
Research Article
Thromb Haemost Res. 2021; 5(2): 1057.
Performance Metrics of the AGGRESTAR PL-12® Platelet Function Analyser in Patients with TIA or Ischaemic Stroke on Commonly-Prescribed Antiplatelet Regimens
Offiah C1,2, Delaney S1,2, Smith DR1,3, Murphy SJX1,2, Ryan DJ2,4, Collins DR2,4, McCarthy AJ1,2, Egan B5, Cox D6,7 and McCabe DJH1,2,3,7,8,9*
1Department of Neurology, Tallaght University Hospital (TUH)/The Adelaide and Meath Hospital, Dublin, Incorporating the National Children’s Hospital (AMNCH), Dublin, Ireland
2Stroke Service, TUH/AMNCH, Ireland
3Vascular Neurology Research Foundation, C/O Department of Neurology, TUH/AMNCH, Dublin, Ireland
4Department of Age-Related Health Care, TUH/AMNCH, Dublin, Ireland
5Department of Vascular Surgery, TUH/AMNCH, Dublin, Ireland
6School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, Dublin, Ireland
7Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, Dublin, Ireland
8Department of Clinical Neurosciences, Royal Free Campus, UCL Queen Square Institute of Neurology, London, U.K
9Academic Unit of Neurology, School of Medicine, Trinity College Dublin, Dublin, Ireland
*Corresponding author: Prof. Dominick JH McCabe, Vascular Neurology Research Foundation, c/o Department of Neurology, Tallaght University Hospital/ AMNCH, Tallaght, Dublin 24, Ireland
Received: March 15, 2021; Accepted: April 12, 2021; Published: April 19, 2021
Abstract
Introduction: The optimal time interval after venepuncture to perform platelet function/reactivity testing at low shear stress on the novel AGGRESTAR PL-12® platelet function analyser in non-Chinese Cerebrovascular Disease (CVD) patients is unknown.
Methods: Twelve TIA/ischaemic stroke patients were recruited to this cross-sectional, methodological study: 3 on aspirin monotherapy, aspirindipyridamole combination therapy, clopidogrel monotherapy and aspirinclopidogrel combination therapy, respectively. The PL-12 (‘mode 2’) was used to calculate the % maximum aggregation rate to fixed doses of arachidonic acid (%MARAA) and adenosine diphosphate (%MARADP). Samples were analysed every 15 minutes from 30-135 minutes, and every 30 minutes between 165-225 minutes after venepuncture to calculate the time interval providing optimal interassay Coefficients of Variation (CVs).
Results: Mean CVs were ≤ 7.37% for the %MARAA assay in patients on aspirin monotherapy or combination therapy, and ≤ 10.24% for the %MARADP assay in patients on clopidogrel monotherapy or combination therapy if assays were performed between 90-120 minutes post-venepuncture. CVs ≤ 10% were also obtained from assays performed between 90-165 minutes postvenepuncture on aspirin monotherapy or combination therapy.
Discussion: Reliable and reproducible platelet function/reactivity data can be obtained with the AGGRESTAR PL-12 analyser in non-Chinese CVD patients on commonly-prescribed antiplatelet monotherapy or combination therapy regimens between 90-120 minutes post-venepuncture.
Keywords: AGGRESTAR PL-12® platelet function analyser; High ontreatment platelet reactivity; Performance metrics; Co-efficient of variation; Ischaemic stroke; Transient ischaemic attack
Introduction
Antiplatelet therapy is one of the cornerstones of secondary preventive therapy in patients with a non-cardioembolic Transient Ischemic Attack (TIA) or ischaemic stroke [1-3]. However, an important proportion of these patients with ischaemic Cerebrovascular Disease (CVD) are not protected from recurrent vascular events with commonly-prescribed, antiplatelet regimens which are chosen based on evidence derived from populations of participants in randomised controlled trials [2]. An emerging body of data indicate that identification of ex vivo antiplatelet Highon- Treatment Platelet Reactivity (HTPR) may predict the risks of recurrent vascular events or outcomes in CVD patients, but we do not currently have evidence to alter and personalise antiplatelet regimens based on an individual CVD patient’s antiplatelet-HTPR status [4,5]. Furthermore, the prevalence of antiplatelet-HTPR varies according to the device used and the shear stress levels to which platelets are exposed [6-8]. Therefore, there is a strong argument in favour of simultaneously assessing antiplatelet-HTPR status with more than one test of platelet function/reactivity until it is clarified which are the most informative at predicting the risk of recurrent vascular events on commonly-prescribed antiplatelet therapy [5].
Platelet function/reactivity may be assessed with different laboratory tests which may provide discordant results when assays are performed simultaneously [2,6-9]. The original method of platelet aggregometry is time-consuming, labour intensive and operator-dependent [10,11]. Therefore, one needs ease of access to user-friendly tests of platelet function/reactivity, which are reliable, reproducible and cost-effective, with methodology optimised to suit specific disease states.
The AGGRESTAR PL-12® is a novel, platelet function analyser designed to assess ex vivo aspirin-HTPR status at low shear stress in response to stimulation with fixed doses of Arachidonic Acid (AA), and P2Y12 receptor antagonist-HTPR status following stimulation with Adenosine Diphosphate (ADP) within a disposable cuvette (SINNOWA Medical Science & Technology Co., Nanjing, China). The device uses a sensitive ‘sequential platelet counting method’ based on an automated impedance technique to quantify platelet aggregation in 3.8% citrate-anticoagulated whole blood [10,12]. The device can record the ‘% maximum aggregation rate (%MAR)’ and average aggregation rate (%AAR) 6.5 minutes after the addition of respective agonists. Preliminary data suggest that assessment of antiplatelet- HTPR status with a precursor of the PL-12 (PL-11®) may assist with prediction of the response to antiplatelet therapy in the clinical setting in Chinese patients with ischaemic heart disease [13,14] and TIA and ischaemic stroke also [1]. Furthermore, a recent randomised trial suggested that using data from the PL-12 to personalise antiplatelet therapy can significantly improve outcomes in the 6 month period after percutaneous coronary intervention [15]. Most studies on the PL-11 and PL-12 reported that samples were analysed within 2 hours of venepuncture, implying that samples in these studies were stable at any stage from immediately after venepuncture to up to 2 hours after venepuncture [1,10,13,14,16,17]. To our knowledge, the performance metrics of the PL-12 over an extended time interval have not been studied in CVD patients on antiplatelet therapy outside China.
The main aim of this pilot, ‘methodology-focused’ study was to establish the optimal time interval after venepuncture to perform platelet function/reactivity testing on the PL-12 in non-Chinese CVD patients on commonly-prescribed antiplatelet treatment regimens to produce the most reliable and reproducible data from this device with the lowest coefficients of variation. Based on preliminary work in our lab in healthy controls, we hypothesised that the results of platelet function/reactivity testing would vary over time from venepuncture on this device, and that optimal time intervals after venepuncture would be identifiable to guide future studies with the PL-12 in CVD and non-CVD patient populations.
Methodology
Study design
This single centre, prospective, cross-sectional observational study enrolled 12 eligible patients with prior TIA or ischaemic stroke at our secondary and tertiary referral university teaching hospital between November 2018 and December 2020. Patients were recruited from the Rapid Access Stroke Prevention Clinics, Neurology, Acute Stroke, Age-Related Health Care or Vascular Surgery services.
The study was fully approved by the St James’s Hospital/AMNCHTallaght University Hospital Research Ethics Committee (REC Reference: 2011/35/03). Written informed consent was obtained from all patients.
Inclusion criteria
We included eligible patients in the early or late phases after a transient ischaemic attack or ischaemic stroke who were on commonly-prescribed antiplatelet therapy for at least ≥ 7 days (aspirin monotherapy, aspirin-dipyridamole combination therapy, clopidogrel monotherapy, or aspirin-clopidogrel combination therapy; N=3 in each group). All patients were under the care of one of the participating consultants, and the diagnosis was clinically confirmed in all cases by the assessing consultant and an experienced vascular neurology research registrar.
Exclusion criteria
Patients were excluded if they had a myocardial infarction or venous thromboembolism within the preceding 3 months; ongoing unstable angina or unstable symptomatic peripheral vascular disease; platelet count < 100 x 109/L; known bleeding or clotting diathesis, including known platelet-related bleeding disorders; active proven vasculitis; active neoplasia; Non-Steroidal Anti-Inflammatory Drug (NSAID) intake other than aspirin, alone or in combination with dipyridamole or clopidogrel, in the preceding 11 days.
Medication adherence
Adherence to antiplatelet therapy in inpatients was confirmed by checking the inpatient prescription chart to ensure the medication had been dispensed and taken for ≥ 7 days. Adherence in all outpatients was assessed by history taking alone by phoning participants the day prior to their scheduled visit to confirm complete medication adherence for ≥ 7 days before venepuncture in this particular study. If complete adherence was not initially confirmed, any issues potentially affecting adherence in outpatients were discussed and the study visit postponed for 11 days until full adherence was subsequently verbally confirmed.
Venepuncture and Sample Collection
After resting for >20 minutes to minimise platelet activation in vivo, careful, ‘atraumatic venepuncture’ was performed using a standardised protocol [18]. Blood was drawn from a free-flowing antecubital vein using a sterile 21G Butterfly® needle (VenisystemsTM, Hospira) and a Vacutainer® system with a luer adaptor (Becton Dickinson Vacutainer® Systems, UK). The tourniquet was applied to the arm and was released during collection of the first 3 ml of blood into a 3 ml sterile vacuette containing 3.2% sodium citrate, which was subsequently discarded. Eleven further vacuettes containing 3.8% sodium citrate (Sinnowa, China) were filled, gently inverted twice and gently placed in a rack at room temperature until each individual sample was used for testing once at 11 pre-planned time intervals after venepuncture. We performed assays every 15 minutes from 30-135 minutes, and then at 30-minute intervals, so that data were available for each patient at 30, 45, 60, 75, 90, 105, 120, 135, 165, 195 and 225 minutes after venepuncture.
Platelet agonists
1000 mM stock powder of arachidonic acid (AA) was stored at -20°C. After removal from the -20°C freezer, the stock powder was diluted with 1 ml of distilled H2O. 70μl of AA (2 mg/ml) was prepared for each assay, but the machine was calibrated to dispense only 25μl for each PL-12 AA test (see below). Sufficient aliquots of AA were stored in a 4°C fridge to be used during these quality control and timing experiments; the remaining aliquots were stored at -20°C for future use. The stock solution of Adenosine 5’-Diphosphate (ADP) (50 umol/L) was stored in a 4°C fridge.
PL-12 assays: There are 4 modes/study paradigms on the PL-12. Mode 1 is ‘automated’ so that all platelet agonists are pre-prepared and placed in the device at the beginning of the assay. A single blood sample is used to test the platelet aggregation response to individual agonists (AA and ADP, respectively) during the automated protocol. Mode 2 uses separate blood samples (S1 and S2) for each assay and requires more manual involvement of the operator to individually add the relevant agonists to assess platelet aggregation in response to AA (from reagent container 1 [R1]) and ADP (from reagent container 2 [R2]), respectively. However, mode 2 confers the advantage that the platelet agonists can be quickly removed from the 4°C storage fridge for use and then immediately returned to the fridge in between individual assays to limit their exposure time to the ambient temperature prior to the conduct of the platelet function analysis, thus potentially preserving agonist quality. Mode 3 can be used to manually add a single agonist to a single blood sample. Mode 4 automatically adds a single agonist to a single blood sample. Based on exploratory work on modes 1 and 2 in healthy controls who were not on antiplatelet therapy to suit the timing of completion of other concurrently-conducted platelet function/reactivity tests, mode 2 was deemed to produce the most reproducible results for the AA assays in our laboratory, so this mode was chosen for all assays in our CVD patients.
Mode 2 was selected from the dropdown menu. Although the device only dispenses 25μl of agonist during each test, 70μl of AA was pipetted into agonist container R1, and 70μl of ADP into agonist container R2 to counteract any potential ‘dead space’ in the containers, as per the manufacturer’s advice. A 3.8% citrate-anticoagulated whole blood sample was then immediately gently inverted 4-5 times, and 250μl of blood was pipetted into the first slot of a ‘twin’ polycarbonate cuvette in position S1, and a separate 250μl of blood was pipetted into the adjoining slot of the twin cuvette in position S2; the handle of the twin polycarbonate cuvette was positioned to point outwards towards the door of the machine. The assay was started by pressing the ‘start button’, and sample analysis was completed in approximately 13 minutes.
The PL-12 measures platelet function/reactivity via a ‘sequential platelet counting method’ over a fixed time period based on an automated electrical impedance principle [10,12]. The analyser automatically counts platelets 5 different times: 2 counts are taken before and 3 counts are taken after the automated dispensing of 25μl of the relevant agonist into the pre-positioned sample tubes. The agonists induce platelet aggregation; because aggregates are too large to be counted as single platelets [15], the recorded platelet count in the sample decreases over time following agonist stimulation proportional to the degree of platelet inhibition in the sample with the antiplatelet regimen in question [12].
The device calculates the % Maximal Platelet Aggregation Rate (MAR) according to the following formula [1,10]:
High on-Treatment Platelet Reactivity (HTPR) on aspirin has been defined in a ‘case-control/cross-sectional’ manner as a %MAR induced by AA (%MARAA) of ≥30% [12], and clopidogrel-HTPR has been defined as a % MAR induced by ADP (%MARADP) of ≥55% in Chinese patients [12,14].
Statistical analysis
Descriptive statistics were used to describe the demographic and vascular risk factor profiles of recruited patients using excel software (Version 16.16.27, Retail License 2016). The standard deviation and mean of 2 x %MARs from each patient at 2 consecutive time points after venepuncture were initially calculated i.e. from 30 and 45 minutes, 60 and 75 minutes, 90 and 105 minutes, 120 and 135 minutes, and 165 and 195 minutes, and 195 and 225 minutes after venepuncture. These standard deviations were then divided by the mean values and multiplied by 100 to calculate the ‘intra-assay CV’ for each of the above time intervals. We then calculated the mean ‘interassay CVs’ of the %MARAA and the %MARADP data by averaging the respective %MAR intra-assay CVs obtained from 3 patients on each antiplatelet regimen at each of the above time periods. The intra-assay SEM (standard error of the mean) was also calculated by dividing the standard deviation by the square root of the sample size at each of the above time intervals. We then calculated the mean inter-assay SEM of the %MARAA and the %MARADP data by averaging the respective %MAR intra-assay SEMs obtained from 3 patients on each antiplatelet regimen at each of the above time periods. We subsequently grouped our %MAR intra-assay CV data into broader time categories and then calculated the mean %MAR inter-assay CVs using data obtained from 3 patients on each antiplatelet regimen during these broader time categories. These analyses were performed to explore the optimal and practical time intervals at which clinicians or scientists could perform the PL-12 assays after venepuncture in CVD patients. We defined the optimal interval a priori as a time period during which the mean inter-assay CVs for the %MAR on the relevant assays were rounded to ≤ 10%, if at all possible, because ≤ 10% is often considered to be an acceptable CV for a test of platelet function [19,20]. For descriptive and exploratory purposes, we calculated the proportion of patients who had antiplatelet-HTPR on the relevant assays on each of the 4 different treatment regimens, using the case-control/cross-sectional definitions of antiplatelet-HTPR outlined above. However, this study was not designed or powered to reliably assess the prevalence of antiplatelet-HTPR in TIA/ischaemic stroke patients. Graphs were generated with Prism Graph Pad, Version 9.0.0.
Results
Twelve Caucasian patients were recruited, 8 of whom were women, with 3 patients on each antiplatelet regimen, respectively. The demographic and vascular risk factor profiles of participants are outlined in Table 1. The mean age of participants was 62.5 years (minimum-maximum range: 45-82 years). The time interval from last TIA or ischaemic stroke to recruitment ranged from 7-3832 days (median = 634.5 days; interquartile range [IQR]: 66.3-1278.5 days). One patient had an initial TIA or stroke whilst on antiplatelet therapy (aspirin 75mg daily) leading to a change to short-term aspirinclopidogrel combination therapy, and two patients had a recurrent TIA or stroke whilst on antiplatelet therapy following their initial cerebrovascular event, prompting a change to clopidogrel by their treating physician prior to recruitment (Table 1).
Parameter
Aspirin
Monotherapy
(N = 3)Aspirin +
Dipyridamole MR
(N = 3)Clopidogrel
Monotherapy
(N = 3)Aspirin +
Clopidogrel
(N = 3)Median Age in Years [IQR]
61 [58-64.5]
49 [47–58.5]
62 [59.5-70]
78 [62.5-80]
Female Sex
3 (100%)
2 (66%)
2 (66%)
1 (33%)
White European
3 (100%)
3 (100%)
3 (100%)
3 (100%)
TIA as Presenting Cerebrovascular Event
3 (100%)
1 (33%)
3 (100%)
0
Ischaemic Stroke as Presenting Event
0
2 (66%)
0
3 (100%)
Median interval since last TIA/Ischaemic Stroke and Duration on current Antiplatelet Regimen in Days [IQR]
154 [83.5-781.5]
3100 [2168-3466]
1009 [634.5-1062]
12 [9.5-48]
Prior History of TIA or Ischaemic Stroke
1 (33%)
0
0
2 (66%)
History of Recurrent TIA/Stroke on Antiplatelet Therapy
0
0
2 (66%)
0
Hypertension
1 (33%)
1 (33%)
1 (33%)
3 (100%)
IHD
0
0
0
1 (33%)
Diabetes Mellitus
0
0
1 (33%)
0
Hyperlipidaemia*
3 (100%)
3 (100%)
3 (100%)
3 (100%)
Migraine
1 (33%)
1 (33%)
1 (33%)
0
Atrial Fibrillation
0
0
0
0
Prior VTE
0
0
0
0
PVD
0
0
0
0
Statin Therapy
3 (100%)
3 (100%)
3 (100%)
3 (100%)
Antihypertensive Medication
1 (33%)
1 (33%)
1 (33%)
3 (100%)
Never Smoked
3 (100%)
1 (33%)
3 (100%)
2 (66%)
Ex-Smoker
0
1 (33%)
0
0
Current Smoker
0
1 (33%)
0
1 (33%)
Legend for Table 1: TIA: Transient Ischaemic Attack; Hyperlipidaemia (established diagnosis on lipid-lowering treatment or based on ‘secondary prevention targets’) = Total cholesterol >3.5mmol/L, LDL cholesterol >1.8mmol/L or triglycerides >1.7mmol/L; VTE = Venous Thromboembolism, including deep vein thrombosis or pulmonary embolism; IHD = Ischaemic Heart Disease, including prior angina, myocardial infarction or coronary revascularisation; PVD = Peripheral Vascular Disease.
Table 1: Demographic and vascular risk profiles of study participants at enrolment. Values are medians [Interquartile Range (IQR)] or absolute values (%), where appropriate.
All CV data which are discussed in the results section are interassay CVs, unless stated otherwise. The mean CVs [SEMs] of the relevant assays are outlined in Tables 2a-2d. The range of mean CVs (minimum-maximum) were 1.54-11.15 % for the MARAA data and 1.13-6.25 % for the MARADP data in patients on aspirin monotherapy between 30-225 minutes after venepuncture (Table 2a), with the lowest CVs on both assays from grouped data between 90-120 minutes after venepuncture (Table 3). The mean CVs for both the MARAA (1.79-5.45 %) and MARADP (1.31-6.37 %) data were excellent between 30-225 minutes after venepuncture on aspirin-dipyridamole (Table 2b). The mean CVs in patients on clopidogrel monotherapy were 8.79-26.08 % for the MARAA data and 8.42-16.34 % for the MARADP data overall, with optimal mean CVs for the most important MARADP data on this treatment regimen between 105-120 minutes (8.42%) or 135-165 minutes (9.35%) (Table 2c), but acceptable mean CVs from grouped MARADP data between 90-120 minutes after venepuncture (10.24%; Table 3). The mean CVs were 6.94-18.93 % for the MARAA data and 5.89-13.95 % for the MARADP data overall in patients on aspirin-clopidogrel combination therapy, with acceptable CVs of ≤ 10.06% for both the MARAA and MARADP data between 90-165 minutes after venepuncture (Table 2d). Having established that the CVs from some assays were sub-optimal <90 minutes after venepuncture on certain treatment regimens, we grouped the mean CV data into broader time intervals to establish practical and optimal intervals to perform relevant assays after venepuncture. The lowest mean CVs were obtained for most relevant assays between ≥ 90-120 minutes, with acceptable CVs also obtained between ≥ 90-165 minutes after venepuncture on aspirin monotherapy, aspirin-dipyridamole or aspirin-clopidogrel combination therapy (Table 3).
Time interval after venepuncture on Aspirin Monotherapy (minutes)
Inter-assay CVs for %MARAA [SEM]
Inter-assay CVs for %MARADP [SEM]
30-45
4.53 [2.46]
2.02 [1.23]
60-75
4.25 [2.38]
4.18 [2.56]
90-105
3.78 [2.16]
3.23 [1.98]
105-120
3.91 [2.3]
1.13 [0.7]
120-135
8.31 [4.56]
6.19 [3.51]
135-165
11.15 [6.18]
6.25 [3.58]
165-195
1.54 [0.9]
2.29 [1.41]
195-225
2.29 [1.36]
2.78 [1.68]
Table 2a: Mean inter-assay CVs for the %MARAA and %MARADP data, with the corresponding inter-assay Standard Error of the Mean [SEM] derived from 3 patients on Aspirin Monotherapy at each time interval after venepuncture.
Time interval after venepuncture on Aspirin and Dipyridamole Combination Therapy (minutes)
Inter-assay CVs for %MARAA [SEM]
Inter-assay CVs for %MARADP [SEM]
30-45
3.85 [2.08]
1.31 [0.71]
60-75
4.75 [2.53]
3.13 [1.81]
90-105
5.45 [2.83]
2.60 [1.41]
105-120
4.82 [2.53]
3.71 [2.05]
120-135
3.82 [2.2]
6.32 [3.46]
135-165
3.13 [1.8]
6.37 [3.3]
165-195
3.22 [1.8]
3.48 [1.81]
195-225
1.79 [0.98]
4.03 [2.25]
Table 2b: Mean inter-assay CVs for the %MARAA and %MARADP data, with the corresponding inter-assay SEM derived from 3 patients on Aspirin and Dipyridamole combination therapy at each time interval after venepuncture.
The proportion of patients who had antiplatelet-HTPR on the relevant AA or ADP assays was quantified by calculating the mean %MAR in each patient, derived from 5 different assay time points on their respective antiplatelet regimens: 90 mins, 105 mins, 120 mins, 135 mins and 165 mins after venepuncture (Figure 1). All 3 patients on aspirin monotherapy and aspirin-dipyridamole combination therapy had aspirin-HTPR (mean %MARAA ≥ 30%). One of the patients on clopidogrel monotherapy had clopidogrel-HTPR, with a mean %MARADP ≥ 55%. Amongst the 3 patients on aspirinclopidogrel combination therapy, 1 (33%) had aspirin-HTPR and 2 (66%) had clopidogrel-HTPR.
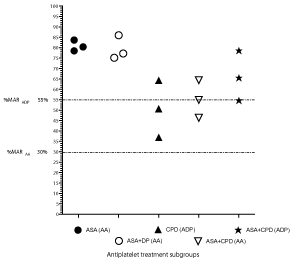
Figure 1: Antiplatelet-HTPR status based on the mean %MAR in each patient on his/her respective antiplatelet regimen using Mode 2 on the PL-12 (derived from
5 different assay time points in each patient between 90-165 minutes after venepuncture - see main text). Aspirin-HTPR was defined as a %MARAA ≥30% [14] and
clopidogrel-HTPR as a %MARADP ≥55% based on prior literature [12,14]. ASA: Aspirin; DP: Dipyridamole; CPD: Clopidogrel.
Discussion
To our knowledge, this is the first study to assess the performance of this relatively novel, user-friendly PL-12 device in a non-Chinese population with CVD. The optimal time interval after venepuncture to assess the %MAR in white European CVD patients on commonlyprescribed antiplatelet regimens on mode 2 of the PL-12 is between 90-120 minutes, with mean CVs of ≤ 7.37% for the %MARAA assay in patients on aspirin monotherapy or combination therapy, and ≤ 10.24% for the %MARADP assay in patients on clopidogrel, alone or in combination with aspirin (Table 3). However, the time interval for sample analysis can easily be extended to between 90-135 minutes, and if required, to between 90-165 minutes after venepuncture to provide reliable %MARAA results in patients on aspirin monotherapy, or aspirin in combination with dipyridamole or clopidogrel, thus facilitating operator flexibility if an error were to arise during analysis which necessitated preparation and processing of samples once again. Although we did not do simultaneous assessment of platelet function/ reactivity with other testing platforms during this ‘methodologyfocused study’, the inter-assay CVs compare favourably with the intra-assay CVs obtained on other common user-friendly tests of platelet function/reactivity in our laboratory [7,21].
The most stable CVs across the entire time spectrum of our experiments from 30-225 minutes after venepuncture were observed in the subgroup of patients on aspirin-dipyridamole combination therapy (1.31-6.37 %). This might potentially reflect the additional inhibitory effects of dipyridamole over aspirin alone on platelet function/reactivity ex vivo which has been observed in healthy controls [22], and also in CVD patients [23,24]. The CVs for the %MARADP assays in patients on clopidogrel monotherapy were lowest between 105-120 minutes and 135-165 minutes after venepuncture, but our grouped data indicated that 90-120 minutes is also the optimal and most practical time at which to plan to perform this assay in conjunction with other assays (CV of ≤ 10.24%; Table 3). The CVs for the %MARAA assays were not optimal in patients on clopidogrel monotherapy in our lab between 90-120 minutes (21.54%) or 90- 165 minutes (25.47%) after venepuncture. However, these %MARAA data are not likely to be of clinical importance in CVD patients if one uses case-control/cross sectional definitions of HTPR because the %MARADP data are the most relevant data in patients on P2Y12 receptor antagonists. It is not clear why there were worse intra-assay CVs on the %MARAA assays in patients on clopidogrel monotherapy compared with those on aspirin-clopidogrel combination therapy, but it might well relate to the inhibition and stabilisation of AAinduced platelet reactivity by aspirin in those on aspirin-clopidogrel combination therapy.
Importantly, we observed slightly higher mean CVs when samples were analysed within 75 minutes after venepuncture for the %MARAA assay in patients on aspirin-clopidogrel combination therapy (Table 2d), and before 105 minutes for the %MARADP assay in patients on clopidogrel monotherapy (Table 2c). Therefore, based on our grouped data (Table 3), we advise that one should not analyse samples too early and should aim to commence sample analysis in European CVD patients after 90 minutes, and ideally between 90- 120 minutes after venepuncture, as outlined above. Prior studies in Chinese patients with the PL-11 or PL-12 analysed samples ‘within 120 minutes of venepuncture’, but the precise analysis time intervals during this 120 minute period were not specified [1,10,14-17]. The authors did not report on whether they performed similar preliminary timing experiments to those outlined in our study, so we cannot comment on whether the potential variability in sample results early (< 90 minutes) after venepuncture differs between European and Chinese patients.
Time interval after venepuncture on Clopidogrel Monotherapy (minutes)
Inter-assay CVs for %MARAA [SEM]
Inter-assay CVs for %MARADP [SEM]
30-45
21.81 [4.88]
12.04 [3.13]
60-75
14.82 [3.45]
16.19 [4.13]
90-105
26.08 [4.31]
14.2 [3.9]
105-120
16.82 [4.08]
8.42 [2.05]
120-135
15.57 [3.45]
14.65 [4.81]
135-165
20.67 [7.58]
9.35 [3.15]
165-195
18.71 [6.01]
10.67 [3.83]
195-225
8.79 [2.65]
16.34 [5.66]
Table 2c: Mean inter-assay CVs for the %MARAA and %MARADP data, with the corresponding inter-assay SEM derived from 3 patients on Clopidogrel monotherapy at each time interval after venepuncture.
Time interval after venepuncture on Aspirin and Clopidogrel Combination Therapy (minutes)
Inter-assay CVs for %MARAA [SEM]
Inter-assay CVs for %MARADP [SEM]
30-45
13.44 [3.21]
6.61 [3.08]
60-75
13.8 [5.08]
6.1 [2.61]
90-105
6.94 [2.73]
5.89 [3.1]
105-120
8.09 [3.33]
9.68 [4.81]
120-135
9.01 [3.38]
6.95 [2.98]
135-165
9.84 [3.4]
10.06 [4.15]
165-195
7.64 [2.65]
13.95 [4.96]
195-225
18.93* [6.75]
7.24* [2.17]
Table 2d: Mean inter-assay CVs for the %MARAA and %MARADP data, with the corresponding inter-assay SEM derived from 3 patients on Aspirin and Clopidogrel combination therapy at each time interval after venepuncture. *Mean CVs for %MAR values were derived from 2 patients at this late time interval between 195-225 minutes in this treatment subgroup.
Time interval after Venepuncture (minutes)
Antiplatelet Regimen (Agonist)
Mean CVs for %MAR
90-120
Aspirin (AA)
4.5
90-135
Aspirin (AA)
8.2
90-165
Aspirin (AA)
7.85
90-120
Aspirin (ADP)
2.43
90-135
Aspirin (ADP)
4.84
90-165
Aspirin (ADP)
4.55
90-120
Aspirin + Dipyridamole (AA)
4.6
90-135
Aspirin + Dipyridamole (AA)
4.44
90-165
Aspirin + Dipyridamole (AA)
4.67
90-120
Aspirin + Dipyridamole (ADP)
4.25
90-135
Aspirin + Dipyridamole (ADP)
5.08
90-165
Aspirin + Dipyridamole (ADP)
5.79
90-120
Clopidogrel (ADP)
10.24
90-135
Clopidogrel (ADP)
13.99
90-165
Clopidogrel (ADP)
13.22
90-120
Clopidogrel (AA)
21.54
90-135
Clopidogrel (AA)
19.79
90-165
Clopidogrel (AA)
25.47
90-120
Aspirin + Clopidogrel (AA)
7.37
90-135
Aspirin + Clopidogrel (AA)
9.2
90-165
Aspirin + Clopidogrel (AA)
10.1
90-120
Aspirin + Clopidogrel (ADP)
7.07
90-135
Aspirin + Clopidogrel (ADP)
9.97
90-165
Aspirin + Clopidogrel (ADP)
9.52
Table 3: Mean inter-assay CVs for the %MAR data at ‘grouped time intervals’ between 90-120 minutes, 90-135 minutes, 90-165 minutes on different antiplatelet regimens using AA or ADP as the agonists (N = 3 patients on each regimen). Higher CVs (>10.5) are highlighted in bold and italics.
A recent systematic review by our group reported a prevalence of antiplatelet-HTPR ex vivo in CVD patients with a range of platelet function/reactivity assays of 3-65% with aspirin, 8-56% with clopidogrel and 1.8-35 % with aspirin and clopidogrel in patients on aspirin-clopidogrel combination therapy, but PL-12 data were not included in that analysis [5]. Our current methodology-focused study was not designed or powered to reliably assess the prevalence of antiplatelet-HTPR in European CVD patients with the PL-12. However, aspirin-HTPR was observed in all 3 patients (100%) on aspirin monotherapy and aspirin-dipyridamole combination therapy and in 1 patient (33%) on aspirin-clopidogrel combination therapy, with clopidogrel-HTPR observed in 1 patient (33%) on clopidogrel monotherapy and in 2 (66%) on aspirin-clopidogrel combination therapy. These pilot prevalence figures are not explained by poor adherence to antiplatelet medication due to the measures taken to optimise adherence to antiplatelet therapy prior to testing in this study, and all were established on their treatment regimen for at least 7 days. Further, larger studies are clearly warranted to address this issue in non-Chinese CVD patients.
The limitations of this study include the small number of patients who were recruited and studied once, mainly in the late phase after symptom onset, so we cannot comment on the reproducibility of the PL-12 assays between the early and late phases after TIA/stroke. However, this is balanced by the detailed timing experiments and large number of assays which were performed on consecutive blood samples taken from each participant to mirror precise experimental conditions during future planned studies on this device. The casecontrol/ cross-sectional definitions of HTPR for the AA and ADP assays were based on data derived from a Chinese patient population, so we are uncertain as yet whether these definitions/thresholds are appropriate for a non-Chinese CVD population. However, as stated above, assessment of the prevalence of HTPR was not the main aim of this study; this issue clearly needs to be addressed in a much larger study of non-Chinese CVD patients with this device.
Conclusion
The optimal and most practical time to perform all novel AGGRESTAR PL-12® platelet function/reactivity assays in European CVD patients on commonly-prescribed antiplatelet regimens in our laboratory is between 90-120 minutes after venepuncture on mode 2 of the device. These data reinforce the importance of performing independent timing experiments on any new device which is being introduced into a research laboratory, and should be informative to platelet scientists and clinician scientists to enable them to obtain the most reliable and reproducible data from the AGGRESTAR PL-12 in non-Chinese CVD patients.
Acknowledgment
Dr. Offiah’s research was funded by The Meath Foundation and The Vascular Neurology Research Foundation Ireland. The vacuettes, AA and ADP platelet agonists, the PL-12 device and reagents for the experiments were supplied via an unrestricted educational grant from Sinnowa, China. Dr Delaney’s and Dr. Smith’s research were funded by The Vascular Neurology Research Foundation Ireland. Dr. Murphy’s research was funded by the Trinity College Dublin Innovation Bursary, The Meath Foundation, Joint Irish Institute of Clinical Neuroscience/Merck Serono Fellowship in Neuroscience Grant, The Vascular Neurology Research Foundation Ireland, and by an unrestricted educational grant from Bayer HealthCare, Ireland and Verum Diagnostica, GmbH. None of the above charities or funding bodies had any influence on the design or conduct of this study, or had any influence on the decision to submit the final manuscript for publication. The manuscript has not been submitted elsewhere and has not been published elsewhere in whole or in part.
Declaration of Interest
The vacuettes, AA and ADP platelet agonists, the PL-12 device and reagents for the experiments were supplied via an unrestricted educational grant from Sinnowa, China to Prof. McCabe’s Vascular Neurology Research group at AMNCH-TUH/TCD. However, the study design, study recruitment, data analysis and data interpretation were performed by the authors completely independently of Sinnowa, China, and the company had no influence on the decision to submit the manuscript for peer-review. The manuscript was not shown to any designated representatives of Sinnowa prior to submission for peer-review. The authors report no conflict of interest and have nothing else to disclose.
References
- Zhang L, Hu X, Zhu J, Cai X, Kong Y, Wang H, et al. Adequate platelet function inhibition confirmed by two inductive agents predicts lower recurrence of ischemic stroke/transient ischemic attack. BioMed Res Int. 2017; 2017: 3504950.
- Lim ST, Coughlan CA, Murphy SJ, Fernandez-Cadenas I, Montaner J, Thijs V, et al. Platelet function testing in transient ischaemic attack and ischaemic stroke: A comprehensive systematic review of the literature. Platelets. 2015; 26: 402-412.
- Zhang Q, Wang C, Zheng M, Li Y, Li J, Zhang L, et al. Aspirin plus Clopidogrel as secondary prevention after stroke or transient ischemic attack: A systematic review and meta-analysis. Cerebrovasc Dis. 2015; 39: 13-22.
- Fiolaki A, Katsanos AH, Kyritsis AP, Papadaki S, Kosmidou M, Moschonas IC, et al. High on treatment platelet reactivity to aspirin and clopidogrel in ischemic stroke: A systematic review and meta-analysis. J Neurol Sci. 2017; 376: 112-116.
- Lim ST, Thijs V, Murphy SJX, Fernandez-Cadenas I, Montaner J, Offiah C, et al. Platelet function/reactivity testing and prediction of risk of recurrent vascular events and outcomes after TIA or ischaemic stroke: systematic review and meta-analysis. J Neurol. 2020; 267: 3021-3037.
- Harrison P, Segal H, Blasbery K, Furtado C, Silver L, Rothwell PM. Screening for aspirin responsiveness after transient ischemic attack and stroke: comparison of 2 point-of-care platelet function tests with optical aggregometry. Stroke. 2005; 36: 1001-1005.
- Kinsella JA, Tobin WO, Cox D, Coughlan T, Collins R, O’Neill D, et al. Prevalence of ex vivo high on-treatment platelet reactivity on antiplatelet therapy after transient ischemic attack or ischemic stroke on the PFA-100® and VerifyNow®. J Stroke Cerebrovasc Dis. 2013; 22: e84-92.
- Murphy SJX, Lim ST, Kinsella JA, Tierney S, Egan B, Feeley TM, et al. Relationship between ‘on-treatment platelet reactivity’, shear stress, and micro-embolic signals in asymptomatic and symptomatic carotid stenosis. J Neurol. 2020; 267: 168-184.
- Harrison P, Segal H, Silver L, Syed A, Cuthbertson FC, Rothwell PM. Lack of reproducibility of assessment of aspirin responsiveness by optical aggregometry and two platelet function tests. Platelets. 2008; 19: 119-124.
- Guan J, Cong Y, Ren J, Zhu Y, Li L, Deng X, et al. Comparison between a new platelet count drop method PL-11, light transmission aggregometry, VerifyNow aspirin system and thromboelastography for monitoring short-term aspirin effects in healthy individuals. Platelets. 2015; 26: 25-30.
- Agarwal S, Coakley M, Reddy K, Riddell A, Mallett S. Quantifying the effect of antiplatelet therapy: a comparison of the Platelet Function Analyzer (PFA- 100) and modified Thromboelastography (mTEG) with light transmission platelet aggregometry. Anesthesiology. 2006; 105: 676-683.
- Zhang JJ, Gao XF, Ge Z, Tian NL, Liu ZZ, Lin S, et al. High platelet reactivity affects the clinical outcomes of patients undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2016; 16: 240.
- Wu Y, Yu H, Tang HQ, Su Y, Shi TL, Liu S, et al. PXR polymorphisms have impact on the clinical efficacy of clopidogrel in patients undergoing percutaneous coronary intervention. Gene. 2018; 653: 22-28.
- Gao XF, Lu S, Ge Z, Zuo GF, Wang ZM, Wang F, et al. Relationship between high platelet reactivity on clopidogrel and long-term clinical outcomes after drug-eluting stents implantation (PAINT-DES): a prospective, propensity score-matched cohort study. BMC Cardiovasc Disord. 2018; 18: 103.
- Zheng YY, Wu TT, Yang Y, Hou XG, Gao Y, Chen Y, et al. Personalized antiplatelet therapy guided by a novel detection of platelet aggregation function in stable coronary artery disease patients undergoing percutaneous coronary intervention: a randomized controlled clinical trial. Eur Heart J Cardiovasc Pharmacother. 2020; 6: 211-221.
- Yang Y, Chen W, Pan Y, Yan H, Meng X, Liu L, et al. Ticagrelor is Superior to Clopidogrel in Inhibiting Platelet Reactivity in Patients With Minor Stroke or TIA. Front Neurol. 2020; 11: 534.
- Ma L, Chen W, Pan Y, Yan H, Li H, Meng X, et al. Comparison of VerifyNow, thromboelastography, and PL-12 in patients with minor ischemic stroke or transient ischemic attack. Aging. 2021; 13: 8396-8407.
- Lim ST, Tobin WO, Murphy SJX, Kinsella JA, Smith DR, Lim SY, et al. Profile of reticulated platelets in the early, subacute and late phases after transient ischaemic attack or ischaemic stroke. Platelets 2020; Dec 21: 1-9 (doi: 10.1080/09537104.2020.1850670. Online ahead of print).
- Al Ghaithi R, Mori J, Nagy Z, Maclachlan A, Hardy L, Philippou H, et al. Evaluation of the Total Thrombus-Formation System (T-TAS): application to human and mouse blood analysis. Platelets. 2019; 30: 893-900.
- van der Meer PF, Karssing-van Leeuwen W, Kurtz J, Spengler HP, Blair A, Devine D, et al. A flow cytometric method for platelet counting in platelet concentrates. Transfusion. 2012; 52: 173-180.
- Tobin WO, Kinsella JA, Coughlan T, Collins DR, O’Neill D, Murphy RP, et al. High on-treatment platelet reactivity on commonly prescribed antiplatelet agents following transient ischaemic attack or ischaemic stroke: results from the Trinity Antiplatelet Responsiveness (TRAP) study. Eur J Neurol. 2013; 20: 344-352.
- Heptinstall S, Fox S, Crawford J, Hawkins M. Inhibition of platelet aggregation in whole blood by dipyridamole and aspirin. Thromb Res. 1986; 42: 215-223.
- Serebruany VL, Malinin AI, Sane DC, Jilma B, Takserman A, Atar D, et al. Magnitude and time course of platelet inhibition with Aggrenox and Aspirin in patients after ischemic stroke: the AGgrenox versus Aspirin Therapy Evaluation (AGATE) trial. Eur J Pharmacol. 2004; 499: 315-324.
- Tobin WO, Kinsella JA, Collins DR, Coughlan T, O’Neill D, Egan B, et al. Enhanced ex vivo inhibition of platelet function following addition of dipyridamole to aspirin after transient ischaemic attack or ischaemic stroke: first results from the Trinity Antiplatelet responsiveness (TRAP) study. Br J Haematol. 2011; 152: 640-647.