
Research Article
Thromb Haemost Res. 2021; 5(1): 1054.
Biological Markers Associated with Pulmonary Embolism in COVID-19
Moises J1,2, Blanco I1,2, Garcia AR1,2, Sanchez M3, Aibar J4, Benegas M3, Andrea R5, Badia JR1,2, Castro P4, Fernandez J6, Jimenez S7, Soriano A8, Reverter JC9, Barbera JA1,2*
11Department of Pulmonary Medicine, University of Barcelona, Spain
2CIBER de Enfermedades Respiratorias (CIBERES), Spain
3Department of Radiology, University of Barcelona, Spain
4Department of Internal medicine, University of Barcelona, Spain
5University of Barcelona, Cardiovascular Institute, Hospital Clinic de Barcelona, Spain
6Department of Hepatology and Gastroenterology, University of Barcelona, Spain
7Department of Emergency, University of Barcelona, Spain
8Department of infectious Diseases, University of Barcelona, Spain
9Department of Hemotherapy and Hemostasis, University of Barcelona, Spain
*Corresponding author: Joan Albert Barbera, Department of Pulmonary Medicine, University of Barcelona, Hospital Clínic de Barcelona, IDIBAPS, Barcelona, Spain
Received: February 17, 2021; Accepted: March 08, 2021; Published: March 15, 2021
Abstract
SARS-CoV-2 infection may predispose to thrombotic complications. Most frequently Pulmonary Embolism (PE). The aim of this study was to identify predictive biomarkers for developing PE in patients with COVID-19.
Methods: This study analysed retrospectively the data of patients with COVID-19 admitted to our institution that underwent CTPA scan due to suspected PE. Relevant laboratory data and radiology images were collected for each patient.
Results: 100 patients with COVID-19 were included. 75 (75%) were male with a mean age of 65±15.3 years-old. Among 33 (33%) patients with confirmed PE, 22 (67%) had peripheral PE (subsegmental or segmental PE). There were no demographic or major clinical differences between patients with and without PE. Only systemic arterial hypertension was more frequent among non-PE patients. D-dimer levels were higher and ferritin levels were lower at the time of CTPA in the PE group compared to the non-PE group (9400ng/ml vs 4000ng/ ml; P<0.001 and 680ng/mL vs 1027ng/mL; P=0.013, respectively). 13 (13%) patients presented haemorrhagic complications without statistically significant differences regarding the type of anticoagulation administered. Mortality rate was 12% with no differences between PE and non-PE groups. On multivariable analysis, the D-dimer/ferritin ratio ≥6 (OR 7.17, 95% CI 2.6-19.4; P<0.001) emerged as independent variable associated to PE.
Conclusions: The D-dimer/ferritin ratio in patients with COVID-19 may help identifying those at an increased risk of PE.
Keywords: COVID-19, SARS-CoV-2; Pulmonary embolism; Biomarkers; Thromboembolic disease; Hemorrhage
Abbreviations
COPD: Chronic Obstructive Pulmonary Disease; COVID-19: Coronavirus Disease 2019; CRP: C-Reactive Protein; CTPA: Computed Tomography Pulmonary Angiography; ICU: Intensive Care Unit; LDH: Lactate Deshydrogenase; LMWH: Low Molecular Weight Heparin; PE: Pulmonary Embolism; RT-PCR: Real Time Polymerase Chain Reaction; UsTnI: Ultra-Sensitive Troponin I.
Introduction
On January 30th, 2020, the World Health Organization (WHO), following the recommendations of the Emergency Committee of the International Health Regulations (IHR, 2005), declared the outbreak of a new coronavirus SARS-CoV2 (responsible of COVID-19 disease).
Due to the rapid extension and progression of the pandemic, clinicians have struggled to identify different complications in these patients. One of the most relevant is Pulmonary Embolism (PE). Almost 20% of patients with SARS-CoV2 pneumonia exhibit abnormal coagulation parameters, which has been related to greater mortality [1-3]. It has been hypothesized that arterial and venous thromboembolisms are related to extreme inflammation and intravascular coagulation, both triggered by the virus infection [4,5]. Series from China, Netherlands and France, have reported a prevalence of PE around 30% in patients with COVID-19 and clinical suspicion, even those who received thromboprophylaxis [4,6,7]. In autopsy series the incidence of thrombotic events has been even higher (58%), the majority, in patients in whom venous thromboembolism was not suspected (8). Accordingly, the International Society on Thrombosis and Haemostasis (ISTH) developed an algorithm for managing this coagulopathy stratifying the risk based on different parameters such as D-dimer, prothrombin time, fibrinogen levels and platelet count, recommending prophylactic treatment with Low Molecular Weight Heparin (LMWH) to all patients requiring hospital admission for COVID-19 [9].
The D-dimer is a degradation product of fibrin that reflects blood clot formation. Although it has a high sensitivity for thrombotic disease, its specificity is poor. In COVID-19, several studies have shown a strong association between increased D-dimer levels and disease severity and prognosis [10-12]. Even though the current standard for PE diagnosis is Computed Tomography (CT) Pulmonary Angiography (CTPA), but in some circumstances it might be difficult to perform or could pose the patient at risk. Therefore, there is a need to increase the pre-test probability of PE in patients with COVID-19. The aim of this study was to identify predictive factors for developing PE in patients with COVID-19.
Methods
We conducted a retrospective, single center study at Hospital Clinic of Barcelona, Spain. We analyzed the first 100 consecutive patients with COVID-19 that underwent CTPA examination to rule out PE from March 9th to April 6th, 2020. The Institutional Ethics Committee of the Hospital Clinic of Barcelona approved the study.
Interstitial-alveolar pattern, alveolar consolidation and/or peripheral ground glass opacities were considered as COVID-19 pneumonia. The majority of patients (85/100) had been diagnosed by Real Time Polymerase Chain Reaction (RT-PCR) for SARS-CoV-2 obtained by nasopharyngeal swab. In the remaining 15 patients, COVID-19 was diagnosed based on typical CT features in the current epidemiological context [13].
CTPA was requested by the treating physician if PE was suspected in patients with persistently elevated D-dimer levels and/or impaired gas exchange not justified by radiological findings.
Epidemiological data, clinical characteristics and anticoagulant treatment were recorded. Thromboprophylaxis with Low Molecular Weight Heparin (LMWH) was classified as: a) standard dose (Subcutaneous (sc) enoxaparin 40mg/24h or equivalent) or b) higher/extended dose (sc enoxaparin 60mg/24 h or 1mg/kg/24 h or equivalent), which was indicated by protocol in patients weighing over 80Kg, and/or additional risk factors for thromboembolic disease, and/or persistently high D-dimer values. Hemorrhagic complications were classified as major bleeding episodes according to the definition of the Control of Anticoagulation Subcommittee of the ISTH [14].
D-dimer, C-Reactive Protein (CRP), Lactate Dehydrogenase (LDH), ferritin levels and platelet count, were evaluated on admission and at time of CTPA. Ultrasensitive-troponin and serum creatinine levels were recorded when CTPA was performed.
Results were expressed as mean and SD for quantitative variables that follow a normal distribution and as median and IQR otherwise. Qualitative variables are expressed as total number and percentages. Fisher exact test was used to compare qualitative variables. The univariable analysis was included in the corresponding multivariable logistic regression backward stepwise model. Strongly correlated variables were excluded from the analyses. All tests were performed with a bilateral significance level of P 0.05. Statistical analysis was done with SPSS statistical software (version 25.0; Chicago, Illinois, USA).
Results
During the study period 1391 patients with COVID-19 were admitted in the hospital, CTPA was performed in 100 (7.2%) of them. Most of the patients were male (75%), with a mean age of 65 years-old. Eighty-five patients (85%) presented some comorbidity. No significant differences were found regarding smoking history. Only one patient had previous history of Venous Thromboembolism (VTE). There were no demographic or major clinical differences between patients with and without PE. Only systemic arterial hypertension was more frequent among non-PE patients (Table 1).
Characteristics
PE patients
N= 33Non-PE patients
N= 67P-value
Age, yrs, mean (SD)
61.6 (15.3)
66.3 (11.9)
0.091
Male sex, n (%)
23 (70)
52 (78)
0.39
Weight, mean (SD)
79 (18.8)
81 (14.9)
0.519
Current smoker, n (%)
Former/never smoker, n (%)5 (15.2)
28 (84.8)11 (16.4)
56 (83.6)0.871
COPD, n (%)
3 (9)
5 (7.5)
0.528
Diabetes, n (%)
5 (15)
11(16.4)
0.56
Arterial Hypertension, n (%)
13 (39.4)
42 (63)
0.034
Cardiovascular disease, n (%)
6 (18)
9 (13)
0.56
Chronic renal failure, n (%)
2 (6)
6 (9)
0.472
Cancer history, n (%)
2 (6)
5 (6.5)
0.579
SpO2/FIO2 on admission, mean (SD)
299 (112.2)
379 (105.3)
0.015
Days from admission to CTPA, median (range)
6 (0-21)
8 (0-26)
0.076
Days from symptoms to CTPA, median (range)
16.5 (4-27)
17 (4-38)
0.662
General condition at the time of CTPA
Immunomodulatory treatment, n (%)
14(42)
42(63)
0.086
ICU admission, n (%)
18 (54.6)
36 (53.7)
0.833
Mechanical Ventilation, n (%)
7(21)
20(30)
0.252
Type of prophylaxis*, n (%)
Standard
High/Extended
16 (48)
10 (30)
38 (57)
27 (70)
0.475
Blood test analysis
CRP (mg/dl) at the time of CTPA
3.61 [0.76-7.4]
1.84 [0.4-4.96]
0.125
ΔCRP (mg/ml)*
8.4 [4.8-13.5]
5.5 [1.7-16.6]
0.378
D-dimer (ng/ml) at the time of CTPA
9400 [4800-10000]
4000 [1500-9900]
<0.001
Δ D-dimer (ng/ml)*
4850 [800-8800]
1750 [300-5125]
0.08
Ferritin (ng/ml) at the time of CTPA
680 [346-1272]
1027 [670-1704]
0.013
Δ Ferritine (ng/ml)*
72 [-206-466]
25 [-348-336]
0.525
LDH [U/L) at the time of CTPA
362 [261-459]
347 [300-422]
0.458
Δ LDH (mg/ml)*
68.5 [-18.8-155.3]
14 [-57-76.5]
0.035
Platelets (x109/L) at the time of CTPA
283 [234-354]
259 [191-358]
0.416
Δ Platelets (x109/L)*
-59 [-143- -17.5]
-66.5 [-193 – -19.8]
0.653
D-dimer/ferritin ratio at the time of CTPA
9.6 [6.7-20]
3.5 [1.7-7.3]
<0.001
Δ D-dimer/ferritin ratio*
6.3 [2.4-12.5]
1.8 [0.5-4.6]
0.038
Creatinine (mg/dl) at the time of CTPA
0.84 [0.58-1.14]
0.99 [0.86-1.25]
0.304
UsTnI (ng/ml) at the time of CTPA
8.3 [4.2-108]
14.3 [7.3-21.8]
0.145
Outcomes
Hemorrhagic complications, n (%)
6 (18)
7 (10)
0.219
Severity of hemorrhagic complications, n (%)
Major
Non-Major
4/13 (31)
2/13 (15)
3 (23)
4 (31)
0.383
Mortality, n (%)
2 (6)
10 (15)
0.2
*Excluding patients diagnosed with PE on admission.
COPD: Chronic Obstructive Pulmonary Disease; COVID-19: Coronavirus Disease 2019; CRP: C-Reactive Protein; CTPA: Computed Tomography Pulmonary Angiography; ICU: Intensive Care Unit; LDH: Lactate Dehydrogenase; PE: Pulmonary Embolism; UsTnI: Ultra-Sensitive Troponin I.
Table 1: Main characteristics in COVID-19 patients with suspected PE.
Most of the cases were treated with a combination of Lopinavir/ Ritonavir (94%) and hydroxychloroquine (97%). Immunomodulatory treatment that included tocilizumab, siltuximab and anakinra were administered in 56 patients. A higher proportion of patients in the non-PE group received immunodulatory treatment, even though the differences did not reach statistical significance (P=0.086).
Four patients were receiving anticoagulation for atrial fibrillation before admission. In sixteen patients the CTPA was performed at admission. Eighty-two patients received thromboprophylaxis with LMWH since admission, 48 at standard dose and 34 at extended dose. In 3 patients, intermittent pneumatic compression was additionally used.
The median time from admission to CTPA examination was 7.5 days. PE was diagnosed in 33 patients (33%). Four patients had concomitant Deep Vein Thrombosis (DVT). Only one patient had DVT without PE.
Among confirmed PE, 11 (33%) patients had central and 22 (67%) peripheral PE. Six patients (18%) had pulmonary infarction and 2 (6%) showed right ventricular enlargement. All patients diagnosed with PE received full dose anticoagulation, 30 with sc LMWH, and 3 with intravenous Unfractionated Heparin (UFH).
On admission, patients with PE had worse oxygenation, showed by lower oxygen saturation to fraction of inspired oxygen (SpO2/ FiO2) ratio. No statistically significant differences in ICU admission or mechanical ventilation was found between PE and non-PE groups.
Significant reduction in CRP and LDH levels and increasing of D-dimer levels and platelet count at the time of CTPA were found. Additionally, we analysed the Δ-value between admission and CTPA for this variables. No significant differences in Δ-value was found between PE and non-PE groups.
At the time of CTPA study, D-dimer levels were significantly higher in the PE group compared with the non-PE group, whereas ferritin was lower in the PE-group. Accordingly, the D-dimer/ferritin ratio was higher in patients with PE (9.6 vs 3.5; P<0.001). There were no differences among platelet count, troponin or creatinine levels (Figure 1A&1B).
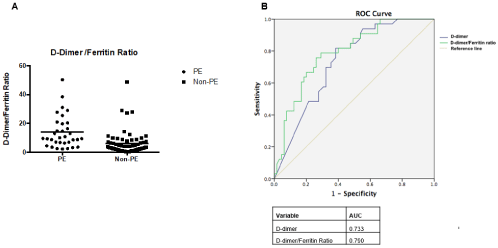
Figure 1:
PE predicting values of D-dimer and ferritin were assessed by the area under the curve ROC (AUC). The AUC of D-dimer for predicting PE was 0.733 and that of ferritin for excluding PE was 0.654. The ratio between D-dimer and ferritin had an AUC of 0.79 for predicting PE.
Several variables such as a previous history of systemic hypertension, age, D-dimer/ferritin ratio and immunomodulatory treatment were included in a multivariable logistic regression analysis. A D-dimer/ferritin ratio ≥6 emerged as an independent variable associated with PE (OR 7.17, 95% CI 2.6-19.4; p<0.001).
Hemorrhagic complications were found in 13 patients in the whole cohort, without differences among patients with and without PE (18 and 10%, respectively (p=0.219). Most of the hemorrhagic complications were major (54%). No statistically significant differences among patients receiving full dose anticoagulation and prophylaxis treatment was found (P=0.191). Although mortality was numerically different in PE and non-PE patients this difference was not statistically significant (6% vs 15%; P=0.2).
Discussion
The present study shows a PE prevalence of 33% in patients with COVID-19 who underwent assessment with CTPA. We showed lower ferritin and higher D-dimer levels in the group with PE than in those without PE, and that the D-dimer/ferritin ratio clearly differed between groups. Cui et al. [6] have previously proposed a D-dimer cut-off ≥3000 ng/dl for predicting PE in patients with COVID-19, but in our series this cut-off had high sensitivity but low specificity. Sensitivity and specificity were increased when analysing the D-dimer/ ferritin ratio, which also had greater AUC in the ROC analysis. Furthermore, multivariate analysis showed that the D-dimer/ferritin ratio ≥6 was independently associated with PE.
In our cohort patients with COVID-19 in general had an intense acute phase response as evidenced by elevated levels of ferritin and D-dimer. Even though the mechanisms leading to thrombotic events in these patients are unclear, and probably inflammation is involved [15], our data suggests that a disproportional increase of D-dimer, probably due to a marked thrombi formation, should raise awareness of thromboembolic complications in COVID-19. The misbalance between acute phase reaction and thrombus formation in this clinical scenario remains a hypothesis and further studies are needed for evaluating these biomarkers and other coagulation proteins like fibrinogen and Von Willebrand with a potentially dual function [16]. Remarkably, in contrast with previous publications [17], hemorrhagic complications occurred in 13% of the patients regardless of type of anticoagulant treatment.
Conclusion
Our study suggests an out-of-proportion procoagulant state in patients with COVID-19 assessed by an increased D-dimer/ferritin ratio should raise awareness on the presence of PE. In patients with COVID-19, hemorrhagic complications are frequent, reinforcing the recommendation to rule out VTE before full dose anticoagulation.
Ethics approval and consent to participate
The Institutional Ethics Committee of the Hospital Clinic of Barcelona approved the study and due to the nature of retrospective chart review, waived the need for inform consent from individual patients (Comite Etic d’Investigacio Clinica; HCB/2020/0273).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contributions
JM, IB, JAB participated in the conceptualization of the study. MS, MB participated in the acquisition and analysis of radiological data, JCR participated in the acquisition of laboratory data. JRB, JF, RA, PC, SJ, AS participated in the acquisition of clinical data. AG, JM, IB drafted the manuscript. All the authors read and approved the final manuscript.
Acknowledgement
We would like to thank all the hospital staff members during the pandemic.
References
- Huang Ch, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020; 395: 497-506.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020; 395: 507-513.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020; 18: 844-847.
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191: 145-147.
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020; 191: 9-14.
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020; 18: 1421-1424.
- Leonard-Lorant I, Delabranche X, Severac F. Acute Pulmonary Embolism in COVID-19 Patients on CT Angiography and Relationship to D-Dimer Levels. Radiology. 2020.
- Wichmann D, Sperhake J-P, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020.
- Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020; 18: 1023-1026.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, The Lancet. 2020; 395: 1054-1062.
- Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020; 382: 1708-1720.
- Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, et al. Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. International Journal of Infectious Diseases. International Society for Infectious Diseases. 2020; 94: 128-132.
- Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020.
- Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, The Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015; 13: 2119-2126.
- Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020; 217: 135-137.
- Thachil J. Forum article: The protective rather than prothrombotic fibrinogen in COVID-19 and other inflammatory states. J Thromb Haemost.2020.
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. I Intensive Care Med. 2020: 1-10.