 5 mg BID and 2.5 mg BID were superior to placebo (HR 1 to 2) as compared to rivaroxaban 20 mg OD to placebo (HR 5). Consequently, apixaban has been approved to become the first treatment option for treatment of acute DVT, low risk Pulmonary Embolism (PE) and extended anticoagulation for prevention of DVT recurrence (Leiden University Medical Center, Dr Huisman personal communication February 2017 and Guys and St Thomas Hospital London Dr Cohen personal communications 2016 and 2017). A total of 3365 patients were included in the extended EINSTEIN CHOICE study (median treatment duration, 351 days) of Bayer Pharmaceuticals comparing rivaroxaban 20 mg OD vs 10 mg OD against aspirin 100 mg during a median treatment duration 351 days [44]. The primary VTE efficacy outcome occurred in 1.5% of 1107 vs 1.2% of 1127 patients receiving 20 mg vs 10 mg OD of rivaroxaban respectively as compared with 4.4% of 1131 patients (4.4%) receiving aspirin (hazard ratio for 20 mg of rivaroxaban vs. aspirin, 0.34;95% CI, 0.20 to 0.59; hazard ratio for 10 mg of rivaroxaban vs. aspirin, 0.26; 95% CI, 0.14 to 0.47; P<0.001 for both comparisons). Rates of major bleeding were 0.5% vs 0.4% vs 0.3% and the rates of Clinically Relevant Nonmajor bleeding (CRNM) were 2.7%, 2.0%, and 1.8% in the group receiving 20 mg vs 10 mg OD of rivaroxaban vs aspirin 100 mg respectively, but a control arm was lacking [44]. The conclusion from the EINSTEIN CHOICE study is that the efficacy/safety profiles of rivaroxaban 20mg vs 10mg OD are equally effective but safer than aspirin but a control arm against which extended rivaroxaban 10mg OD should have been assessed was lacking. The conclusion of the Einstein Choice study DVT extension study should be that the Hazard Ratio (HR) of 5 for Rivaroxaban 20 mg OD vs placebo would not have been shortened by Rivaroxaban 10mg OD in the metaanalysis of Cohen et al for extended anticoagulation in figure 3. consequently, The Einstein Choice investigators should come up with a direct comparison of Rivaroxaban 10mg OD vs 5mg BID vs placebo. Large randomized clinical trials comparing the three Xa inhibtors in FDA approved dosing each apart will not solve the postulate that BID over OD for all Xa inhibitors is superior in terms of major and RCNM bleeds. Frost et al showed in a randomized direct comparison of the pharmacokinetics and pharmacodynamics of apaxaban BID vs rivaroxaban OD that apixaban 2.5mg BID demonstrated less intersubject variability in exposure, lower Area Under the Curve (AUC), and higher trough and smaller peakto- trough fluctuations in plasma concentration suggesting more constant anticoagulation compared with rivaroxaban 10mg OD [45]. The clinical impact of these differences on the relative efficacy and safety of apixaban and rivaroxaban remains to be determined. The Pharmaceutical Research & Development (R&D) and Academic clinical investigators are obliged to take full responsability to reanalyse the precilinical and clinical basis research and dose findings studies and to perform quality driven prospective direct comparisons of rviaroxaban 20mg OD versus 10mg BID and edoxaban 60mg OD versus 30mg BID in the treatment of acute DVT and PE; and to come up with RCTs directly comparing rivaroxaban 20 mg OD versus 10 mg BID and edoxaban 30 mg OD versus 15mg BID in DVT and PE extension studies for prevention of DVT recurrence and the postthrombotic syndrome. Based on equal efficacy and suprior safety profile apixaban should be considered to become the first treatment option for treatment of acute DVT, recebral embolic prevention atrial fibrillation and extended anticoagulation for prevention of DVT recurrence and PTS.
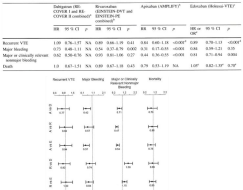
Figure 4: Direct comparison of Hazard Ratios (HR) Apixaban (A) vs Dabigatran (D), Rivaroxaban (R) and Edoxaban (E); and Rivaroxaban (R) versus Dabigatran
(D) and Edoxaban (E) [40].
Table top. Results acute VTE treatment for 3 to 6 months (Hazard ratios: HR compared to VKA) in randomized clincal trials with Dabigatran 150 mg BID,
Rivaroxaban 15 mg BID 3 weeks then 20 mg QD; Apixaban 10 mg BID 7 days then 5 mg BID; and Edoxaban 60 mg QD or 30 mg QD adjusted in terms of recurent
VTE, major bleeding major or clinically relevant nonmajor bleeding and death [39].
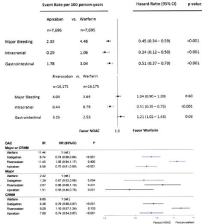
Figure 5: Forest plot depicting the hazard ratio for each paired propensity medication apixaban vs warfarin and rivaroxaba vs warfarin with regard to major
bleeding, intracranial bleeding and gastrointestinal bleeding in patients with nonvalvular atrial fibrillation [42].
Forest blot depicting the hazrd ratio for dabigatran, rivaroxaban and apixaban versus Oral Anticoagulation (OAC) with vitamin K antagonist for major or Clinical
Relevant Non-Major (CRNM) bleeding for major bleeding and CRNM in patients with nonvalvular atrial fibrillation [43].
Take home message
A novel clinical concept for the assessment of acute Deep Vein Thrombosis (DVT) and the Post-Thrombotic Syndrome (PTS) by DUS in routine clinical practice at 1, 3 to 6 months. And at one year post-DVT will separates post-DVT patients in 4 groups. Group 1: rapid complete recanalization within 3 months, no reflux at 6 months post-DVT, and no. PTS for which anticoagulation and MECS can be discontinued at 6 month post-DVT. Group 2, no PTS with reflux of the deep venous system and no PTS when wearing MECS for which anticoagulation should be continued until re-evaluation at 1 year post DVT. Group 3 and 4 PTS with reflux and incomplete recanalization or obstruction at 6 to 12 months post-DVT are candidates for longterm anticoagulation and MECS for at least 2 years or even longer to prevent DVT recurrence to prevent progression of PTS. A large scale prospective study is warranted to fine-tune and prove this concept.
Declaration of Interest
The first author is a founder of the Goodheart Intitute & Foundation in Nature Medicine & Health, Rotterdam, The Netherlands, Freedom of Science and Education, European Free University Network. JJ Michiels is co-founder of the Central European Vascular Forum (CEVF) and serves as consultant professor in the Bloodcoagulation, Hemostasis Research Laboratory (co-founder VWF-VWD research program) at the department of Hematology University Hospital, Antwerp; as consultant professor in Hematology and Bloodcoagulation, Comenius University, Bratislava, Slovakia; consultant to the Dutch Society of Internal Medicine and Ministery of Public Health; consultant of quality driven Industrial and Pharmaceutical Medicine; as an editor of 2 Medical Journals and as a guest editor on request and by self initiation.
References
- Freyburger G, trilaud H, Labrouche S, Gauthier P, Javorschi S, Bernard P, et al. D-dimer strategy in thrombosis exclusion – a gold standard study in 100 patients with suspected deep vein thrombosis or pulmonary embolism: 8 d-dimer methods compared. Thromb Haemostas. 1998; 79: 32-37.
- Van der Graaf F, van den Borne H, van der Kolk M, de Wild PJ, Janssen GW, van Uum SH. Exclusion of deep vein thrombosis with D-dimer testing – comparison of 13 D-dimermethods in 99 outpatients with suspected deep vein thrombosis using venography as reference standard. Thromb Haemostas. 2000; 83: 191-208.
- Perrier A, Desmarais S, Miron MJ, de Moerloose P, Lepage R, Slosman D, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999; 353: 190-195.
- Oudega R, Moons KG, Hoes AW. Ruling out deep venous thrombosis in primary care. A simplew diagnostic algorithm including D-dimer testing. Thromb Haemostas. 2005; 94: 200-205.
- Oudega R, Hoes AW, Moons KG. The Wells rule does not adequately rule out deep venous thrombosis in primary care patients. Ann Intern Med. 2005; 143: 100-107.
- Schutgens RF, Ackermark P, Haas FJ, Nieuwenhuis HK, Peltenburg HG, Pijlman AH, et al. Combination of a normal D-dimer concentration and a non-high pretest probability score is a safe strategy to exclude4 deep venous thrombosis. 2003; 107: 593-597.
- Wells PS, Brill-Edwards P, Stevens P, Panju A, Patel A, Douketis J, et al. A novel and rapid whole-blood assay for D-dimer in patients with clinically suspected deep vein thrombosis. 1995: 91: 2184-2187.
- Michiels JJ, Gadisseur A, van der Planken M, Schroyens W, de Maeseneer M, Hermsen JT, et al. A critical appraisal of non-invasive diagnosis and exclusion of deep vein thrombosis and pulmonary embolism in outpatients with suspected deep vein thrombosis or pulmonary embolism: how many tests do we need? Int Angiol. 2005; 24: 27-39.
- Michiels JJ, Gadisseur A, Van DerPlanken M, Schroyens W, De Maeseneer M, Hermsen JT, et al. Different accuracies of rapid enzyme-linked immunoabsorbant, turbidimetric, and agglutination D-dimer assays for thrombosis exclusion: impact on diagnostic work-ups of outpatients with suspected deep vein thrombosis and pulmonary embolism. Sem Thromb Hemostas. 2006; 32: 678-693.
- Oudega R, Toll DB, Bulten RJ, Hoes AW, Moons KGM. Different cut-off values for two D-dimer assays to exclude deep venous thrombosis in primary care. Thromb Haemostas. 2006; 95: 744-746.
- Kraaijenhagen RA, Piovelli F, Bernardi E, Verlato F, Beckers EA, Koopman MM, et al. Simplification of the diagnostic management of suspected deep vein thrombosis. Arch Intern Med. 2002; 162: 907-911.
- Tick LW, Ton E, Voorthuizen TH, Hovens MM, Leeuwenburgh I, Lobatto S, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography and D-dimer test. Am J Med. 2002; 113: 630-635.
- Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep vein thrombosis. N Eng J Med. 2003; 349: 1227-1235.
- Kearon C, Ginsberg JS, Douketis J, Crowther MA, Turpie AG, Bates SM, et al. A randomized trial of diagnostic strategies after normal proximal vein ultrasonography for suspected deep vein thrombosis: D-dimer testing compared with repeated ultrasonography. Ann Intern Med. 2005; 142: 490-496.
- Meissner MH, Manzo RA, Bergelin RO, Markel A, Strandness E. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surgery. 1993; 18: 596-608.
- Markel A, Manzo RA, Bergelin RO, Strandness DE. Valular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg. 1992; 15: 377-384.
- Markel A. Origin an natural history od deep vein thrombosis of the legs. Semin Vasc Surg. 2005; 5: 65-74.
- Siragusa S, MalatoA, Anastasio R, Cigna V, Milio G, Amato C, et al. Residual vein thrombosis to establish duration of antiocoagulation after a first episode of deep vein thrombosis. 2008; 112: 511-516.
- Prandoni P, Lensing AWA, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002; 137: 955-960.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Pancani C, Coccheri S. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemostas. 2002; 87: 7-12.
- Palareti G, Legnani C, Cosmi B, Valdré L, Lunghi B, Bernardi F, et al. Predictive value of D-dimer test for recurrent venous thromboembolism after anticoagulant withdrawal in subjects with a previous idiopathic event in carriers of congenital thrombophili. 2003; 108: 313-318.
- Eichinger S, Minar E, Bialonczyk C, Hirschl M, Quehenberger P, Schneider B, et al. D-dimer levels and risk of recurrent venous thromboembolism. 2003; 290: 1071-1074.
- Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, et al. D-dimer testing to determine the duration of anticoagulant therapy. New Eng J Med. 2006; 355: 1780-1789.
- Brandjes DP, Buller HR, Heijboer H, Huisman MV, de Rijk M, Jagt H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein-thrombosis. 1997; 349: 759-762.
- Prandoni P, Lensing AWA, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome. A randomized controlled trial. Ann Intern Med. 2004; 141: 249-256.
- Eklof B, Rutherford RB, Bergan J, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004; 40: 1248-1252.
- Widmer LK, Plechl SC, Leu HJ, et al. Venenkrankungen bei 1800 Berufstätigen. Basel Studie II. Schweiz Med Wochensch. 1967; 97: 107-110.
- Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000; 31: 1307-1312.
- Prandoni P, Lensing AWA, Cogo A, Cuppini S, Villalta S, Carta M, et al. The long-term clinical course of acute deep vein thrombosis. Ann Intern Med. 1996; 125: 1-7.
- Bernardi E, Bagatella P, Frulla M, Simioni P, Prandoni P. Postthrombotic syndrome: incidence, prevention, and management. Sem Vasc Med. 2001; 1: 71-79.
- Pesavento R, Bernardi E, Concolato A, Dalle Valle F, Pagnan A, Prandoni P. Postthrombotic syndrome. Sem Thromb Hemostas. 2006; 32: 744-751.
- Roumen-Klappe EM, den Heyer M, Janssen MCH, van der Vleuten C, Thien T, Wollersheim H. The post-thrombotic syndrome: incidence and prognostic value of non-invasive venous examinations in a six-year follow-up study. Thromb Haemostas. 2005; 94: 825-830.
- Wentel TD, Neumann HAM. Management of the postthrombotic syndrome: The Rotterdam approach. Semin Thromb Hemost. 2006; 32: 814-821.
- Michiels JJ, Moosdorff W, Michiels JM, Barth JB, Maasland H, Lao MU, et al. Duplex ultrasound, clinical score, thrombotic risk, and D-dimer testing for evidence based diagnosis and management of deep veinthrombosis andalternative diagoses in the primary care setting and outpatient ward. Int Angiol. 2014; 33: 1-19.
- Palareti G, Cosmi B, Legnani C, Antonucci E, De Micheli V, Ghirarduzzi A, et al. D-dimer to guide the duration of anticoagulation in patients with venous thromboembolism: a management study. Blood. 2014; 124: 196–203.
- Latella J, Desmarrais S, Miron MJ, Roussin A, Joyal F, Kassis J, et al. Relation between D-dimer level, venous valvular reflux and the development of post-thrombotic syndrome after deep vein thrombosis. J Thromb Haemostas. 2010; 8: 2169-2175.
- Michiels JJ, Moossdorff W, Lao MU, Maasland H, Michiels JM, Neumann HAM, et al. Diagnosis and treatment of DVT and prevention of DVT recurrence and the PTS: bridging the gap between DVT and PTS in the primary care setting or outpatient ward. J Vasc Diagn Intervent. 2017; 5: 21-34.
- Cohen A, Imfeld S, Rider T. Phase III trials of new oral anticoagulants in the acute treatment and secondary prevention of VTE: comparison and critique of study methodology and results. Adv Ther. 2014;31:473-493.
- Cohen AT, Hamilton M, Bird A, Mitchell SA, Horblyuk R, Batson S, et al. Comparison of the Bon-VKA oral anticoagulants apixaban, dabigatran and rivaroxaban in the extended treatment and prevention of VTE: systemic review and Network meta-analysis. Plos One. 2016; 11: 160064.
- Mantha S, Ansell J. Indiret comparison of dabigatran, rivaroxaban, apixaban and endoxaban fort he treatment of acute venous thromboembolism. J Thromb Thrombolysis. 2015; 39: 155-165.
- Baker WL, Phung QJ. Systemic review and adjusted indirect comparison of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012; 5: 711-719.
- Halvorson S, Ghanima W, Tvete IF, Hoxwash C, Falck P, Solli O, et al. A nationwide registry stufy to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulation. Eur Heart J Cardiovasc Pharmacother. 2017; 3: 28-36.
- Yao X, Abraham NS, Sangarahingham LR, Bellolio MF, McBamr RD, Shah ND, et al. Effectivenes and safey of dabigatran, rivaroxaban and apixaban vesus warfarin in nonvalvular atrial fibrillation. Am Heart J. 2016; 5: 003725.
- Weitz J, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameax H, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Eng J Med. 2017.
- Frost C, Sony Y, Barrett YC, Dursly J, Boyd RA, LaCreta F, et al. A randomized direct comparison of pharmackinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharm Adv Apllic 2014; 6: 179-187.