
Research Article
Thromb Haemost Res. 2021; 5(1): 1055.
The Global Hemostatic Thrombodynamics Assay in Healthy Children
Seregina EA1,2*, Kumskova MA3, Gracheva MA1, Poletaev AV1, Kopilov KG3, Ataullakhanov FI1,2,4,5, Smetanina NS1,6, Balandina AN1,2
1Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Russia
2Center for Theoretical Problems of Physicochemical Pharmacology RAS, Russia
3National Medical Research Center of Hematology, Russia
4Department of Physics, Moscow State University, Russia
5The Faculty of Biological and Medical Physics, Moscow Institute of Physics and Technology, Russia
6Pirogov Russian National Research Medical University, Moscow, Russia
*Corresponding author: Elena A Seregina, Laboratory of Clinical Hemostasis, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, 117198 Samory Mashela str., 1, Moscow, Russia
Received: January 25, 2021; Accepted: March 24, 2021; Published: March 31, 2021
Abstract
Background: There is evidence that the concentrations of clotting and anticoagulant factors in children depend on age and differ from those found in adults. The results of APPT, TT, PT, fibrinogen are similar in children and adults in some studies, while PT and APTT show differences in others. Recent studies on global hemostatic assays Thromboelastography (TEG) revealed no significant differences in test results between healthy children and adults, while the thrombin generation test (TGT) showed significant differences. The Thrombodynamics (TD) assay is a new global hemostasis assay that considers the spatial organization of coagulation.
Methods: APTT, TT, PR, fibrinogen and TD assays were performed in 102 healthy children between the ages of 1 and 17 years who underwent annual medical examinations and in 91 healthy adult volunteers. The following TD assay parameters were determined: lag time (Tlag), initial clot growth velocity (Vi), stationary clot growth velocity (Vs), clot size 30 minutes after the start of clot growth (CS) and clot Density (D). Written consent was obtained from all participants or their parents after they received complete information about the tests.
Results: Age-specific reference values for the TD assay in healthy children aged 1-17 years are presented. No significant differences were observed between different age groups of children (15 years, 6-10 years, and 11-17 years) or between all children (1-17 years) and adults. Significant differences were not observed between genders.
Conclusions: The TD assay results revealed no age-specific differences in the parameters between children aged 1-17 years and adults.
Introduction
Global hemostatic tests, such as Thromboelastography (TEG), Rotational Thromboelastometry (ROTEM) and the Thrombin Generation Test (TGT), are increasingly used in clinical practice [1- 3]. The Thrombodynamics (TD) assay is a new test that provides a global assessment of hemostasis from clot initiation and development in the plasma sample volume to the localization of the plasma clot [4]. The ability of the TD assay to predict thrombotic and hemorrhage complications has been reported for the septic patients [5], the patients hemolytic anemia [6,7], during pregnancy complications [8], and patients with hemophilia [9].
As of yet, no study has been conducted to define the parameters of the TD assay in healthy children older than 1 year. The current research in the field of pediatric hematology has revealed that hemostasis in children changes as they age, especially during the first year of life [10]. Moreover, Andrew et al. [10-12] showed significant differences in the concentrations of clotting and anticoagulant factors between children aged 1-17 years and adults. However, the results of coagulation screening tests (the activated partial prothrombin time- APPT, the international normalized ratio-INR, and the fibrinogen and prothrombin time-PT) were similar between children aged 1-17 years and adults [12-14]. The authors explained that the changes in concentrations of clotting and anticoagulant factors in children after 1ye old are minor so there is no changes in clotting times [12,14,15]. But some studies revealed difference between children and adults groups in APTT [16] or PT [17,18]. The authors explained that the different changes in different laboratories are related with analyzer and reagent system [16]. Recently published data suggest that there are no differences in TEG parameters between children (1-17 years of age) and adults [19-21]. It is also reported that functional profile of coagulation was not compromised in different age-groups in children from 4 months to 10 years and adults in global ROTEM ExTEM assay. The differences were observed between group of children 3 months and younger and adults, and also between group of children from 11 to 16 years and adults [22]. In contrast, the TGT revealed a nearly 15% decrease in endogenous thrombin potential in children aged 0.5- 17 years compared to adults [23,24]. Thus, our study was designed to evaluate the hemostatic status of healthy children using the TD assay and to determine reference values for this new global test for children who are 1-17 years of age.
Methods
Patients
The clinical protocol was approved by the Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology Ethics Committee (approval number 10/2014). After obtaining written informed parental consent, blood samples were collected from 102 healthy children who were undergoing annual clinical examinations. The children had neither acute illnesses nor known inherited coagulation deficiencies; however, 18% of the children had minor iron deficiency anemia, 12% had alimentary allergies, 10% had adenoid hypertrophy, and 60% were considered completely healthy. No children had none conditions affected coagulation system (Table 1). These children were not receiving any anticoagulant therapy and had no history of bleeding or thrombotic disorders. Their complete blood count results were in the normal ranges. The age groups used in this study (1-5 years, 6-10 years, and 11-17 years) are in agreement with those used in previous studies outlining age-specific hemostatic reference ranges [12]. Blood was also collected from 91 healthy adult volunteers (median age of 32 years and age range of 18-70 years; 74 women and 20 men) to compare pediatric and adult reference values. All healthy adult donors had no changes in blood tests (basic metabolic panel and complete blood count), no inherited illnesses and no inflammation at the point of examination.
Age group
Mean
years
M/F*
Number of children with diagnosis
Iron deficiency anemia, N (%)
Alimentary allergy, N (%)
Adenoid hypertrophy, N (%)
Healthy children, N (%)
1-5 years
3.2
18/14
9 (28%)
5 (15%)
4 (13%)
14 (44%)
6-10 years
7.8
20/11
4 (13%)
3 (10%)
5 (16%)
19 (61%)
11-17 years
14.4
24/15
6 (15%)
5 (13%)
1 (3%)
27 (69%)
Adults (>18 Years)
35.4
17/74
-
-
-
-
*M=Males, F=Females.
Table 1: Clinical and demographic data of the children and the adults.
Reagents
SynthASil, RecombiPlasTin 2G, Fibrinogen-C, and Thrombin Time were obtained from HemosIL, Instrumentation Laboratory, Massachusetts, USA, and the TD kit was obtained from LLC HemaCore, Russia.
Blood collection and plasma preparation
Blood samples were drawn into 3-mL vacuum tubes (Monovette, Sarstedt, Germany) with 0.106 M sodium citrate buffer. The blood samples were processed by centrifugation at 1,500xg for 15 min to obtain Platelet-Poor Plasma (ppp), and part of the plasma was subsequently subjected to centrifugation at 10,000xg for 5 min to obtain Platelet-free Plasma (pfp).
Clotting time tests, fibrinogen assays
The following tests were performed using frozen ppp samples and the aforementioned reagents: Activated Partial Thromboplastin Time (APTT), Prothrombin Time (PT), Thrombin Time (TT), and fibrinogen concentration. All tests were performed in the Coagulation Laboratory of the Federal Research and Clinical Center of Pediatric Hematology, Oncology and Immunology, using an ACL TOP-700 (Instrumentation Laboratory, Massachusetts, USA) automated analyzer, according to the manufacturer’s instructions.
Thrombodynamics assay
The general concept of the test was previously described in [5,6]. A schematic diagram of the assay is shown in Figure 1. Briefly, coagulation is activated in a thin layer of plasma when it is brought into contact with Tissue Factor (TF) that has been immobilized on a plastic surface. Clot formation starts at the activator and propagates into the bulk of plasma where there is no TF present. Light scattering by fibrin allows observation of the spatial clot formation in real time by using time-lapse imaging (Figure 1a). The main parameters of clot growth in space are lag time (Tlag) and the initial (Vi) and stationary (Vst) rates of clot growth. Tlag is defined as the time between clotting initiation and the actual appearance of the fibrin clot (Figure 1c). Vi is measured as the slope of the curve on a clot size vs. time graph representing 2–6 minutes of clot growth when coagulation is occurring in the region where the diffusion of the active factors from the activator plays the major role. The most distinct parameter is Vst, which is measured as the slope of the curve on a clot size vs. time graph representing the interval 15-25 min after the initiation of clot growth. During this interval, the coagulation process is occurring far from the activating surface without direct contact with TF, and it is determined by only the plasma protein properties. Based on the clot size dynamics (Figure 1c), the clot size at 30 minutes (CS) and the time of spontaneous clot appearance (Tsp) were calculated. In normal plasma, the clot grows only from the activator, while in plasma with procoagulant activity; we observed activator-independent clotting in the plasma volume, with the appearance of spontaneous clots caused by circulating microparticles [25]. The clot density (D) was calculated as the plateau of intensity in the plot of the light scattering intensity vs. the distance of clot growth (Figure 1b). The Thrombodynamics Analyser System and Thrombodynamics kit (HemaCore LLC, Moscow, Russia) include the necessary reagents for sample preparation and coagulation activation and were used for all the experiments.
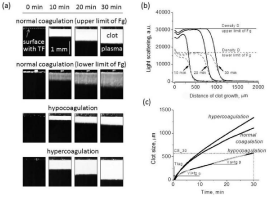
Figure 1: Thrombodynamics Test: Sensitivity to Different States of the
Hemostatic System.
a: Typical light scattering time-lapse images of clot growth initiated by
immobilized TF in healthy donor plasma (2 upper panels with upper and lower
limits of fibrinogen) and in plasma from patients in hypercoagulation (lower
panel) or hypocoagulation (middle panel) states. The TF-coated activator
surface is seen as a horizontal black strip at the top of each image.
b: Plot of the light scattering intensity vs. the distance of clot growth.
C: Plot of clot size vs. time for the experiments shown in (a). The curves also
indicate the following parameters used for analysis throughout the study: the
lag time; the time to clot growth initiation; the initial clot growth velocity, Vi (tg
a); and the stationary clot growth velocity, Vs (tg β).
Statistical analysis
The statistical analysis of the differences between the datasets were performed using the Wilcoxon signed-rank test for statistical significance (p<0.05). Pearson’s correlation test was used for correlation calculations. For all calculations, the program OriginPro 8.0 (OriginLab Corporation, MA, USA) was used.
Results
In total, 102 healthy children underwent an annual clinical examination. Reference values for the TD assay were determined for different age groups and are shown in Table 2, expressed as the mean and 5th-95th percentiles. Similar to coagulation test APTT, TT, INR and fibrinogen level (Table 4), no significant differences were observed between age groups (Table 2) and gender groups (Table 3) for the TD assay. There was a significant difference in PT values for all children groups compared to adults but no differences between all children groups (Table 4).
Parameter
1-5 years
(N=32)6-10 years
(N=31)11-17 years
(N=39)Adults (N=91)
Tlag, min
0.9 (0.8-1.0)
0.9 (0.8-1.1)
0.9 (0.8-1.1)
0.9 (0.7-1.1)
Vi, μm/min
51 (46-57)
51 (44-57)
51 (45-56)
52 (45-57)
Vs, μm/min
25 (22-29)
24 (21-28)
25 (21-29)
26 (22-30)
CS_30, μm
1064 (933-1191)
1068 (940-1230)
1055 (920-1991)
1108(955-1230)
D, a.u.
23362 (17646-29771)
23789 (18445-28209)
23891 (17765-29905)
24011 (18155-30595)
Tsp, min
No spontaneous clots observed
No spontaneous clots observed
No spontaneous clots observed
No spontaneous clots observed
*No significant differences among all children and adults age groups (P>0.05).
Table 2: Thrombodynamics reference values.
Parameter
1-5 years
6-10 years
11-17 years
M (N=18)
F (N=14)
M (N=20)
F (N=11)
M (N=24)
F (N=15)
Tlag, min
0.9 (0.7-1)***
0.9 (0.8-1.1)
0.9 (0.8-1.1)
0.9 (0.8-1.2)
0.9 (0.8-1.1)
0.9 (0.8-1.2)
Vi, μm/min
51 (45-58)
51 (47-57)
51 (43-58)
52 (48-57)
51 (46-56)
49 (45-56)
Vs, μm/min
24 (21-27)
25 (22-28)
24 (21-27)
25 (22-28)
25 (21-29)
24 (22-27)
CS_30, μm
1058
1078
1056
1093
1059
1032
(907-1162)
(970-1168)
(920-1221)
(1012-1223)
(925-1189)
(906-1180)
D, a.u.
22716
24413
24079
23262
23931
24200
(17450-29930)
(20908 -28394)
(20467-26966)
(17240-28331)
(17906-30216)
(20643-27214)
*No significant differences among all children and adult gender groups (P>0.05).
Table 3: Thrombodynamics values for gender groups (F=Female, M=Male, the mean value (5-95 percentiles) are presented).
Test
1-5 years
6-10 years
11-17 years
Adults
(N=32)
(N=33)
(N=39)
(N=90)
APPT, sec
35.7 (31.1-39.3)
36.9 (31.9-42.0)
35.1 (30.4-41.1)
34.3 (29.1-39.6)
TT, min
23 (20-25)
23 (19-25)
23 (20-26)
20 (17-22)
Fibrinogen, g/l
2.6 (2.0-3.5)
2.7 (2.1-3.7)
2.6 (2.0-3.3)
2.9 (2.1-3.8)
INR
1 (0.9-1.2)
1 (0.9-1.2)
1 (1.0-1.3)
0.9 (0.8-1.0)
PT, %
92 (75-105)*
97 (79-108)*
94 (71-120)*
115 (102-120)
P-value PT**
0.03
0.05
0.04
-
*No significant differences between all children and adult age groups (P>0.05) except PT shows the difference between children and adults but no difference between all children groups (P>0.05). Actual P-values compared to adults group are presented.
Table 4: Standard coagulation tests.
We found a strong correlation between the D and the concentration of the fibrinogen clot for all ages (Figure 2). Because lipemia may interfere with optical clot detection in the TD assay, only postprandial lipemia-free plasma was used for the calculations of this correlation. This finding is consistent with previous in vitro data [26].
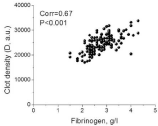
Figure 2: The correlation between the clot density (D) and the fibrinogen concentration.
Pearson’s correlation test was used for the correlation calculation.
Discussion
This study was the first to provide reference values for the TD assay in healthy children. We found no age-related differences in the TD assay parameters, which was in agreement with previous results regarding the TEG [19], ROTEM [22] and other coagulation tests (APPT, INR, fibrinogen, PT) [12,14,15]. According to Andrew et al. [10-12], compared to adults, children have higher levels of some clotting factors and inhibitors and lower levels of others; compared to adults, children have 18% lower levels of FII, FVII, FIX, FX, Protein C, and heparin cofactor II and 18% higher levels of FXIIa and a2- macroglobulin.
Therefore, why are the standard coagulation tests, TEG and TD assays not sensitive to these differences in levels? A possible explanation lies in the fact that the coagulation system is known to be robust and is insensitive to changes in the concentrations of its components unless they are by at least an order of magnitude [27].
Interestingly, the TGT revealed a nearly 15% lower Endogenous Thrombin Potential (ETP) in children compared with adults, which is in disagreement with the results obtained with other assays. A possible explanation for this is that TGT parameters are linearly proportional to the prothrombin concentration [28]. This agrees well with the previous report of an 18% lower [11-14] prothrombin concentration in healthy children than in adults. So the changes in TGT may caused by a reduction of thrombin conversion, an increase of thrombin inactivation, or both [29]. In contrast, TEG and TD are not focused on the concentration of one specific factor but rather reveal the clotting process as a whole.
Children under 1 year of age were not involved in this study, so further investigations are required to examine the hemostatic system in children younger than 1 year of age in detail, as it is during this period that most developmental changes occur.
Developmental and maturation of hemostasis system is universally accepted and introduced by Andrew et al. in the late 1980’s [10-12]. However, coagulation analysers and reagents have changed significantly since that. Coagulation testing is known to be sensitive to changes in individual reagents and analyzers system, the methods used (mechanical or optical), blood collection method, lipemia. In the world practice it is known that the age-specific reference ranges are more appropriate to utilise in a modern coagulation laboratory. Our study was designed to determine children references for our laboratory system of reagents and analyzer. But there were no difference (p>0.05) for standard coagulation tests (APTT, TT, fibrinogen, INR) as in other similar studies [12-14]. However, PT shows the difference between children and adults groups for our laboratory system as described in some other papers [17,18]. Interestingly, there was no significant difference for INR (Table 4). A possible explanation is that the changes in PT may caused by a reduction of thrombin conversion as for TGT. Whereas INR is not linearly proportional to the prothrombin concentration. In this study, we generated standard reference values for children in different age groups for the TD assay. Previously, a small group of healthy volunteers was used as a control group for adult patients with severe disorders in the TD assay [5,6]. In summary, we present reference values for the TD assay for healthy children aged 1-17 years and adults. The reference values for adults in our study are consistent with those found in groups of healthy adults in previous studies.
References
- Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016; 174: 503-514.
- Bolliger D, Seeberger MD, Tanaka KA. Principles and Practice of Thromboelastography in Clinical Coagulation Management and Transfusion Practice. Transfus Med Rev. 2012; 26: 1-13.
- Tripodi A. Thrombin Generation Assay and Its Application in the Clinical Laboratory. Clin Chem. 2016; 62: 699-707.
- Panteleev MA, Ovanesov MV, Kireev DA, Shibeko AM, Sinauridze EI, Ananyeva NM, et al. Spatial Propagation and Localization of Blood Coagulation Are Regulated by Intrinsic and Protein C Pathways, Respectively. Biophys J. 2006; 90: 1489-1500.
- Soshitova NP, Karamzin SS, Balandina AN, Fadeeva OA, Kretchetova AV, Galstian GM, et al. Predicting prothrombotic tendencies in sepsis using spatial clot growth dynamics: Blood Coagul Fibrinolysis. 2012; 23: 498-507.
- Seregina EA, Nikulina OF, Tsvetaeva NV, Rodionova MN, Gribkova IV, Orel EB, et al. Laboratory tests for coagulation system monitoring in a patient with β-thalassemia. Int J Hematol. 2014; 99: 588-596.
- Seregina EA, Tsvetaeva NV, Nikulina OF, Zapariy AP, Erasov AV, Gribkova IV, et al. Eculizumab effect on the hemostatic state in patients with paroxysmal nocturnal hemoglobinuria. Blood Cells Mol Dis. 2015; 54: 144-150.
- Ataullakhanov F, Koltsova E, Balandina A, Serebriyskiy I, Vuimo T, Panteleev M. Classic and Global Hemostasis Testing in Pregnancy and during Pregnancy Complications. Semin Thromb Hemost. 2016; 42: 696-716.
- Ovanesov MV, Krasotkina JV, Ul’yanova LI, Abushinova KV, Plyushch OP, Domogatskii SP, et al. Hemophilia A and B are associated with abnormal spatial dynamics of clot growth. Biochim Biophys Acta. 2002; 1572: 45-57.
- Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, et al. Development of the human coagulation system in the full-term infant. Blood. 1987; 70: 165-172.
- Andrew M, Paes B, Johnston M. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol. 1990; 12: 95- 104.
- Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992; 80: 1998-2005.
- Toulon P. Developmental hemostasis: laboratory and clinical implications. Int J Lab Hematol. 2016; 38: 66-77.
- Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, et al. Developmental haemostasis: Impact for clinical haemostasis laboratories. Thromb Haemost. 2006; 95: 362-372.
- Rodgers GM. Developmental hemostasis: age-specific differences in the levels of hemostatic proteins: a rebuttal. J Thromb Haemost. 2014; 12: 285.
- Appel IM, Grimminck B, Geerts J, Stigter R, Cnossen MH, Beishuizen A. Age dependency of coagulation parameters during childhood and puberty: Pediatric reference levels of coagulation parameters. J Thromb Haemost. 2012; 10: 2254-2263.
- Flanders MM. Pediatric Reference Intervals for Seven Common Coagulation Assays. Clin Chem. 2005; 51: 1738-1742.
- Castellone DD. Establishing reference intervals in the coagulation laboratory. Int J Lab Hematol. 2017; 39: 121-127.
- Miller BE, Bailey JM, Mancuso TJ, Weinstein MS, Holbrook GW, Silvey EM, et al. Functional maturity of the coagulation system in children: an evaluation using thrombelastography. Anesth Analg. 1997; 84: 745-748.
- Pivalizza EG, Pivalizza PJ, Gottschalk LI, Kee S, Szmuk P, Abramson DC. Celite-activated thrombelastography in children. J Clin Anesth. 2001; 13: 20- 23.
- Chan K-L, Summerhayes RG, Ignjatovic V, Horton SB, Monagle PT. Reference Values for Kaolin-Activated Thromboelastography in Healthy Children: Anesth Analg. 2007; 105: 1610-1613.
- Oswald E, Stalzer B, Heitz E, Weiss M, Schmugge M, Strasak A, et al. Thromboelastometry (ROTEM®) in children: age-related reference ranges and correlations with standard coagulation tests. Br J Anaesth. 2010; 105: 827-835.
- Haidl H, Cimenti C, Leschnik B, Zach D, Muntean W. Age-dependency of thrombin generation measured by means of Calibrated Automated Thrombography (CAT). Thromb Haemost. 2006; 95: 772-775.
- Devreese K, Wijns W, Combes I, Van kerckhoven S, Hoylaerts MF. Thrombin generation in plasma of healthy adults and children: chromogenic versus fluorogenic thrombogram analysis. Thromb Haemost. 2007; 98: 600-613.
- Lipets E, Vlasova O, Urnova E, Margolin O, Soloveva A, Ostapushchenko O, et al. Circulating Contact-Pathway-Activating Microparticles Together with Factors IXa and XIa Induce Spontaneous Clotting in Plasma of Hematology and Cardiologic Patients. El-Maarri O, editor. PLoS ONE. 2014; 9: e87692.
- Ovanesov MV, Ananyeva NM, Panteleev MA, Ataullakhanov FI, Saenko EL. Initiation and propagation of coagulation from tissue factor-bearing cell monolayers to plasma: initiator cells do not regulate spatial growth rate*. J Thromb Haemost. 2005; 3: 321-331.
- Panteleev MA, Balandina AN, Lipets EN, Ovanesov MV, Ataullakhanov FI. Task-Oriented Modular Decomposition of Biological Networks: Trigger Mechanism in Blood Coagulation. Biophys J. 2010; 98: 1751-1761.
- Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, et al. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. 2002; 88: 576-582.
- Kremers R, Wagenvoord R, de Laat H, Monagle P, Hemker H, Ignjatovic V. Low paediatric thrombin generation is caused by an attenuation of prothrombin conversion. Thromb Haemost. 2016; 115: 1090-1100.