
Review Article
Thromb Haemost Res. 2018; 2(1): 1010.
Endothelial Wnt-Pathway Activation Protects the Blood- Retinal-Barrier during Neurodegeneration-Induced Vasoregression
Kolibabka M¹*, Acunman K¹, Riemann S¹, Huang H², Gretz N³, Hoffmann S³, Feng Y² and Hammes HP1,4
¹5th Medical Department, Heidelberg University, Germany
²Institute for Experimental Pharmacology and Toxicology, Heidelberg University, Germany
³Medical Research Center, Medical Faculty Mannheim, Heidelberg University, Germany
4European Center for Angioscience (ECAS), Germany
*Corresponding author: Kolibabka M, 5th Medical Department, Heidelberg University, Medical Faculty Mannheim, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany
Received: March 08, 2018; Accepted: April 27, 2018; Published: May 18, 2018
Abstract
Vasoregression and impairment of the blood-retinal-barrier are hallmarks of diabetic retinopathy and especially the loss of pericytes is believed to be in part responsible for increased capillary permeability. However, heterogeneity in retinal permeability despite homogeneous pericyte loss raise doubts concerning this responsibility. In this study, we identify the mechanisms preserving bloodretinal barrier integrity despite the loss of pericytes. In male homozygous Polycystic Kidney Disease (PKD) rat, a model of neurodegeneration-induced vasoregression, we demonstrate the loss of pericytes without increased permeability by quantitative retina morphometry and immunofluorescence staining. Expression profiling of adherens junction associated genes via qPCR arrays revealed the up regulation of Wnt-pathway dependent factors as possible underlying mechanism. A gene regulatory network analysis identified the Lymphoid Enhancer binding Factor 1 (Lef1), a transcription factor in non-canonical Wnt-signaling, as upstream effectors for the observed gene regulation. Lef1 expression was up regulated in PKD rats and repression of Lef1using siRNA resulted in increased permeability in Human Umbilical Vein Endothelial Cells (HUVECs) in vitro. Regulation of Lef1 gene expression during the course of vasoregression was mediated by a demethylation of the Lef1 promoter in PKD rats as demonstrated by methylation dependent qPCR. Our data demonstrate that capillary endothelial cells are able to preserve the integrity of the blood-retinal-barrier despite a loss of pericytes by promoterdemethylation and consecutive up regulation of Lef1. This novel principle may lead to new therapeutic approaches by targeting endothelial Lef1 in diseases with impaired vascular barriers.
Keywords: Retinopathy; Pericyte loss; Wnt-pathway; Lymphoid enhancer binding factor 1; Vasoregression
Abbreviations
PKD: Polycystic Kidney Disease; qPCR: Quantitative Real Time Polymerase Chain Reaction; Wnt: Wingless-type MMTV Integration Site Family; Lef1: lymphoid Enhancer binding Factor 1; siRNA: small interfering Ribonucleic Acid; HUVEC: Human Umbilical Vein Endothelial Cell; BRB: Blood-Retinal Barrier; VEGF - Vascular Endothelial Growth Factor; VE-Cadherin: Vasculo-Endothelial cadherin; SD: Sprague Dawley; Cq: Cycle of quantification; Hnf4a: Hepatocyte nuclear factor 4 alpha; TMB: 3,3’,5,5’-Tetramethylbenzidine; gDNA: genomic Desoxyribonucleic Acid; MMP2: Matrix Metalloproteinase 2.
Introduction
Retinal degeneration in the pathogenesis of retinopathies affects every compartment of the neurovascular unit. The impairment of the retinal vasculature is the basis of a variety of retinopathies, most prominently in forms of macular edema and in diabetic retinopathy [1]. Increased permeability, para- and transcellular, loss of pericytes and formation of acellular capillaries are the most prominent vascular aberrations in the early phases of experimental retinopathies [2-4].
Increased capillary permeability in diabetic retinopathy primarily arises as response to increased levels of retinal Vascular Endothelial Growth Factor (VEGF). However, the loss of pericytes is assumed an additional factor in the Breakdown of The Blood-Retinal-Barrier (BRB) [5-7].
The polycystic kidney disease (CMV-HA-PKD2(1-703), PKD) rat is an experimental model for neurodegeneration-induced vasoregression. Due to a primary degeneration of photoreceptor cells, the retinal oxygen consumption decreases over the course of the degeneration in the PKD rat [8,9]. Due to the reduced oxygen demand, the PKD retina does not develop ischemia, despite vasoregression. Without ischemia, the retinal VEGF levels in the PKD rat remain on low to normal levels, allowing the investigation of the impact of pericyte loss on blood-retinal permeability without the influence of VEGF [10]. In contrast to a recently published study, in which a minor loss of pericytes was associated with a slightly increased capillary permeability, the vasoregression in the PKD rat is severely aggravated, leading to faster dynamics in vascular remodeling processes [11].
BRB integrity is dependent on a variety of factors, mainly inter-cellular junction molecules on different levels and capillary blood flow, locally regulated by pericytes [7,12]. The inter-cellular communication within the endothelium and between endothelial cells and pericytes deserves special attention. Junctional components of note are inter-endothelial tight and adherens junctions and especially adherens junctions are connected to an intracellular signaling network, which mediates endothelial-pericyte communication, too [13-17]. Whereas tight junctions represent a more restrictive barrier than adherens junctions, the versatility of the later make them a potential target for interventions in diabetic retinopathy [7]. Apart from their physical resistance, adherens junction associated VEcadherin acts as intracellular beta-caten in trap and is able to influence the activity of Wnt-signaling pathways via the release of beta-catenin [18]. Altered Wnt-pathway activity is involved in the pathogenesis of diabetic retinopathy in patients and experimental models and, in these models, has been linked to impaired VE-cadherin beta-catenin interactions [19-22]. In addition to Wnt-pathway activity, betacatenin is involved in the regulation of Notch-signaling pathway activity via association with presenilin-1[23]. Notch-signaling is not only crucial in angiogenesis, but also in maintenance of vascular stability and barrier integrity by regulating adherens junctions [24- 26].
In this study, we analyze the mechanistic connections between BRB integrity and adherens junctions in a normoglycemic model of neurodegeneration-induced vasoregression with negligible impact of VEGF signaling.
Materials and Methods
Animals
Male homozygous polycystic kidney disease (CMV-HA-PKD2 (1-703), PKD) rats were generated and genotyped as previously described [8]. The animals were kept in a 12 hours light-dark cycle with food and water ad libitum. At 4 and 8 weeks of age animals were killed, the eyes enucleated, snap-frozen in liquid nitrogen and stored at -80°C. Age-matched Sprague Dawley (SD) rats (https://www. janvier-labs.com/rodent-research-models-services/research-models/ per-species/outbred-rats/product/sprague-dawley.html) served as controls (Janvier Labs, Le Genest-Saint-Isle, France). All animal experiments were conducted in compliance with the EC directive 2010/63/EU, the guidelines of the statement for the use of Animals in Ophthalmic and Visual Research (ARVO) and have been reported following the ARRIVE guidelines. This study was approved by the federal animal welfare committee (Regierungspräsidium Karlsruhe, Karlsruhe, Germany).
Quantitative retina morphometry
Quantitative retina morphometry was performed on retinal digest preparations to analyze acellular capillaries and pericytes as previously published [27]. Investigators were simply blinded for quantification.
Albumin immunofluorescence
Vertical 6μm paraffin sections were stained for rat albumin at 8 weeks of age. After deparaffinization, antigen retrieval was performed using citrate buffer. Sections were rehydrated, permeabilized and unspecific protein binding was blocked using 0.5% Triton X-100 (Sigma Aldrich, Steinheim, Germany) and 2% horse serum (Dako, Santa Clara, USA). Sections were incubated overnight at 4°C with a sheep anti-rat albumin antibody (9 0220-2424, 1:100, Biotrend, Cologne, Germany). Donkey anti-sheep Alexa Fluor 555 (A21436, 1:200, Thermo Fisher, Waltham, USA) was used as secondary antibody. Images of three animals per group with 5 slides per animal were obtained using a confocal microscope (Leica TCS SP8, Wetzlar, Germany).
qPCR of adherens junction genes
RNA was isolated using Trizol reagent according to the manufacturer’s protocol (Invitrogen, Karlsbad, USA). Reverse transcription was performed using the RT² First Strand Kit according to the manufacturer’s protocol with a total input of 1 μg RNA per sample (Qiagen, Hilden, Germany). For the qPCR of adherens junction associated genes, the RT² Profiler PCR Array Rat Adherens Junction was used in combination with the RT² SYBR Green ROX qPCR Mastermix (Qiagen, Hilden, Germany). The targets and RefSeqs are listed in Supplementary file 1. Relative expression and fold-regulations were calculated using the 2-ΔΔCq method with multiple reference genes. B2m was excluded from the reference gene calculation due to significant regulation.
qPCR of transcription factor genes
Reverse transcription was performed using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). Subsequent qPCR was performed with hydrolysis probes for Lef1 (NM_130429.1, Rn01639120_m1) and Hnf4a (NM_001270931.1, NM_001270933.1, NM_022180.2, Rn04339144_m1). Gapdh (NM_017008.4, Rn99999916_s1) was used as reference gene (all primers purchased from Thermo Fisher, Waltham, USA). Fold regulations were calculated using the 2-ΔΔCq method.
Permeability assay
Human umbilical vein endothelial cells (HUVECs, Thermo Fisher, Waltham, USA) were cultured in 6-well plate hanging inserts with 1μm pores (Merck, Darmstadt, Germany). After reaching 90% confluence, HUVECs were transfected with siRNAs against Lef1 (Hs02_00349169) or Hnf4a (Hs01_00124507, both from Sigma Aldrich, Steinheim, Germany) using TransIT-siQUEST transfection reagent (Mirus, Madison, USA). The siRNA Universal Negative Control #1 served as control (Sigma Aldrich, Steinheim, Germany). After 24 hours, medium was changed and horseradish peroxidase was added to a final concentration of 50μg/mL (Sigma Aldrich, Steinheim, Germany) to the upper wells. After an incubation of 30 min, 10μL medium was collected from the lower wells and added to 90μL substrate solution containing 54μg 3,3’,5,5’-tetramethylbenzidine (TMB, Sigma Aldrich, Steinheim, Germany). After 5 minutes 20μL of 1mM sulfuric acid were added to stop the reaction (Sigma Aldrich, Steinheim, Germany) and the absorption at 450nm was measured using a microplate reader (Tecan, Männedorf, Switzerland).
Lef1 promoter methylation qPCR
Retinae were dissected from frozen eyes and gDNA was isolated using a commercially available kit (Thermo Fisher, Waltham, USA). DNA was digested using methylation sensitive and methylation dependent restriction enzymes (Qiagen, Hilden, Germany) and subsequently subjected to SYBR green ROX qPCR using assay optimized primers (Lef1 CpG island 104915, EPRN104915-1A and Lef1 CpG island 104916, EPRN104916-1A, both Qiagen, Hilden, Germany). Methylated and non-methylated fractions were calculated according to the manufacturer’s protocol.
Statistical analysis
All data are reported as mean±standard deviation unless stated otherwise. Statistical analyses were performed using GraphPad Prism v6.01 (GraphPad Software, La Jolla, USA). For comparisons between groups over different time-points, two-way ANOVA with Sidak’s multiple comparison tests were used. For the permeability assay, a one-way ANOVA with Holm-Sidak’s multiple comparison test was used. A p<0.05 was considered statistically significant. One data point was eliminated from the quantitative retina morphometry due to a bleached preparation.
Results and Discussion
Early vasoregression with intact blood-retinal-barrier
Retinal digestion preparations demonstrated an early onset of vasoregression, aggravating with 8 weeks of age. Formation of acellular capillaries was significantly higher in PKD compared to SD rats from 4 weeks of age on, with an additional elevation at 8 weeks of age (4 weeks: 12±1 vs 29±1, p<0.0001; 8 weeks: 17±1 vs 51±4, p<0.0001, SD vs PKD, n=4-5, Figure1a). In line, the number of pericytes was significantly lower from 4 weeks on, but showed no additional decrease with 8 weeks of age (4 weeks: 2763±195 vs 1915±65, p=0.0005; 8 weeks: 2532±243 vs 1798±81, p=0.0012, SD vs PKD, n=4-5, Figure 1b). This severe pericyte loss and high numbers of acellular capillaries are in line with previous studies and comparable to the retinal phenotype observed in diabetic rat models [28,29]. As the loss of pericytes is thought to be a crucial process in bloodretinal- barrier breakdown, the permeability of retinal capillaries was assessed with albumin staining. Despite the significantly decreased numbers of pericytes in PKD rats, no extra-vasation of albumin was observed at any time point (n=4, Figure 1c). The absence of increased permeability despite the severe pericyte loss, even without elevated VEGF levels seems to be in contrast with previously published results [11]. Differences of note between these studies include the higher grade of inflammation and the changes in Notch-expression in the PKD rat [30].
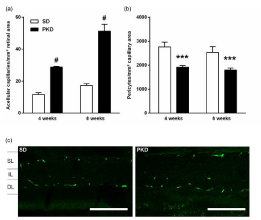
Figure 1: Vasoregression with preserved BRB integrity. (a) Increased
numbers of acellular capillaries in PKD rats from 4 weeks of age on.
Aggravating vasoregression in 8 weeks old PKD rats. (b) Pericyte numbers
are significantly reduced in PKD rats similarly at 4 and 8 weeks of age. (c)
Albumin immunofluorescence stainings of vertical paraffin sections show no
sign of extravasated albumin in 8 weeks old SD and PKD rats. (a)+(b) n=4,
***p<0.001, #p<0.0001. (c) Scale bar=100μm.
Upregulation of adherens junction-associated Wntpathway components
Expression profiling of adherens junction associated genes showed significantly different regulated genes in PKD rats with an age-dependency, changing from 4 to 8 weeks of age (n=6, Figure 2a). At 4 weeks, 7 of 84 genes were significantly regulated in PKD rat retinae with Cdh3 being the only transcript identified as upregulated (Figure 2b), Table 1). At 8 weeks of age, 13 of 84 genes were significantly regulated (Figure 2b, Table 1). None of the genes identified at 4 weeks was persistently regulated at 8 weeks of age. In contrast to the earlier time-point, 12 of 13 genes were up- and only one transcript was down regulated (Figure 2b, Table 1). Due to the tight relations between adherens junctions and the Wnt-pathway as well as the observed gene regulatory pattern, the gene regulatory network analysis predicted an increased activation of the Wnt-pathway in 8 weeks old PKD rat retinae. The association between vascular barrier function and Wnt-pathway activity has been reported in mice lacking Fzd7, a key receptor in canonical Wnt-pathway activation, which is associated with adherens-junctional VE-Cadherin (Cadh5) and β-catenin (Ctnnb1). Fzd7 knock-out lead to an increase in vascular permeability, whereas overexpression of Ctnnb1 and subsequent re-/over-activation of the Wnt-pathway rescued the phenotype, normalizing vascular permeability similar to our data [31].
Gene Symbol
Fold regulation 1m
p-value 1m
Fold regulation 2m
p-value 2m
Actn3
1.32
0.156
3.00
0.004
Cdc42
1.32
0.862
1.89
0.024
Cdh1
-1.21
0.592
6.03
0.028
Cdh2
-1.20
0.385
2.37
0.006
Cdh3
2.10
0.022
1.49
0.325
Ctnna3
-2.40
0.329
2.38
0.006
Dsg1
2.64
0.220
9.49
0.022
Exoc2
-4.81
0.150
-34.59
0.001
Notch1
1.05
0.745
1.88
0.024
Pik3cg
1.05
0.739
5.99
0.024
Pnn
-1.51
0.226
1.87
0.025
Ppap2b
-1.20
0.427
1.90
0.024
Pvrl3
-1.91
0.114
1.89
0.025
Rala
-1.51
0.048
1.18
0.515
Rhoa
-1.51
0.048
1.18
0.527
Tjp1
-1.51
0.049
1.19
0.309
Vcl
-1.51
0.049
1.19
0.287
Vezt
-1.92
0.045
-1.06
0.734
Was
1.05
0.679
3.03
0.047
Yes1
-1.51
0.049
1.19
0.490
Table 1: Expression of relevantly regulated adherens junction associated genes. Significantly regulated genes are listed in alphabetical order. Fold regulation PKD vs SD, significant regulation in bold, non-significant regulation in regular italics. Two-way ANOVA with Sidak’s multiple comparison analysis, p<0.05 was considered statistically significant.
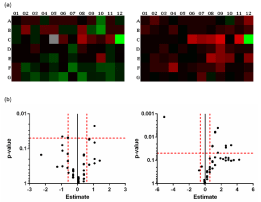
Figure 2: Gene expression of adherens junction associated genes. (a)
Heatmap analysis of 84 adherens junction associated genes in 4 weeks old
(left) and 8 weeks old (right) old SD and PKD rat retinae. Illustrated is upregulation
(red) to down-regulation (green), PKD vs SD. (b) Volcano plot of
adherens junction associated genes in 4 weeks (left) and 8 weeks old (right)
SD and PKD rat retinae. Red dashed lines indicate thresholds for significant
(y-axis: p=0.05) and relevant regulation (x-axis: estimate= -0.5849 and
0.5849 corresponding to a fold regulation= -1.5 and 1.5).
Lef1 repression increases endothelial permeability
For the identification of possible upstream effectors responsible for the observed gene expression in PKD rats, we performed a gene regulatory network analysis with the significantly regulated genes using a freely available online tool (GNCPro, SABiosciences Qiagen, Hilden, Germany). Based on the gene regulation, two Wnt-pathway related transcription factors were identified as possible upstream effectors by the in silico analysis, Hnf4a and Lef1 (Figure 3a). In order to validate the prediction, we performed qPCRs for Hnf4a and Lef1 in 4 and 8 weeks old rat retina. Neither at 4 nor at 8 weeks did Hnf4a show a significant regulation in PKD rat retinae (4 weeks: 1.48±0.32- fold of SD, p=0.1677, 8 weeks: 1.35±0.39-fold of SD, p=0.2406, n=4, Figure 3b). At 4 weeks of age, Lef1 expression showed no significant differences between SD and PKD rats (1.28±0.14-fold of SD, p=0.1161, n=4). However, with 8 weeks Lef1 was significantly up regulated by over 150% in the retinae of PKD rats (2.58±0.24-fold of SD, p<0.0001, n=4, Figure 3c). The role of Lef1 in the vasculature had yet to be studied in detail. During angiogenesis, endothelial Lef1 plays multiple roles. It regulates endothelial cell invasion and thereby the initial steps of angiogenesis by modulating MMP2 expression when targeted by active β-catenin [32]. During vasculogenesis, Wnt3a signaling determines mesodermic specialization toward endothelial cells via Lef1, indicating its key role in the formation of vessels in endothelial cells [33]. The balance between Notch and Wnt signaling is crucial in angiogenesis, determining retinal vascular density. The Wnt signaling activity in this process is mediated by Lef1 activity in angiogenic stalk cells, with loss of Lef1 resulting in vasoregression [34].
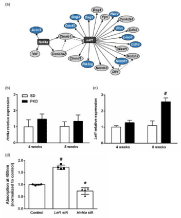
Figure 3: Predicted upstream effectors and impact on endothelial
permeability. (a) Gene regulatory network analysis identifies Wnt pathway
components Hnf4a and Lef1 as relevant upstream effectors for the observed
gene expression changes in 8 weeks old PKD rats. Blue=relevantly regulated
genes, dashed arrows indicate predicted up-regulation, arrows indicate
predicted transcription factor activity. (b) Hnf4a shows no significant regulation
in PKD rat retinae at neither time-point. (c) Lef1 is significantly up-regulated
in 8 weeks old PKD rat retinae but not in 4 weeks old. (d) Knock-down of
Lef1 results in significantly increased permeability in HUVECs in vitro. Hnf4a
knock-down leads to significantly increased permeability as assessed by a
HRP diffusion assay. n=4, *p<0.05, #p<0.0001.
In order to confirm the impact of Lef1 on the permeability of endothelial cells, we performed an in vitro permeability assay with knock-outs of Lef1 and Hnf4a in HUVECs. In accordance with the observations in the PKD rat retinae, the knock-out of Lef1 resulted in a significant increase in transendothelial permeability of 70% (1.72±0.09 of control, p<0.0001, n=4). In contrast, knock-out of Hnf4a had only a minor effect, decreasing transendothelial permeability by 25% (0.74±0.14 of control, p=0.0233, n=4, Figure 3d).
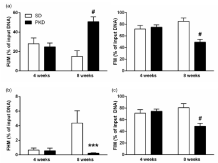
Figure 4: Promoter demethylation underlying Lef1 regulation in PKD rats.
(a) Fraction of non-methylated (FUM, left) Lef1 is significantly increased and,
reversely, methylated Lef1 (FM, right) is significantly decreased in 8 weeks
old PKD rats. (b) Fraction of Hyper-Methylated (FHM) Lef1 increases from 4
to 8 weeks but remains on a similar level in PKD rats, leaving it significantly
lower in 8 weeks old PKD rats. (c) The Fraction of Intermediary Methylated
(FIM) Lef1 is significantly decreased in 8 weeks old PKD rats. n=4, ***p<0.001,
#p<0.0001.
The differences in gene expression between 4 and 8 weeks old rats show the fast dynamics of vascular remodeling and the gene regulatory response to the aggravating vasoregression. The upregulation of Lef1 dependent genes runs parallel to the increase in inflammatory response signaling in the inner retina, possibly posing an additional threat to the integrity of the BRB [30,35].
Decreased Lef1 methylation in PKD rat retinae
Possible regulators of the observed Lef1 regulation included expression changes directly mediated by Ctnnb1 or Csnk2a2 (Figure 3a), an epigenetic regulation like DNA methylation or histone modification or post-translational mechanisms like miRNA mediated regulation. As Ctnnb1 and Csnk2a2 were present in our dataset and were not significantly regulated, their involvement in Lef1 regulation was considered as not relevant.
In order to identify possible histone modifications or miRNAs that could explain the Lef1 regulation in the context of the observed gene expression pattern we performed a gene list enrichment analysis using Enrichr [36,37].Under consideration of the Lef1 dependent gene regulation, no histone modification or candidate miRNA could be identified as possible Lef1 regulator on the background of this gene expression data set.
Next, we analyzed the DNA methylation of the Lef1 gene in the retinae of SD and PKD rats.The rat Lef1 gene possesses two CpG islands as possible DNA methylation sites, one at the translation start site and the other 5’ of the promoter.The CpG island 5’ of the promoter site (primer 104916) showed no methylation in the retinae of either strain. However, the CpG island at the translation start site (primer 104915) showed an age dependent dynamic in its methylation status. At 4 weeks of age, SD and PKD rats both had a methylated fraction of about 70% (71.94±5.91% vs 75.15±3.88%, SD vs PKD, p=0.4034, n=4, Figure 4a). In SD rats, the fraction of methylated DNA increased with 8 weeks by 15%, whereas in PKD rats, the methylated DNA decreased by 25% compared to 4 weeks old rats (85.06±5.87% vs 49.21±5.00%, SD vs PKD, p<0.0001, n=4, Fig 4(a)). This divergence between SD and PKD rats was due to a dual mechanism. In SD rats, the fraction of hyper-methylated DNA showed a 6.5-fold increase from 4 to 8 weeks, which was not observed in PKD rats (4 weeks: 0.66±0.29% vs 0.56±0.37%, p=0.8813, 8 weeks: 4.36±1.70% vs 0.23±0.07%, p=0.0001, SD vs PKD, n=4, Figure 4b). The greatest portion of the methylated DNA was represented by the intermediary methylated DNA, whichin SD rats increased from 4 to 8 weeks by 9%. In contrast to the hyper-methylated fraction, PKD rats not only showed unchanged, but decreased levels of intermediary methylated DNA (4 weeks: 71.28±6.16% vs 74.59±3.85%, p=0.4212, 8 weeks: 80.70±6.99% vs 48.98±4.93%, p<0.0001, SD vs PKD, n=4, Fig4(c)). DNA methylation in promoter regions is associated with transcriptional repression. As a dynamic process, the methylation status of DNA is dependent on the balance of methylation and demethylation and decreased DNA methylation can be the result of either decreased methylation or increased demethylation, shifting this balance [38-40]. In order to determine which one of these applies to the PKD rat, the activity of the enzymes involved would have to be quantified. As the pathogenic processes in the PKD rat connecting neurodegeneration to vasoregression are still not completely understood, the quantification of enzyme activity would have to be performed with retinal tissue and cannot be modelled in vitro [9,30,41]. Given the mechanism demonstrated by our data and the annotation to endothelial cells, the number of target cells is relatively small within the heterogeneous retinal tissue. The number of different enzymes involved in the methylation of DNA is quite small with no described cell-type specific forms in the retina [20,38,39]. Therefore, the expected changes in enzyme activity would be so small, that the precision of the measurement could hardly identify significant differences between SD and PKD rats that are reliably related to the Lef1 methylation.
Conclusion
In this study we demonstrate the preservation of blood-retinalbarrier integrity during vasoregression by an upregulation of Lef1 dependent adherens junction genes. Our data underline the importance of vascular Lef1expression for sustained BRB integrity during retinal vasoregression. Pericyte degeneration or migration alone is not sufficient to impair the BRB, as we demonstrate the endothelial ability to counteract this loss of cell-cell regulation. Increased permeability and pericyte loss are pathogenic hallmarksof a variety of cerebrovascular diseases, whereLef1-mediated Wnt signaling is a potential therapeutic target for their prevention and treatment.
Acknowledgement
This study was funded by the Deutsche Forschungs Gemeinschaft (DFG) with grants to YF (Fe 969/2-1), HPH (Ha 1755-10/1) and within the GRK1874 DIAMICOM (MK, KA, SR, HH, YF and HPH). The authors declare no competing financial interests. The authors would like to thank Nadine Dietrich for conducting the retinal digest preparation.
References
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016; 51: 156-186.
- Hammes H-P, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011; 60: 9-16.
- Hammes H-P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018; 61: 29-38.
- Díaz-Coránguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017; 139: 123-137.
- Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AEB, Al-Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003; 19: 442-455.
- Valdez CN, Arboleda-Velasquez JF, Amarnani DS, Kim LA, D’Amore PA. Retinal microangiopathy in a mouse model of inducible mural cell loss. Am J Pathol. 2014; 184: 2618-2626.
- Cunha-Vaz J. The Blood-Retinal Barrier in the Management of Retinal Disease: EURETINA Award Lecture. Ophthalmologica. 2017; 237: 1-10.
- Gallagher AR, Hoffmann S, Brown N, Cedzich A, Meruvu S, Podlich D, et al. A truncated polycystin-2 protein causes polycystic kidney disease and retinal degeneration in transgenic rats. J Am Soc Nephrol. 2006; 17: 2719-2730.
- Feng Y, Wang Y, Stock O, Pfister F, Tanimoto N, Seelinger MW, et al. Vasoregression linked to neuronal damage in the rat with defect of polycystin-2. PLoS One. 2009; 4.
- Feng Y, Wang Y, Yang Z, Wu L, Hoffmann S, Wieland T, et al. Chronic hyperglycemia inhibits vasoregression in a transgenic model of retinal degeneration. Acta Diabetol. 2014; 51: 211-218.
- Hu J, Dziumbla S, Lin J, Bibli S-I, Zukunft S, de Mos J, et al. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017; 552: 248-252.
- Kisler K, Nelson AR, Rege S V, Ramanathan A, Wang Y, Ahuja A, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci. 2017; 20: 406-416.
- Wisniewska-Kruk J, Klaassen I, Vogels IMC, Magno AL, Lai CM, Van Noorden CJF, et al. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res. 2014; 122: 123-131.
- Trost A, Lange S, Schroedl F, Bruckner D, Motloch KA, Bogner B, et al. Brain and Retinal Pericytes: Origin, Function and Role. Front Cell Neurosci. 2016; 10: 1-13.
- Klaassen I, Van Noorden CJF, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013; 34: 19-48.
- Klaassen I, Hughes JM, Vogels IMC, Schalkwijk CG, Van Noorden CJF, Schlingemann RO. Altered expression of genes related to blood–retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009; 89: 4-15.
- Rho S-S, Ando K, Fukuhara S. Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions. J Nippon Med Sch. 2017; 84: 148-159.
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol. 1995; 129: 203-217.
- Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, et al. Activation of the Wnt Pathway Plays a Pathogenic Role in Diabetic Retinopathy in Humans and Animal Models. Am J Pathol. 2009; 175: 2676-2685.
- Berdasco M, Gómez A, Rubio MJ, Català-Mora J, Zanón-Moreno V, Lopez M, et al. DNA Methylomes Reveal Biological Networks Involved in Human Eye Development, Functions and Associated Disorders. Sci Rep. 2017; 7: 1-15.
- Liu X, Zhang B, McBride JD, Zhou K, Lee K, Zhou Y, et al. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013; 62: 4228-4238.
- Yoon C-H, Choi Y-E, Cha YR, Koh S-J, Choi J-I, Kim T-W, et al. Diabetes-Induced Jagged1 Over expression in Endothelial Cells Causes Retinal Capillary Regression in a Murine Model of Diabetes Mellitus Clinical Perspective. Circulation. 2016; 134: 233-247.
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, et al. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001; 152: 785-794.
- Polacheck WJ, Kutys ML, Yang J, Eyckmans J, Wu Y, Vasavada H, et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017; 552: 258-262.
- Liu Z-J, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003; 23: 14-25.
- Terlizzi V, Kolibabka M, Burgess JK, Hammes HP, Harmsen MC. The Pericytic Phenotype of Adipose Tissue-Derived Stromal Cells Is Promoted by NOTCH2. Stem Cells. 2018; 36: 240-251.
- Hammes H-P, Weiss A, Führer D, Krämer HJ, Papavassilis C, Grimminger F. Acceleration of experimental diabetic retinopathy in the rat by omega-3 fatty acids. Diabetologia. 1996; 39: 251-255.
- Dietrich N, Kolibabka M, Busch S, Bugert P, Kaiser U, Lin J, et al. The DPP4 Inhibitor Linagliptin Protects from Experimental Diabetic Retinopathy. PLoS One. 2016; 11: e0167853.
- Busch S, Kannt A, Kolibabka M, Schlotterer A, Wang Q, Lin J, et al. Systemic Treatment with Erythropoietin Protects the Neurovascular Unit in a Rat Model of Retinal Neurodegeneration. Stitt A, editor. PLoS One. 2014; 9: e102013.
- Feng Y, Wang Y, Li L, Wu L, Hoffmann S, Gretz N, et al. Gene expression profiling of vasoregression in the retina-involvement of microglial cells. PLoS One. 2011; 6.
- Ferreira Tojais N, Peghaire C, Franzl N, Larrieu-Lahargue F, Jaspard B, Reynaud A, et al. Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovasc Res. 2014; 103: 291-303.
- Planutiene M, Planutis K, Holcombe RF. Lymphoid enhancer-binding factor 1, a representative of vertebrate-specific Lef1/Tcf1 sub-family, is a Wnt-beta-catenin pathway target gene in human endothelial cells which regulates matrix metalloproteinase-2 expression and promotes endothelial cell inva. Vasc Cell. 2011; 3: 28.
- Hübner K, Grassme KS, Rao J, Wenke NK, Zimmer CL, Korte L, et al. Wnt signaling positively regulates endothelial cell fate specification in the Fli1a-positive progenitor population via Lef1. Dev Biol. 2017; 430: 142-155.
- Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, et al. Nrarp Coordinates Endothelial Notch and Wnt Signaling to Control Vessel Density in Angiogenesis. Dev Cell. 2009; 16: 70-82.
- Altmann C, Schmidt MHH. The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. International Journal of Molecular Sciences. 2018.
- Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles G, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013; 14: 128.
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016; 44: 90-97.
- Schübeler D. Function and information content of DNA methylation. Nature. 2015; 517: 321-326.
- Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013; 502: 472-479.
- Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986; 321: 209-213.
- Vogler S, Pannicke T, Hollborn M, Grosche A, Busch S, Hoffmann S, et al. Müller Cell Reactivity in Response to Photoreceptor Degeneration in Rats with Defective Polycystin-2. PLoS One. 2013; 8.