
Research Article
Thromb Haemost Res. 2021; 5(1): 1056.
Real-Life Applications and Challenges of Andexanet Alfa for Life-Threatening Bleeding and Reversal Prior to Surgery
Modi K¹*, Attar D¹, Rimsans J², Connors JM², Lekura J¹, Kuriakose P¹, Kaatz S¹ and Sylvester K²
1Henry Ford Hospital, 2799 W Grand Blvd, Detroit, USA
2Brigham and Women’s Hospital, 75 Francis St, Boston, USA
*Corresponding author: Krishna Modi, Henry Ford Hospital, 2799 W Grand Blvd, Detroit, MI 48202, USA
Received: March 15, 2021; Accepted: April 09, 2021; Published: April 16, 2021
Abstract
Andexanet alfa is a targeted reversal agent for apixaban and rivaroxaban for life-threatening or uncontrolled bleeding. There are few multicenter, real world studies that also include patients with off-label use who require emergent surgery. The objective was to describe hemostatic efficacy, thrombotic events, clinical applications, pharmaceutical challenges, and mortality associated with reversing apixaban and rivaroxaban with andexanet alfa in clinical practice. Retrospective descriptive observational cohort study of andexanet alfa use at 2 academic medical centers in the United States from July 1, 2018 to September 30, 2019. Ninety patients received 91 doses of andexanet alfa including 6 for reversal prior to surgery. Effective hemostasis was achieved in 72.9% of bleeding episodes and all patients that received andexanet alfa preoperatively were deemed to have effective hemostasis. Thrombotic events occurred in 7 of 90 patients (7.7%) and 2 of these events occurred the day after administration. Incorrect high-dose andexanet alfa was given 11 times with an estimated excess expenditure of $272,250. Thirty-two of 90 (35.5%) patients died, and most deaths occurred during the initial hospitalization. Our real-world experience with andexanet alfa in bleeding patients is similar to the non-comparative trial that led to Food and Drug Administration approval, and our findings show good hemostatic efficacy in a small number of patients requiring emergent surgery. We highlight the importance of appropriate dose based on time of ingestion and factor Xa inhibitor dose. Our 2 institutions spent over a quarter of a million dollars on excess andexanet alfa in a year and a half.
Keywords: Apixaban; Andexanet alfa; Anticoagulation; Bleeding; Reversal; Rivaroxaban
Introduction
Direct oral anticoagulants including Factor Xa inhibitors (FXai) are guideline preferred for treatment of Venous Thromboembolism (VTE) and non-valvular atrial fibrillation thromboprophylaxis [1,2]. A concern for prescribing FXai was the availability of a targeted reversal agent. Non-specific reversal strategies, such as 4-Factor Prothrombin Complex Concentrates (4F-PCC), have been used but there are no large prospective head-to-head studies or randomized controlled trials to evaluate efficacy and safety. Hemostatic efficacy of 4F-PCC has been reported to range from 65% to 95% in bleeding patients, with thrombotic event rates of 0% to 8%, and mortality ranging from 14% to 32% [3-7].
Coagulation factor Xa (recombinant), inactivated-zhzo (commonly known as andexanet alfa) is an US Food and Drug Administration (FDA)-approved reversal agent for FXai, apixaban and rivaroxaban, for life-threatening or uncontrolled bleeding [8]. It is a modified human factor Xa decoy protein that binds to and neutralizes FXai [9] without catalytic activity. It is administered intravenously and can be given as a low-dose of a 400mg intravenous bolus, followed by an intravenous infusion at a rate of 4mg per minute for up to 2 hours; or a high dose, 800mg intravenous bolus, followed by an intravenous infusion given at a rate of 8mg per minute for up to 2 hours. The dose of andexanet alfa is determined by the FXai used, dose and time since last FXai ingestion [10]. In healthy volunteers, andexanet alfa is effective in reducing anti-factor- Xa activity by 92% to 94% [11]. In the landmark ANNEXA-4 trial, 82% of patients achieved hemostasis when reversed with andexanet alfa for life-threatening bleeding, with thrombotic events observed in 10% of patients during a 30-day period following drug administration [12]. Of note, no patients requiring emergent surgery or invasive procedures were included in the ANNEXA-4 trial.
Studies demonstrating the real-life applications of andexanet alfa are limited, with a lack of data on the role of andexanet alfa in the perioperative setting. The purpose of this study is to describe the hemostatic efficacy, thrombotic events, clinical applications, pharmaceutical challenges, and mortality associated with reversing apixaban and rivaroxaban with andexanet alfa in clinical practice.
Methods
This retrospective observational cohort study included all adult patients treated with andexanet alfa from July 1, 2018 to September 30, 2019 at Henry Ford Hospital in Detroit, and Brigham and Women’s Hospital in Boston. This study was approved by the institutional review boards of each institution.
The Henry Ford Hospital protocol recommends andexanet alfa for reversal of apixaban and rivaroxaban in cases of life-threatening bleeding and includes use for emergent life-saving procedures or surgeries. Brigham and Women’s Hospital’s guidelines allow for the use of andexanet alfa for reversal of life-threatening major bleeding but does not include use for urgent or emergent surgery as the sole indication. In the Brigham and Women’s Hospital guideline, if apixaban or rivaroxaban was taken >18 hours prior to presentation, if the indication is for an emergent surgery or procedure, or for an FXai other than apixaban or rivaroxaban, review and approval from a hematologist is required.
Manual abstraction of electronic medical records included patient demographics, FXai prescribed, anticoagulation indication and reason for reversal including location of bleeding or type of surgery. All patients aged 89 or above were listed as 89 years old for patient confidentiality. Timing of last FXai dose and andexanet alfa doses were collected. Outcomes included hemostatic efficacy, development of a thrombotic event (including type, location, and timing), resumption of anticoagulation (including regimen and timing), and mortality. For operational metrics, the time for order entry to medication administration was calculated and the appropriateness of the andexanet alfa dosing based on FDA labeling.
Our major endpoint was hemostasis efficacy. We were unable to use the definition used in the ANNEXA-4 study12 because of lack of documentation of key variables in this retrospective analysis. For Intracranial Hemorrhage (ICH), hemostasis was considered achieved if post-reversal imaging, either computed tomography or magnetic resonance imaging, demonstrated minimal or no expansion of the bleed and additional surgical procedures were not required to control bleeding. For patients who had an extraventricular drain placed, notes were referenced to determine if the procedure was done to stop bleeding or for standard post-ICH care. If surgical procedure was performed at initial evaluation, the definition of effective hemostasis was based on follow-up imaging, and neurology or neurosurgery documentation. If there was no repeat imaging available due to the patient’s status changing to comfort measure only, hemostasis was not considered effective. If, however, a computed tomography scan showed no further expansion of the bleed, but patient code status was changed due to residual effects of the initial bleed, hemostasis was considered effective.
For extracranial bleeding, hemostasis was defined as ineffective if the patient required supportive blood products or concentrated clotting factors within 48 hours after andexanet alfa receipt to manage bleeding, or if there was a hemoglobin drop of =2gm/dL in the setting of bleeding. For surgery-related reversal where blood loss is anticipated, hemostasis was considered effective if estimated blood loss did not exceed 30% of expected blood loss of performed procedure.
The major safety endpoint was the development of thrombotic events, including VTE, stroke, myocardial infarctions, and allcause mortality within 30 days after andexanet alfa. Data regarding resumption of anticoagulation, including therapeutic dosing or VTE chemoprophylaxis were recorded.
A barrier to this being a retrospective descriptive study was incomplete documentation to assess our endpoints. This was documented accordingly during data collection to limit bias.
Categorical variables are presented as number and percentages. Continuous variables are presented as mean (standard deviation) or median (interquartile range) as appropriate.
Results
Ninety patients received 91 doses of andexanet alfa between July 1, 2018 to September 30, 2019; 32 patients (35.6%) at Brigham and Women’s Hospital and 58 patients (64.4%) at Henry Ford Hospital. Patients’ demographics are noted in Table 1. The majority were male (n = 55, 61.1%) and white (n = 66, 73.3%). Half of our patients were older than 75 years, with multiple patients being 89 years or older. The youngest patient was 23 years old. The major indications for anticoagulation were atrial fibrillation and VTE, and most patients were receiving apixaban (73.3%).
n (%)
Total number of patients
90
Total number of andexanet alfa administrations
91
Male
55 (61.1)
Age at andexanet alfa administration, years
75.5 (66.5-83)*
Race (n=90)
White
66 (73.3)
African American
12 (13.3)
Hispanic
3 (3.3)
Unknown
5 (5.6)
Other
4 (4.4)
Indication for anticoagulation (n=90)
Atrial fibrillation
64 (71.1)
Venous thromboembolism treatment
20 (22.2)
Venous thromboembolism prophylaxis after surgery
1 (1.1)
Peripheral arterial disease
3 (3.3)
Arterial thrombus
1 (1.1)
Atrial fibrillation and venous thromboembolism
1 (1.1)
Anticoagulant reversed (n=90)
Apixaban
66 (73.3)
Rivaroxaban
24 (26.7)
Location within hospital where andexanet alfa given (n=91)
Emergency department
59 (64.8)
Intensive care unit
17 (18.7)
General provider unit
7 (7.7)
Perioperative area
8 (8.8)
*Median (interquartile range) when appropriate.
Table 1: Characteristics of patient population.
Andexanet alfa was given primarily in the emergency department (Table 1). It was given in 85 cases for life-threatening bleeding and 6 for preoperative reversal. Figure 1 illustrates the location of bleeding, which was most frequently ICH (n = 42, 49.4%), gastrointestinal bleeding (n = 15, 17.6%), or aortic aneurysm or dissection (n = 5, 5.9%). Other locations of bleeding included the intraperitoneal (n = 4), retroperitoneal (n = 4), thoracic cavity (n = 2), and pericardial space (n = 2). There was also hemoptysis related to malignancy of the head and neck or lung (n = 3), an intramuscular hematoma (n = 1), bleeding in the pelvis (n = 1), and an unclear genitourinary or gastroenterological source (n = 1). There was 1 case of both ICH and gastrointestinal bleed, and another case in which the source of bleeding was not identified. There were 3 cases of postoperative bleeding.
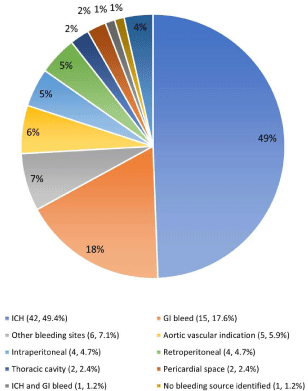
Figure 1: Location of bleeding when andexanet alfa is used for reversal in
setting of life-threatening bleeding. Abbreviations: GI: Gastrointestinal; ICH:
Intracranial Hemorrhage.
The median time from andexanet alfa order to drug administration was 58 minutes (interquartile range 42.5-81). In 78% of reversal cases, 100mg vials were used for preparation and 200mg vials for the remainder. The high dose was given in 18.7% (17/91) administrations and there was adequate information in the chart to determine the dose was incorrect for 11/17 (64.7%) cases. Low dose was given 74 times and there was insufficient charting to determine if this was the correct doses in 5 administrations. All other low-dose administrations were correct. Details regarding all incorrect andexanet alfa dose administrations are noted in Figure 2. Nine bleeding episodes had reversal given >18 hours from the last dose of the FXai, which was an exclusion criterion in the ANNEXA-4 trial [12].
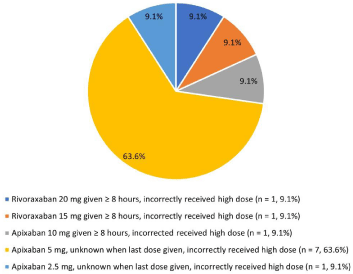
Figure 2: Details regarding factor Xa inhibitors and andexanet alfa doses
when incorrect dose of andexanet alfa given.
Details regarding outcomes are listed in Table 2. There were 85 cases in which andexanet alfa was given for life-threatening bleeding. Effective hemostasis was achieved in 72.9% of encounters (62/85). In those with ICH, the rate of hemostasis was 86.0% (37/43). Procedures were concurrently performed to evaluate and manage bleeding in 38 of these 85 cases (44.7%) and included hematoma evacuation for ICH, upper and lower endoscopies for gastrointestinal bleeding, exploratory laparotomy, interventional radiology guided embolization, and bronchoscopy. In 29.7% of cases (27/91), additional procoagulant products were administered including 4F-PCC (13), fresh frozen plasma (16), cryoprecipitate (4), and activated prothrombin complex concentrates (aPCC) (1). These procoagulant products were often given concurrently with andexanet alfa.
Clinical Outcome
Result, n (%)
Hemostasis achieved
Overall cohort (n=91)
68 (74.7)
Bleeding cohort (n=85)
62 (72.9)
Intracranial hemorrhage* (n=43)
37 (86.0)
Perioperative cohort (n=6)
6 (100)
Thrombotic events within 30 days (n=86)†
7 (7.7)
Deep vein thrombosis
3 (3.3)
Pulmonary embolism
0
Stroke
2 (2.2)
Myocardial infarction
2 (2.2)
In-hospital mortality
29 (32.2)
Mortality within 30 days
32 (35.5)
*One patient had both intracranial hemorrhage and gastrointestinal bleed.
†Unable to determine in 5 patients
Table 2: Clinical efficacy and safety outcomes.
VTE chemoprophylaxis was initiated after reversal in 38.5% of cases (35/91) on an average of 2.8 days after andexanet alfa administration. Treatment dose oral or intravenous anticoagulation was initiated in 30.8% (28/91) cases, on average 7.6 days after reversal. Oral anticoagulation was restarted using the same dose of apixaban or rivaroxaban in 71.4% of cases (20/28), and a lower dose or alternative oral agent was started in 25% of cases (7/28). A single patient was discharged on subcutaneous low molecular weight heparin.
Thrombotic events were documented in 7/91 encounters (7.7%) within 30 days of andexanet alfa administration, including 3 deep vein thromboses, 2 strokes, and 2 myocardial infarctions. Details regarding these events are listed in Table 3. Two patients that received high doses of andexanet alfa when not indicated developed thrombotic events the same day as receiving andexanet alfa. An additional 2 patients had received 4F-PCC along with andexanet alfa and were noted to have thrombotic events at days 2 and 7. Twentynine (32.2%) patients died during the initial hospitalization with 58.6% (17/29) of the deaths attributable to the bleeding episode. Three additional patients died after hospital discharge, resulting in a 30-day mortality of 35.5%. The 30-day mortality rate for patients presenting with ICH was 20.9% (9/43).
Patient
Dose of Andexanet Alfa Given
Thrombotic Event, and Time After Andexanet Alfa
Received Pro-Coagulation Products?
VTE Prophylaxis Restarted*
Therapeutic Anticoagulation Restarted*
30-Day In Hospital-Mortality
1
Low
DVT, 7 days
Yes, 4F-PCC
Yes, on day 2
Yes
Alive
2
High
DVT, 2 days
Yes, 4F-PCC
Yes, on day 3
No
Alive
3
Low
Stroke, 3 days
No
No
No
Deceased
4
Low
MI and embolic shower to small bowel, same day
No
No†
Yes
Deceased
5
High‡
Stroke, same day
No
No†
Yes
Unknown
6
High‡
MI, same day
No
No†
Yes
Alive
7
Low
Upper extremity DVT, 30 days
No
No
No
Alive
4F-PCC: 4-Factor Prothrombin Complex Concentrates; DVT: Deep Vein Thrombosis; MI: Myocardial Infarction; VTE: Venous Thromboembolism.
*VTE chemoprophylaxis and therapeutic anticoagulation was restarted at some point during this hospitalization, but not necessarily prior to thrombotic event.
†VTE prophylaxis not initiated because therapeutic anticoagulation restarted.
‡High dose of andexanet alfa given when patients should have received low dose.
Table 3: Characteristics and outcomes of the 7 patients who developed thrombotic events.
The preoperative indications for andexanet alfa included exploratory laparotomies for incarcerated or strangulated hernias (n = 3), repair of gastric ulcers (n = 1), small bowel resection and repair (n = 1), and a femoral endarterectomy (n = 1). Estimated blood loss did not exceed 30% of expected in any of the patients and no bleeding complications were documented in operative notes. This demonstrates appropriate hemostasis in 100% of patients receiving andexanet alfa for reversal prior to surgery. Five of the 6 patients restarted anticoagulation in-hospital, and none developed thrombotic events. Within 30 days, 1 patient had died (1/6) due to causes unrelated to the index bleeding event.
One patient received andexanet alfa on 2 separate occasions. The patient had metastatic lung cancer with unilateral pulmonary embolism <3 months prior to presentation, treated with apixaban and presented to the hospital twice with massive hemoptysis. The patient achieved hemostasis with the first andexanet alfa administration and was discharged on therapeutic dose apixaban; however, the patient was re-admitted and died from fatal massive hemoptysis despite their second course of andexanet alfa.
Discussion
In this analysis, the hemostatic efficacy rate after reversal with andexanet alfa for life-threatening bleeding was 72.9%, and 86% in ICH-specific hemostasis, similar to rates seen in the ANNEXA-4 trial at 82% and 80%, respectively [12]. Our data provide reassurance and validity to current available literature describing its use. We do note that a small proportion of patients included in this analysis received other pro-coagulant therapies, including 4F-PCC, fresh frozen plasma, cryoprecipitate, and aPCC, as well as concurrent procedures performed in an effort to manage bleeding. Based on the exclusion criteria from the ANNEXA-4 trial, the use of 4F-PCC/aPCC was outside both hospital guidelines for andexanet alfa use due to lack of data in relation to thrombotic events [12,13]. As our hospitals are tertiary care centers, patients may have received 4F-PCC/aPCC at the referring hospital where andexanet alfa was not available, reflecting real-world practice. We do note that 2/7 patients who received both andexanet alfa and 4F-PCC developed thrombotic events within a week of administration, despite resumption of VTE chemoprophylaxis. Use of both therapies concurrently presents a challenge; further studies need to be performed to evaluate the risks when both therapies are used simultaneously.
All 6 cases in which andexanet alfa was administered preoperatively, estimated blood loss did not exceed 30% of expected blood loss, and these patients were not noted to have bleeding complications. Although this is an off-label use for andexanet alfa, these results are in accordance with previously published retrospective reports where patients underwent reversal prior to surgery [14-17]. With successful use documented in preoperative cases, questions arise in regards to expanding use and larger studies to assess the efficacy and safety of preoperative reversal of FXai, especially for major surgeries or those requiring intraoperative anticoagulation, such as during cardiopulmonary bypass, are needed.
The rate of thrombotic events observed was 7.7%, which was lower than the 10% observed in the ANNEXA-4 trial [12]. We note that in 4/7 thrombotic events, patients were given either high doses of andexanet alfa when not indicated or had received 4F-PCC in addition to the correct dose of andexanet alfa. This emphasizes the importance of ensuring patients receive the correct dose of the reversal agent to minimize possible risks for thrombotic events. It also raises a concern that the risk of thrombotic events may increase when both andexanet alfa and 4F-PCC are used together [3-7].
The 30-day mortality rate in this analysis was 35.5% compared to 14% in the ANNEXA-4 trial [12]. The ANNEXA-4 trial excluded patients with life expectancy <1 month, large ICH volume, defined as >60mL, or patients with Glasgow Coma Scale score of <7. Most of the deaths in our analysis were in-hospital, of which 58.6% were related to the initial bleeding episode. The higher mortality observed likely reflects a critically ill patient population.
We found that a high number of patients were ordered and administered a dose of andexanet alfa that was not recommended in the FDA labeling. Dosing the drug can be challenging, and often insufficient information regarding the patient’s FXai regimen is available on initial presentation in which is needed for proper dose selection. In certain time critical situations, providers may prefer the higher dose in the face of uncertainty. No patients inappropriately received the lower dose. We were missing documentation in 5 patients in order to classify appropriate dosing. Given the effect of andexanet alfa on the tissue factor pathway, there may have been an increased thrombotic risk in patients with high dosing when it is not indicated [8]. The costs associated with andexanet alfa is frequently in the spotlight. The cost difference between low and high doses of andexanet alfa is approximately $24,750 [10], which resulted in an excess expenditure of $272,250. Ongoing education to medical providers, increased presence of clinical pharmacists, clinical decisions support, pharmacy stewardship, and a mandatory requirement for consultation may improve appropriate use of this drug.
Our large real-world data evaluating the use of andexanet alfa highlights the challenges in using this drug. It also reveals challenges in which patients receive other pro-coagulant therapies that were not studied in conjunction with andexanet alfa. Limitations of our study include the retrospective design and missing data that precluded us from operationalizing a trial based hemostatic efficacy assessment and dose/time of last FXai.
Conclusion
In conclusion, our real-world experience with andexanet alfa at 2 academic medical centers found similar hemostatic effectiveness as the trial that led to the FDA approval of andexanet alfa, with lower rates of thromboembolism but higher mortality in daily practice. The use of andexanet alfa in patients requiring emergent surgery or procedures is evolving and may be considered in individual circumstances. The addition of mandatory consultation with a pharmacist or hematologist may help achieve appropriate use by mitigating incorrect use of the higher dose, which may lead to less thromboembolic events and prevent excessive cost expenditures.
References
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016; 149: 315-352.
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019; 140: e125-e151.
- Tao J, Bukanova EN, Akhtar S. Safety of 4-factor Prothrombin Complex Concentrate (4F-PCC) for emergent reversal of factor Xa inhibitors. J Intensive Care. 2018; 6: 34.
- Smith MN, Deloney L, Carter C, Weant KA, Eriksson EA. Safety, efficacy, and cost of Four-Factor Prothrombin Complex Concentrate (4F-PCC) in patients with factor Xa inhibitor-related bleeding: a retrospective study. J Thromb Thrombolysis. 2019; 48: 250-255.
- Schulman S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018; 118: 842-851.
- Majeed A, Ågren A, Holmström M, Bruzelius M, Chaireti R, Odeberg J, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017; 130: 1706- 1712.
- Berger K, Santibañez M, Lin L, Lesch CA. A low-dose 4F-PCC protocol for DOAC-associated intracranial hemorrhage. J Intensive Care Med. 2020; 35: 1203-1208.
- US Food and Drug Administration. ANDEXXA prescribing information. 2020.
- Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013; 19: 446-451.
- Beik N, Reddy P, Sylvester KW, Connell NT, Giugliano RP, Piazza G, et al. Andexanet alfa (Andexxa) formulary review. Crit Pathw Cardiol. 2019; 18: 66-71.
- Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015; 373: 2413-2424.
- Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019; 380: 1326-1335.
- US Food and Drug Administration. ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo). 2020.
- Brown CS, Scott RA, Sridharan M, Rabinstein AA. Real-world utilization of andexanet alfa. Am J Emerg Med. 2020; 38: 810-814.
- Stevens VM, Trujillo T, Mueller SW, MacLaren R, Reynolds PM, Kiser TH. Coagulation factor Xa (recombinant), inactivated-zhzo (andexanet alfa) hemostatic outcomes and thrombotic event incidence at an academic medical center. Clin Appl Thromb Hemost. 2019; 25: 1076029619896619.
- Culbreth SE, Sylvester KW, Rimsans J, Connors JM. Coordinating emergent procedures after andexanet alfa. Am J Hematol. 2019; 94: E278-e282.
- Barra ME, Das AS, Hayes BD, Rosenthal ES, Rosovsky RP, Fuh L, et al. Evaluation of andexanet alfa and Four-Factor Prothrombin Complex Concentrate (4F-PCC) for reversal of rivaroxaban- and apixaban-associated intracranial hemorrhages. J Thromb Haemost. 2020; 18: 1637-1647.