
Special Article - Venous Thrombosis
Thromb Haemost Res. 2019; 3(3): 1028.
Endovascular Recanalisation of Chronic Iliofemoral Obstructions with Dedicated Venous Stents
Lichtenberg M*
Department of Angiology, Klinikum Arnsberg, Germany
*Corresponding author: Michael Lichtenberg, Klinikum Hochsauerland, Karolinenhospital, Klinik für Angiologie, Stolte Ley 5, D-59759 Arnsberg, Germany
Received: July 09, 2019; Accepted: August 07, 2019; Published: August 14, 2019
Abstract
Approximately 60% of patients with acute iliofemoral deep vein thrombosis recover without further symptoms. However, 40% will have some degree of Post-Thrombotic Syndrome (PTS), and 4% will develop severe PTS. PTS is the most common complication; it reduces quality of life and increases DVT-related costs. The clinical symptoms and severity of PTS may vary; the most common symptoms include oedema, pain, hyperpigmentation, lipodermatosclerosis, and ulceration. PTS is based on the principle of outflow obstruction. It may be caused by venous hypertension, and may lead to valve damage and venous reflux or insufficiency. A significant lumen reduction within the iliac vein system is defined as an aspect ratio of = 2. Recent technical developments and new dedicated venous stent techniques have permitted recanalization of complex venous outflow obstructions. First-in-man safety and efficacy data concerning the new dedicated venous stents are very promising, but long-term data are still awaited.
Key Messages
Pathophysiology: A persistent venous outflow obstruction after an iliofemoral thrombosis causes the symptoms of a post-thrombotic syndrome in the large majority of patients. A persistent swelling of the leg and venous claudication may signify a limitation in quality of life for (young) patients.
Endovascular treatment: At specialised clinics, the technically very laborious procedures of endovascular recanalisation reopen chronic and long-standing obstructions of the inferior vena cava and the iliofemoral veins.
New generation of venous stents: We now have a growing body of clinical safety and efficacy data for the new generation of venous stents. The physical properties of a stent should be taken into account when selecting a venous stent. The nine approved venous stents in Europe differ very significantly in terms of their properties.
Introduction
Inadequate recanalisation of venous blood flow after an acute iliofemoral thrombosis leads to a persistent and hemodynamically relevant outflow obstruction with secondary valve insufficiency of the deep communicating veins, later also the saphenous veins of the affected lower extremity, in the large majority of patients [1-4]. The clinical symptoms of the resulting Post-Thrombotic Syndrome (PTS) extend from venous claudication with or without a persistent swelling to the development of venous ulcers. Especially in patients with chronic iliofemoral obstructions, conservative treatment with continued compression therapy fails very often. Thus, it would be advisable to institute causal interventional measures to eliminate the thrombus or the chronic obstruction. Such treatment would prevent valve damage and PTS. Recanalisation treatment is concluded with stent implantation in nearly all cases. We now have nine approved dedicated venous stents in Europe, whose efficacy and safety have been analysed in several studies.
Initially the stents used for arterial stent PTA (percutaneous transluminal angioplasty) were also used for the recanalisation of iliac veins (such as Wallstents or nitinol stents). However, PTA stenting of a post-thrombotic vein with intraluminal scars and frequently also external compression is not comparable with arterial stent PTA in patients with arteriosclerosis. The reason is that, depending on the location of the obstruction in the iliofemoral vein, a number of physical requirements need to be fulfilled.
1. The diameter of veins is larger than the diameter of the corresponding arteries. Stents with a diameter of 14-18 mm are used for reconstructions in the iliac veins [5].
2. Post-thrombotic lesions are usually very long and require longer stents. Currently we have stents with lengths of up to 160 mm. The use of several overlapping stents does not resolve this problem sufficiently because the required flexibility is reduced by overlapping [6,7].
3. Post-thrombotic veins are frequently scarred over longer portions. Furthermore, there may be an additional external compression such as those in patients with May-Thurner syndrome. Therefore, one needs stents with greater radial force.
4. Venous recanalisation calls for highly flexible stents that adjust to the anatomical course of the vein during motion. The largest degree of angulation (up to 90°) occurs at the junction between the external and the common iliac vein in sitting position [8].
Recanalisation procedure
Venous dilatation and stenting are painful and may take considerable time. Performing the intervention in local anaesthesia should be restricted to patients with circumscribed stenoses in the iliac region, such as those with the May-Thurner syndrome. In all other cases it would advisable to use general anaesthesia.
Suitable accesses for iliofemoral and caval recanalisation are the common femoral vein, the superficial femoral vein, the popliteal vein, and the internal jugular vein, as well as the common femoral vein on the contralateral side. As a last resort, one could also use the large saphenous vein or the deep femoral vein as the path of access.
The first step is an ultrasound-guided puncture of the vein. After introducing a delivery sheath by Seldinger technique, the obstruction can be passed through with the aid of various wires and catheters. After passing the wire through, the site of constriction or obstruction is dilated using a balloon with a large lumen. The diameter and length of the vein should be pre-dilated at least to the dimensions of the proposed stent. Post-dilatation is performed after the implantation of a venous stent. The stent PTA should be performed from one healthy segment to the other. Over stenting is essential when treating a stenosis in the common iliac vein. One should refrain from implantation of the stent deep into the inferior vena cava. A control phlebography in two planes is essential. After successful recanalisation, contrast medium flows rapidly through the stented iliac vessel. Collateral vessels should not be seen (examples Figure 1a and 1b).
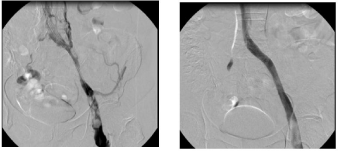
Figure 1a and b: Functional obstruction of iliofemoral flow over a long distance in a 43-year-old woman who had experienced iliofemoral venous thrombosis
treated by conservative means 5 years ago (left). Now a recurrence of the thrombosis three months earlier. Clinically advanced post-thrombotic syndrome with
lipodermatosclerosis and venous claudication (a). After successful recanalisation and implantation of a 14 x 120 mm venous stent, we find no evidence of
collaterals on angiography and sufficient downward flow (b).
Despite successful iliac stent PTA, early thrombotic obstructions are nearly unavoidable when there is insufficient inflow from the superficial and deep femoral veins and/or the large saphenous vein. When the common femoral vein is involved, which signifies limited inflow; it may be necessary to insert an arteriovenous fistula. This can be performed either as a hybrid procedure during the endovascular recanalisation or immediately thereafter.
Venous stents
In addition to clinical safety and efficacy analyses, venous stents have been extensively analysed in respect of their physical properties. The following quantifiable variables have been investigated:
Radial resistive force: This term describes the force needed to compress a stent radially. The greater the required force, the greater the radial resistive force of a stent. One example would be an iliofemoral vein severely affected by thrombosis, which is treated over a long portion with a stent. If the resistive force were too low, the stent would not unfold adequately and the acquired vessel lumen would be insufficient. This would result in a persistent obstruction. The diameter ratio of breadth and height (aspect ratio), determined by an intravascular ultrasound investigation, is used to establish whether a sufficient gain in lumen size has been achieved (Figures 2a and b). An aspect ratio = 2 is correlated with an unfavourable patency rate of the treated vein segment [9].
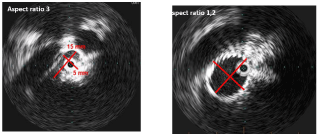
Figure 2 and b: 32-year-old woman with a high-grade post-thrombotic stenosis of the common iliac vein on the left side (aspect ratio of 3 on the intravascular
ultrasound investigation), which led to a persistent swelling of the leg and venous claudication after 150 meters. Following venous stent implantation, the aspect
ratio was reduced to nearly 1.
Crush resistance: This term describes the force needed to compress the strength in radial direction.
a) Cook Zilver Vena Stent: This venous requires at least a 7-French introducer system. The stent has an open-cell design, and is available in lengths of 60-140 mm and diameters of 14-16 mm. Due to its smaller profile the stent has a lower radial force compared to other venous stents while its flexibility is similar and its resistance to local compression (crush resistance) a little lower [10]. The prospective non-randomised VIVO-EU study presented in 2016 [11] showed, in 35 patients with a non-malignant acute or chronic iliofemoral obstruction, a primary patency rate of 87.9% after 12 months. The patients’ VCSS (Venous Clinical Severity Score), VDS (Venous Disability Score), CEAP and CIVIC (quality of life) scores were significantly improved. Parallel to the European study, currently the American IDE (investigational device exemption safety and efficacy) study is in progress; its publication is anticipated in 2020.
b) Veniti Vici Stent: This stent was recently acquired by Boston Scientific Company. It is currently the only venous stent entirely with a closed-cell design, available in lengths of 60 to 120 mm and diameters of 12 to 16 mm. Due to its configuration the stent has a markedly higher radial force than the Zilver Vena stent [10]. Two-hundred patients with acute or chronic obstructions of the iliofemoral veins were included in the recently presented VIRTUS IDE study which included 170 patients. Primary patency rate based on venography was 79.8% in post-thrombotic patients and 96.2% in non-thrombotic patients after 12 months. Baseline VCSS of 10 decreased to 5.5. No safety concerns were reported. A parallel registry of 75 patients by Lichtenberg et al. showed a significant clinical improvement after 12 months measured by VCSS and CEAP score. Primary patency rate of 100% for non-thrombotic lesions, and a primary patency rate of 87% for chronic post-thrombotic obstructions of the iliofemoral veins were reported within this registry [12].Bard Venovo Stent: This open-cell design stent is available in diameters of 10-20 mm, and lengths of 40-160 mm. A typical characteristic of this stent, in addition to its tri-axial delivery system, is that the ends of the stent flair by 2 mm. This permits very good fixation at the time of delivery. The Venovo stent has one of the highest radial force [10]. Recently the VERNACULAR IDE trial with 170 was reported with a primary patency rate of 81.3% for PTS lesions and 96.9% for nonthrombotic lesions after 12 months. A parallel registry by Lichtenberg et al. showed a significant clinical improvement of patients after 6 months, a primary patency rate of 97% for non-thrombotic lesions, and a primary patency rate of 96% for chronic post-thrombotic obstructions of the iliofemoral veins [13].
c) Optimed sinus Venous Stent: A hybrid stent with strong radial force, consisting of powerful rings connected with individual nitinol bridges. This creates an excellent compromise between high radial force and a high degree of longitudinal flexibility [10]. The stent is available in diameters of 10 to 18 mm and lengths of 60-150 mm. A registry study conducted by de Wolf et al. in 2015 showed, in 75 patients with compression syndrome and chronic post-thrombotic obstructions, a primary patency rate of 92% after 12 months and a significant improvement in clinical symptoms [14].
d) Optimed sinus Obliquus Stent: A hybrid stent consisting proximally of a closed-cell design (very high radial force and crush resistance, 10) and peripherally an open-cell design. The proximal part has a characteristic oblique shape (diameter 14-16mm), which makes this stent especially suitable for treating patients with May-Thurner syndrome without over stenting of the contralateral inflow. Due to the very high radial force of the proximal closed-cell portion, the stent is able to resist the high forces exerted in May- Thurner syndrome. It is available in different lengths (80-150mm). Due to its flexible peripheral parts the stent is able to adjust to existing anatomy of the iliofemoral veins. A retrospective registry on the stent has been published recently (Table 1) [15].
Stent
Manufacturer
Diameter and Length
Material
Studies
Zilver Vena
Cook
14-16 mm
60-140 mm
Nitinol
VIVO EU trial concluded:
Primary patency rate of 87.9% after 12 months [11])
VIVO US trial: not concluded yet (awaited in 2020)
sinus-Venous
Optimed
10-18 mm
60-150 mm
Nitinol
Single-centre study in 2015: Primary patency rates of 96% after 6 months and 92% after 12 months [14]
Single-centre study in 2018: Primary patency rate of 68% after 12 months
sinus-Obliquus
Optimed
14-16 mm
80-150 mm
Nitinol
(oblique)
Sinus obliquus-01-NIS:
Recruitment still in progress. Single-
centre register in 2017: Primary patency rates of 92% after 6 months and 83% after 10 months [15]
sinus-XL Flex
Optimed
14-24 mm
40-160 mm
Nitinol
sinus-XL
Optimed
16-36 mm
30-100 mm
Nitinol
Vici
Boston Scientific Group
12-16 mm
60-120 mm
Nitinol
(closed-cell stent design)
Virtus trial (IDE):
Primary patency rate of 84% after 12 months. Single-centre study in 2018: Primary patency rates of 86-100% (PTS/NIVL) after 12 months [12]
Venovo
Bard BD
10-20 mm
40-160 mm
Nitinol
Tri-axial delivery system
Vernacular-Trial (IDE):
Primary patency rate 88.3% after 12 months. Single-centre study in 2018: Primary patency rates 96.6% (NIVL) and 95.7% (PTS) after 12 months [13]
Abre
Medtronic
10-20 mm
40-150 mm
Nitinol
Tri-axial delivery system
ABRE-Trial (IDE):
Recruitment still in progress.
Blueflow
IP medical
12-16mm
60-150mm
Nitinol
(woven)
Blueflow-PMCF:
Recruitment still in progress.
Table 1: Summary of the current CE-approved stents authorised for the iliofemoral venous system. (NIVL: Non Thrombotic Iliac Vein Lesion; PTS: Post Thrombotic Syndrome).
e) Medtronic Abre Venous Stent: Tubular stent, opencell design, with a three-point connection between the individual cells, which provides a high degree of flexibility along with high radial forces in the individual cells. A tri-axial release system similar to that of the Venovo stent. As a result, the stent can be positioned optimally. The minimal sheath size for the stent is 9F. The maximum available diameter is 20mm, and the maximum available length 150 mm. The stent has been approved in Germany (only for the iliac veins) and is currently being analysed in an IDE study (ABRE).
f) Blueflow Venous Stent: This venous stent manufactured by IP medical company (Germany) was approved in spring of 2018. It is the only stent available so far with a woven nitinol design (similar to the Wallstent). The advantage of this design appears to be its high resistance to kinking and fractures, which makes it especially suitable as an extension stent in the region below the inguinal ligament and the common femoral vein, in patients with the post-thrombotic syndrome. The stent is available in a maximum diameter of 16 mm and a maximum length of 150 mm.
Practical Conclusion
In selected cases of iliofemoral obstruction, endovascular treatment is an acceptable option for the prevention or causal treatment of a post-thrombotic syndrome, as evidenced by the positive efficacy and safety data obtained in the present investigation. The treatment should be restricted to specialised centres. In order to obtain favourable clinical results in the long term, the interventionalist must ensure sufficient inflow and outflow in the recanalised vein segment.
Dedicated venous stents should be used for this purpose. Depending on the underlying individual pathology of the respective vein segment, the stents should possess a higher radial force or greater flexibility. The principle of “one stent fits all” does not apply to iliofemoral veins.
References
- White RH. The epidemiology of venous thromboembolism. Circulation. 2003; 107: 14-18.
- Akesson H, Brudin L, Dahlstrom JA, Eklöf B, Ohlin P, Plate G. Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. European journal of vascular surgery. 1990; 4: 43-48.
- Cogo A, Lensing AW, Prandoni P, Hirsh J. Distribution of thrombosis in patients with symptomatic deep vein thrombosis. Implications for simplifying the diagnostic process with compression ultrasound. Archives of internal medicine. 1993; 153: 2777-2780.
- Plate G, Akesson H, Einarsson E, Ohlin P, Eklöf B. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. European journal of vascular surgery. 1990; 4: 483-489.
- Raju S, Buck WJ, Crim W, Jayaraj A. Optimal sizing of iliac vein stents. Phlebology. 2018; 33: 451–457.
- de Graaf R, Arnoldussen C, Wittens CH. Stenting for chronic venous obstructions a new era. Phlebology 2013; 28: 117-122.
- de Graaf R, de Wolf M, Sailer AM, van Laanen J, Wittens C, Jalaie H. Iliocaval confluence stenting for chronic venous obstructions. Cardiovasc Intervent Radiol. 2015; 38: 1198-1204.
- Neglén P, Tackett TP, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008; 48: 1255-1261.
- Lichtenberg, M. Endovascular Toady. 2018; 9-11.
- Dabir D, Feisst A, Thomas D, Luetkens JA, Meyer C, Kardulovic A, et al. Physical Properties of Venous Stents: An Experimental Comparison. Cardiovasc Intervent Radiol. 2018; 41: 942-950.
- O`Sullivan G, Lichtenberg M, Mcann-Brown J, et al. VIVO-EU Results: Prospective European Study of the Zilver® VenaTM Venous Stent in the Treatment of Symptomatic Iliofemoral Venous Outflow Obstruction.
- Lichtenberg M, Breuckmann F, Stahlhoff WF, Neglén P. Placement of closedcell designed venous stents in a mixed cohort of patients with chronic venous outflow obstructions - short-term safety, patency, and clinical outcomes. Vasa. 2018: 1-7.
- Lichtenberg M, de Graaf R, Stahlhoff WF, özkapi A, Rassaf T, Breuckmann F. Venovo venous stent in the treatment of non-thrombotic or post-thrombotic iliac vein lesions: short-term results from the Arnsberg venous registry. Vasa accepted. 2019.
- de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Short-Term Clinical Experience with a Dedicated Venous Nitinol Stent: Initial Results with the Sinus-Venous Stent. Eur J Vasc Endovasc Surg. 2015; 50: 518-526.
- Stuck AK, Kunz S, Baumgartner I, Kucher N. Patency and Clinical Outcomes of a Dedicated, Self-Expanding, Hybrid Oblique Stent Used in the Treatment of Common Iliac Vein Compression. J Endovasc Ther. 2017; 24: 159-166.