
Special Article - Platelets
Thromb Haemost Res. Res. 2019; 3(1): 1019.
Major Depression is Associated with High Platelet Activity and Reactivity
Milovanovic M1,2*
1Department of Internal Medicine, Vrinnevi Hospital, Sweden
2Department of Social and Welfare, LinkÖping University, Sweden
*Corresponding author: Micha Milovanovic, Department of Internal Medicine, Vrinnevi Hospital, S-601 82 NorrkÖping, Sweden
Received: December 06, 2018; Accepted: February 01, 2019; Published: February 08, 2019
Abstract
Objective: Depression exists in mild, moderate and severe depressive attacks. Evidence show that the condition is a risk factor for the expansion of Cardiovascular Disease (CHD). Platelet reactivity is associated with CHD and may well be a thinkable connection between Major Depression (MD) and CHD. The current study examined platelet activity and reactivity in density separated platelets of patient with MD compared to a Control Group (CON) without MD.
Material and Methods: N=24 patients suffering from MD, all individuals were diagnosed with DSM-IV and ICD-10. N=52 healthy individuals without MD served as controls. A flow cytometer was used to measure platelet bound fibrinogen with and without platelet agonists (ADP and TRAP-6). Finally, platelet counts were carried out using a routine cell counter.
Results: When compared to CON, MD displayed a significant higher in vivo platelet bound fibrinogen i.e. no platelet agonists added (p<0.05). After adding of platelets agonists, MD showed significantly more reactive platelets (p<0.05).
Conclusion: Activated platelets are also a feature of CHD. It is therefore to presume that a common pathophysiology exists between these diseases. It is possible that both conditions activate same inflammatory reactions.
Introduction
Depression can be divided into mild, moderate and severe depressive episodes. The disease is frequent in a broad-spectrum of the population, and even more common in those who suffer from chronic disease [1]. Depression is considered as a risk factor for the development of Cardiovascular Disease (CHD) [2-4], although there are shared sentences if cardiovascular disease can cause depression [5]. However, there are evidence of clinical significance, after an acute myocardial infarction, almost 20% of patients obtain a Major Depressive (MD) disorder and additional 20% develop a minor depressive disorder [6]. Previous studies have shown that depression relates to more reactive platelets [7-11]. Platelet reactivity is also linked to CHD [12-17] and could therefore be a possible link between MD and CHD.
Platelets are essential in the hemostatic process by prevent bleeding which is the principal function of the cell. When activated, platelets change shape, redistribute Glycoprotein (GPIb) receptors and expose procoagulant phospholipids on their surface, and initiate the release reaction. The GPIIb/IIIa complex, which is also present on resting platelets, undergoes a conformational change leading to exposure of a binding site for fibrinogen. These activation-dependent platelet surface changes are detectable by whole blood flow cytometry using specific antibodies such as PAC-1 directed against the fibrinogen binding site of GPIIb/IIIa [18].
Circulating platelets devour a density span of 1.04-1.08 kg/l [19,20]. Platelet organelles are assumed to determine its density, high-density platelets are believed to have additional amount of a and dense granules [19]. There is no consensus regarding the density of platelets. Some studies show that the density of platelets increases with age [21,22], while other studies show the opposite [23,24]. Further studies show that density is constant during cell life [25-27]. The clinical effect of platelet heterogeneity has been researched for a lengthy period. Platelet density is boosted in combination with acute myocardial infarctions [28]. High-density platelets are connected with the activity of inflammatory bowel disease [29]. Low peak platelet density characterizes essential thrombocythemia [30]. Preeclampsia is related to large platelets having low peak density [31].
Platelet heterogeneity by investigating platelet density subpopulations in different clinical conditions has been our fundamental areas in our laboratory research. Surface bound fibrinogen in vivo reveal that high density platelets in contrast with the normal platelet population circulate “more activated”, these types of cells also have less P-selectin as an indication of platelet ex vivo a-granula liberation [32]. We have also confirmed that low density platelets circulate “more activated”, however, in difference to high density populations, light platelets contain additional P-selectin and a smaller amount of dense bodies [33]. Low-density platelet populations reveal low in vivo activity [34] and increased serotonin content in Alzheimer’s disease [35]. Normal platelet population of Fibromyalgia contain more serotonin [36], and is associated with elevated in vivo platelet activity [37].
The study aims to investigate if platelet activity and reactivity differ in MD compared to a control group (CON) without MD.
Material and Methods
Subjects
24 patients suffering from Major Depression (MD), aged 50 ± 14 years (mean ± SD) participated in the study. All patients were registered for the study when seeking care at the psychiatry clinic at the local hospital. All included individuals were diagnosed with DSMIV and ICD-10 i.e. the condition has lasted for at least two weeks. DSM-IV specifically emphasizes that the condition should have resulted in a change in the person’s condition. The patients who orally rejected were excluded from the study. 52 healthy individuals, aged 68 ± 4 years (mean + SD) were used as a Control-Group (CON). All of the CON-group were enrolled in the study after requesting medical care at a neighbouring health centre, everyone where free from any depression state. All of CON-group gave informed agreement. The study was sanctioned by the local ethics committee of LinkÖping University, Sweden (reg. number: 2012/269-32).
Laboratory investigations
Each individual donated two blood samples from the antecubital vein into Vacutainer™ tubes (Becton and Dickinson, New Jersey, U.S.A.). The blood of the first tube (7.5 ml) was anticoagulated by adding 2.5 ml platelet-inhibitory solution*, this test tube was used for analysis of platelet in vivo activity. Before measuring platelet in vivo activity, platelets had to be separated according to density, a linear Percoll™ (GE Healthcare Bio-Sciences AB, Sweden) gradient was used [32,33,38]. The subsequent ingredients were varied to prepare the two Percoll™ solutions (1.09 and 1.04 kg/L) for the gradient (Table 1).
PercollTM solutions
1.09 kg/L
1.04 kg/L
PercollTM
32.84 g
8.88 g
H2O
11.42 g
19.14 g
Platelet-inhibitory solution*
4.50 g
3.00 g
Table 1: The subsequent ingredients were varied to prepare the two Percoll™ solutions (1.09 and 1.04 kg/L) for the gradient.
The platelet inhibitory liquid* was made by a mixture of the same quantities of subsequent solutions:
1. 0.15 mol/L Na2 citrate# and 0.13 mmol/L Na3EDTA* (pH 7.4 at 25°C).
2. 0.001 g/L prostaglandin E1 # and 1 mL 95% ethanol in H2O. #Sigma-Aldrich, Missouri, U.S.A.
3. 2.7 mmol/L theophylline# dissolved in 0.15 mmol/L TRIS chloride buffer (pH 7.4 at 25°C). #Sigma-Aldrich, Missouri, U.S.A
A two-chamber gradient maker was put in use to manufacture linear gradients (Biorad, California, U.S.A.). The gradients were made in 50 mL test tubes guarding the density span of 1.09 kg/L to 1.04 kg/L. 7.63 g of the 1.09 kg/L Percoll™ mixture was covered in the lowest part of the test tube. Thereafter, 13.08 g of the 1.09 kg/L and 12.48 g of the 1.04 kg/L Percoll™ mixtures were engaged into two-chamber gradient maker to produce the gradient. Then, the 10 mL anticoagulated whole blood was thoughtfully covered on top of created 50 mL test tube including entirely produced gradient. The 50 mL test tube was then consequently centrifuged at 2767 g for 1½ hours. After centrifugation, the underside of the test tube was pierced and the content was segregated by gravity into 17 different density fractions [31]. All of these density fraction consists of about 2 mL of the test tube substance. In all fractions, platelet counts were analyzed with a CELL-DYN 4000 (Abbott Diagnostics, Illinois, U.S.A.). Platelet bound fibrinogen were measured with a Beckman Coulter EPICS XLMCL Flow Cytometer (Beckman Coulter, Inc., California, U.S.A.) [38]. Platelets were classified with a PE-conjugated antibody against GPIb (Dako AS, Denmark). Since no agonist was used to identify platelet-bound fibrinogen in this setting, it reflects the in vivo activity of this platelet.
The second tube, containing 3,5 ml venous blood anti-coagulated with 0.5 ml 0.129 M disodium citrate were used to measure platelet reactivity i.e. platelet bound fibrinogen with stimulation with platelet agonists. The agonists used were ADP (1.7 μmol/L and 8.5 μmol/L) (Sigma-Aldrich, Missouri, U.S.A.) and Thrombin Receptor Activating Peptide (TRAP-6) (57 μmol/L and 74 μmol/L) (Biotechnology Centre of Oslo, Norway). By this measurement it imitates in vitro platelet reactivity. The number of platelets (%) having more surface bound fibrinogen after stimulation than a negative control (containing 10 mmol/L EDTA and HEPES buffer) was applied as an investigational factor [39].
Statistics
Microsoft Excel®, ver. 12.0.6514.5000 was employed for the statistical assessments. In text and tables are described mean values ± one Standard Deviation (SD). The unpaired Student’s t test was used for estimating quantitative data. p-values = 0.05 were utilized to specify significance.
Results
Platelet activity
The distribution of platelets in the 17 density fractions is shown in Figure 1. There was no demonstrated difference between the groups. Figure 2 shows in vivo platelet bound fibrinogen i.e. platelet activity in 17 different density fractions. MD had significantly more surface-bound fibrinogen among high and normal density platelets (fractions nos. 3-9) compared to CON, p<0.05. In vitro platelet bound fibrinogen, i.e. platelet reactivity is represented in Figure 3. After addition of platelets agonists, ADP (1.7 and 8.5 μmol/L) and TRAP- 6 (57 and 74 μmol/L) MD demonstrated significantly more reactive platelets when matched to CON, p<0.05. Finally, MD patients had larger platelets when evaluated against CON, i.e. Mean Platelet Volume (MPV), p<0.05 (data not shown).
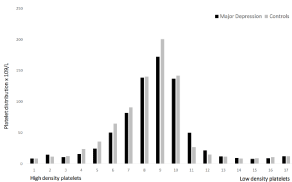
Figure 1: The distribution of platelets (×109/L) in the gradient for MD patients and controls. Fraction no. 1 detains platelets having highest density.
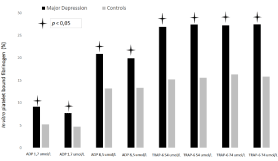
Figure 3: In vitro platelet reactivity i.e. platelet bound fibrinogen (%), is revealed for MD related with controls.
Discussion
This are to be the first description studying different platelet density subpopulations in MD with regarding to platelet bound fibrinogen. Compared with controls MD patients revealed both a significantly higher platelet in vivo activity and in vitro platelet reactivity.
The present study thereby displays significant differences when comparing the two study groups with respect to in vivo platelet activity in the different density fractions (Figure 2). The result indicates that platelets of both high and normal density are activated in MD. MD patients also exhibit higher platelet reactivation compared to CON (Figure 3). Both results thus show the same pattern seen at CHD. Activated platelets are also part of the atherosclerotic plaques that lead to CHD. CHD also exhibits higher platelet activity and platelet reactivity [40,41]. Earlier studies have shown evidence that depression also is linked to an increased risk of CHD. Individuals who have experience from depression have larger probability for myocardial infarction and cardiovascular death [2-4]. Depression and CHD may well allocate a common pathophysiology, as our results indicates that platelets flowing in the blood of patients with major depression are more clearly triggered in comparison with no major depression.
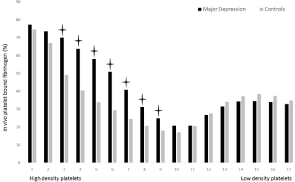
Figure 2: Platelet bound fibrinogen (%) i.e. in vivo platelet activity, is shown for MD patients and healthy controls. The densest platelets are found in fraction no. 1.
It is to hypothesize that platelets also adversely affect nerve cells, for instance, nerve cellular abnormalities are observed in subjects with mood disorders such as depression. Another example may be widespread pain, our previous studies have shown that there is a correlation between platelet activity and pain, though pain is also common in CHD [37]. Consequently, it appears to be a numeral of thinkable shared mechanisms linking platelets with major depression and CHD. Precisely how these mechanisms are related needs more understanding, a pairing might be the inflammatory reaction.
Platelet size i.e. Mean Platelet Volume (MPV) is considered as a replacement biomarker of platelet activity and a suitable predictive test in cardio metabolic disease. MPV relates with their reactivity [42]. For example, MPV is associated with acute myocardial infarction [43] and its prognosis [44], with coronary atherosclerosis [45], and presence short-term prognosis and long-term possibility of stroke [46]. At last, high MPV have been described in patients with hypertension [47]. Our results show that MD patients have a higher MPV compared with the control group. Therefore, results presented in this study suggests that there could be a possible link between clinical depression and platelet activity and reactivity.
If platelets are effortlessly activated in patients with depression, it might be thinkable that SSRI antidepressants may lessen activation of platelets and thereby cardiovascular illness and death in patients with heart disease. This matter might be incredibly stimulating issue to study in a greater prospective randomized study. Our data on platelets have primarily examined patients with major depression, but not CHD patients with depression. This is also a research trail that could be investigated in future studies.
The clinical implication of the monitored platelet heterogeneity stays uncertain; including what may be the source and significance of differences. One cannot estimate the clinical significance of our results founded on the present study. For this reason, greater studies of this subject are necessary to evaluate these questions.
Acknowledgement
Östergötland County Council made this work possible by its funding.
References
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006; 367: 1747-1757.
- Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008; 121: 20-27.
- Van Melle JP, De Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004; 66: 814-822.
- Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomatic Medicine. 2005; 67: 19-25.
- Frasure-Smith N, Lespérance F. Recent evidence linking coronary heart disease and depression. Psychosom Med. 2006; 51: 730-737.
- Carney RM, Rich MW, Freedland KE, Saini J, Velde A, Simeone C, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med. 1988; 50: 627-633.
- Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996; 153: 1313-1317.
- Musselman DL, Marzec UM, Manatunga A, Knight BT, Porter MR, Kasey S, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry. 2000; 57: 875-882.
- Markovitz JH, Shuster JL, Chitwood WS, May RS, Tolbert LC. Platelet activation in depression and effects of sertraline treatment: an open-label study. Am J Psychiatry. 2000; 157: 1006-1008.
- Walsh MT, Dinan TG, Condren RM, Ryan M, Kenny D. Depression is associated with an increase in the expression of the platelet adhesion receptor glycoprotein Ib. Life Sci. 2002; 70: 3155-3165.
- Cassidy EM, Walsh MT, O’Connor R, Condren RM, Ryan M, O'Keane V, et al. Platelet surface glycoprotein expression in post-stroke depression: a preliminary study. Psychiatry Res. 2003; 118: 175-181.
- Rauch U, Osende JI, Fuster V, Badimon JJ, Fayad Z, Chesebro JH. Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences. Ann Intern Med. 2001; 134: 224-238.
- Fitzgerald DJ, Roy L, Catella F, Fitz Gerald GA. Platelet activation in unstable coronary disease. N Engl J Med. 1986; 315: 983-989.
- Smitherman TC, Milam M, Woo J, Willerson JT, Frenkel EP. Elevated beta thromboglobulin in peripheral venous blood of patients with acute myocardial ischemia: direct evidence for enhanced platelet reactivity in vivo. Am J Cardiol. 1981; 48: 395-402.
- Sobel M, Salzman EW, Davies GC, Handin RI, Sweeney J, Ploetz J, et al. Circulating platelet products in unstable angina pectoris. Circulation. 1981; 63: 300-306.
- Wu KK, Hoak JC. Increased platelet aggregates in patients with transient ischemic attacks. Stroke. 1975; 6: 521-524.
- Zahavi J, Zahavi M. Enhanced platelet release reaction, shortened platelet survival time and increased platelet aggregation and plasma thromboxane B2 in chronic obstructive arterial disease. Thromb Haemost. 1985; 53: 105-109.
- Schmitz G, Rothe G, Ruf A, Barlage S, Tschope D, Goodall HA, et al. European working group on clinical cell analysis: consensus protocol for the flow cytometric characterization of platelet function. Thromb Haemost. 1998; 79: 885-896.
- Chamberlain KG, Froebel M, Macpherson J, Penington DG. Morphometric analysis of density subpopulations of normal human platelets. Thromb Haemost. 1988; 60: 44-49.
- Chamberlain KG, Penington DG. Monoamine oxidase and other mitochondrial enzymes in density subpopulations of human platelets. Thromb Haemost. 1988; 59: 29-33.
- Mezzano D, Hwang KL, Catalano P, Aster RH. Evidence that platelet buoyant density, but not size, correlates with platelet age in man. Am J Hematol. 1981; 11: 61-76.
- Boneu B, Vigoni F, Boneu A, Caranobe C, Sie P. Further studies on the relationship between platelet buoyant density and platelet age. Am J Hematol. 1982; 13: 239-246.
- Karpatkin S. Heterogeneity of human platelets II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969; 48: 1083-1087.
- Corash L, Tan H, Gralnick HR, Shafer B. Heterogeneity of human whole blood platelet subpopulations: I. Relationship between buoyant density, cell volume, and ultrastructure. Blood. 1977; 49: 71-87.
- Penington DG, Lee NL, Roxburgh AE, Mc-Gready JR. Platelet density and size: the interpretation of heterogeneity. Br J Haematol. 1976; 34: 365-376.
- Caranobe C, Sie P, Boneu B. Serotonin uptake and storage in human platelet density subpopulations. Br J Haematol. 1982; 52: 253-258.
- Martin JF, Shaw T, Heggie J, Penington DG. Measurement of the density of human platelets and its relationship to volume. Br J Haematol. 1983; 54: 337-352.
- Martin JF, Plumb J, Kilbey RS, Kishk YT. Changes in volume and density of platelets in myocardial infarction. Br Med J. 1983; 287: 456-459.
- Järemo P, Sandberg-Gertzen H. Platelet density and size in inflammatory bowel disease. Thromb Haemost. 1996; 75: 560-561.
- Järemo P. Platelet density in essential thrombocythemia and polycythemia vera. Platelets. 1999; 10: 61-63.
- Järemo P, Lindahl TL, Lennmarken C, Forsgren H. The use of platelet density and volume measurements to estimate the severity of pre-eclampsia. Eur J Clin Invest. 2000; 30: 1113-1118.
- Milovanovic M, Lotfi K, Lindahl T, Hallert C, Järemo P. Platelet density distribution in essential thrombocythemia. Pathophysiol Haemost Thromb. 2010; 37: 35-42.
- Milovanovic M, Lysen J, Ramström S, Lindahl TL, Richter A, Järemo P. Identification of low-density platelet populations with increased reactivity and elevated alpha-granule content. Thromb Res. 2003; 111: 75-80.
- Järemo P, Milovanovic M, Nilsson S, Buller C, Winblad. Low-density platelet populations demonstrate low in vivo activity in sporadic Alzheimer disease. Platelets. 2012: 23: 116-120.
- Milovanovic M, Eriksson K, Nilsson S, Post C, Winblad B, Lindahl TL, Järemo P. Alzheimer and platelets: Low-density populations reveal increased serotonin content in Alzheimers disease. Clin Biochem. 2014: 47:51-53.
- Milovanovic M, Nilsson S, Harakka PI, Gerdle B. Augmented serotonin content in density separated platelets of fibromyalgia patients. Clin Diagn Pathol. 2016.
- Milovanovic M, Nilsson S, Harakka PI, Post C, Gerlde B. High in vivo platelet activity in female fibromyalgia patients. Journal of Biomedical Sciences. 2016: 3: 21.
- Järemo P. Computerised method for recording platelet density distribution. Eur J Haematol. 1995; 54: 304-309.
- Lindahl TL, Festin R, Larsson A. Studies of fibrinogen binding to platelets by flow cytometry: an improved method for studies of platelet activation. Thromb Haemost. 1992; 68: 221-225.
- Willoughby S, Holmes A, Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs. 2002; 1: 273-288.
- Lina Badimon, Teresa Padró, Gemma Vilahur. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012; 1: 60-74.
- Yetkin E. Mean platelet volume not so far from being a routine diagnostic and prognostic measurement. Thromb Haemost. 2008; 100: 3-4.
- Cameron HA, Phillips R, Ibbotson RM, Carson PH. Platelet size in myocardial infarction. Br Med J. 1983; 287: 449-451.
- Kiliçli-Camur N, Demirtunç R, Konuralp C, Eskiser,A, Basaran, Y. Could mean platelet volume be a predictive marker for acute myocardial infarction. Med Sci Monit. 2005; 11: 387-392.
- Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991; 338: 1409-1411.
- Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004; 35: 1688-1691.
- Ordu, S, Ozhan H, Caglar O, Alemdar R, Basar C, Yazici M, et al. Mean platelet volume in patients with dipper and non-dipper hypertension. Blood Press. 2010; 19: 26-30.